Ischemic stroke (infarction) of the spinal cord
- Understanding Spinal Cord Ischemia and Infarction
- Etiology and Pathophysiology of Spinal Cord Infarction
- Clinical Syndromes Caused by Spinal Vessel Lesions
- Diagnosis of Spinal Cord Infarction
- Treatment of Spinal Cord Ischemic Stroke
- Differential Diagnosis of Acute Myelopathy
- Prognosis and Rehabilitation
- When to Seek Urgent Medical Attention
- References
Understanding Spinal Cord Ischemia and Infarction
Ischemic stroke or infarction of the spinal cord is a rare but devastating neurological condition resulting from a disruption of blood supply to the spinal cord, leading to tissue damage (ischemia) and potentially irreversible neuronal death (infarction). Understanding the vascular anatomy of the spinal cord is crucial for comprehending the mechanisms and clinical presentations of these events.
Arterial Blood Supply to the Spinal Cord
The spinal cord receives its arterial blood supply from a complex network of longitudinal and segmental arteries:
- Longitudinal Arteries:
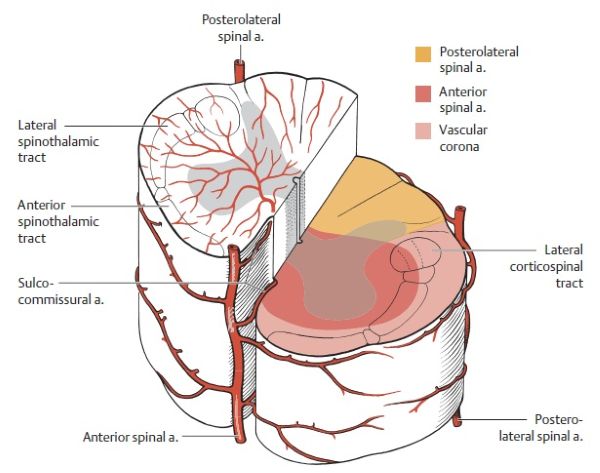
- Anterior Spinal Artery (ASA): Typically, a single artery formed by the union of branches from both vertebral arteries before they merge to form the basilar artery. The ASA runs as a continuous vascular trunk along the anterior median fissure of the spinal cord down to the conus medullaris (terminal cone). It then often loops posteriorly to connect with the posterior spinal arteries. The ASA supplies approximately the anterior two-thirds of the spinal cord, including the anterior and lateral horns of gray matter, and the anterior and lateral white matter columns (corticospinal and spinothalamic tracts).
- Posterior Spinal Arteries (PSAs): Usually, there are two PSAs, also originating from the vertebral arteries or the posterior inferior cerebellar arteries (PICAs). They descend along the posterolateral sulci of the spinal cord, near the entry points of the posterior (dorsal) nerve roots. The PSAs are often not continuous individual vessels but rather form anastomotic chains of smaller arteries where blood can circulate in either direction. They supply the posterior one-third of the spinal cord, including the posterior horns of gray matter and the posterior columns (gracile and cuneate fasciculi, responsible for vibration and proprioception).
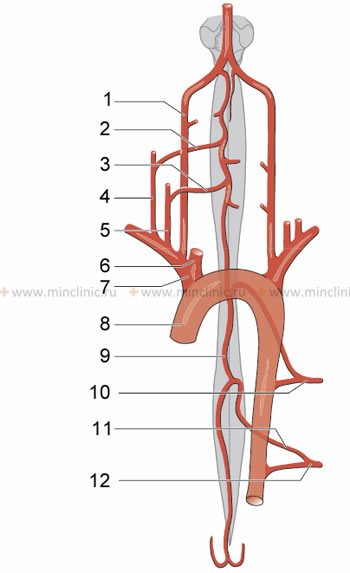
- Segmental (Radicular or Medullary) Arteries: The longitudinal spinal arteries (ASA and PSAs) receive crucial reinforcement from segmental arteries at various levels along the spinal cord. These segmental arteries arise from larger systemic arteries:
- In the cervical region, from branches of the vertebral arteries and the thyrocervical and costocervical trunks of the subclavian artery.
- In the thoracic and lumbar regions, from posterior intercostal and lumbar arteries originating from the aorta.
The anterior spinal artery gives off sulcocommissural branches at small intervals (approximately 200 such branches) that pass horizontally through the anterior median fissure. These branches fan out anterior to the anterior white commissure to supply almost all of the gray matter (anterior and central horns) and the surrounding rim of white matter, including parts of the anterior funiculi. Circumflex (enveloping) branches also arise from the ASA and anastomose with similar branches from the posterior spinal arteries, forming a pial arterial plexus or vascular crown (vasocorona) on the surface of the cord. The anterior branches of this vasocorona supply the anterolateral and lateral funiculi of the spinal cord, including most of the lateral corticospinal (pyramidal) tracts. The main neural structures supplied by the posterior spinal arteries are the posterior funiculi (dorsal columns) and the apex of the posterior horns of the spinal cord.
Diagram illustrating the contribution of segmental arteries to the arterial network of the spinal cord. Key: 1. Vertebral artery, 2. Anterior radicular artery C4-C5, 3. Anterior radicular artery C6-C8, 4. Costo-cervical trunk, 5. Thyro-cervical (shield-neck) trunk, 6. Common carotid artery, 7. Brachiocephalic trunk, 8. Aorta, 9. Anterior spinal (vertebral) artery, 10. Posterior intercostal artery Th4-Th6, 11. Great radicular artery (Adamkiewicz artery), 12. Posterior intercostal artery Th9-L1.
Spinal Cord Venous Drainage
The venous drainage of the spinal cord begins with capillaries within the gray matter, which form groups corresponding to the columns of neurons. These capillaries drain into intrinsic spinal cord veins. Most of these veins run radially towards the periphery of the spinal cord. Veins located closer to the center of the cord initially spread longitudinally, running parallel to the central canal, before exiting the spinal cord deep within its anterior or posterior median sulcus. On the surface of the spinal cord, the veins form extensive pial venous plexuses that deliver blood to longitudinally oriented collecting veins, primarily the anterior and posterior spinal veins. The posterior spinal collecting vein is generally larger and increases in size towards the lower part of the spinal cord. From these spinal collecting veins, blood flows via anterior and posterior radicular veins (typically 5 to 11 on each side of the spinal cord) into the internal vertebral venous plexus (plexus venosus vertebralis internus, also known as Batson's plexus).
Diagram of the venous drainage of the spinal cord. Key: 1. Arachnoid mater, 2. Dura mater, 3. Posterior external vertebral venous plexus, 4. Posterior spinal vein, 5. Posterior central vein, 6. Posterolateral spinal veins, 7. Sulcocommissural vein, 8. Sulcal vein, 9. Periosteum, 10. Anterior and posterior radicular veins, 11. Anterior internal spinal (vertebral) venous plexus, 12. Intervertebral vein, 13. Vertebral veins, 14. Anterior external spinal (vertebral) venous plexus, 15. Basivertebral vein, 16. Anterior spinal vein.
The internal vertebral venous plexus, surrounded by loose connective and adipose tissue, is located in the epidural space (incorrectly stated as subdural in original text; it is extradural/epidural) and is analogous to the dural venous sinuses of the brain. This valveless venous plexus communicates through the foramen magnum with these dural sinuses at the base of the skull. Venous blood also flows out from the internal plexus via intervertebral veins that pass through the intervertebral foramina. Through these intervertebral veins, blood enters the external vertebral venous plexus (plexus venosus vertebralis externus). This external plexus, among other connections, ultimately drains venous blood into systemic veins such as the azygos vein (which connects the superior and inferior vena cava, running to the right of the spine), lumbar veins, and vertebral veins.
Etiology and Pathophysiology of Spinal Cord Infarction
Causes of Ischemia and Stroke
The anterior and posterior spinal arteries themselves are usually not susceptible to primary atherosclerosis, unlike cerebral arteries. However, the spinal cord can suffer infarction (stroke) due to ischemia resulting from various pathological processes affecting its blood supply. Common causes include:
- Aortic Pathology:
- Aortic dissection or thrombosis can cause spinal cord infarction by blocking (occluding) the origins of critical segmental (radicular) arteries that feed the anterior and/or posterior spinal arteries.
- Surgical repair of aortic aneurysms (especially thoracoabdominal) or other aortic procedures can inadvertently compromise spinal cord blood flow.
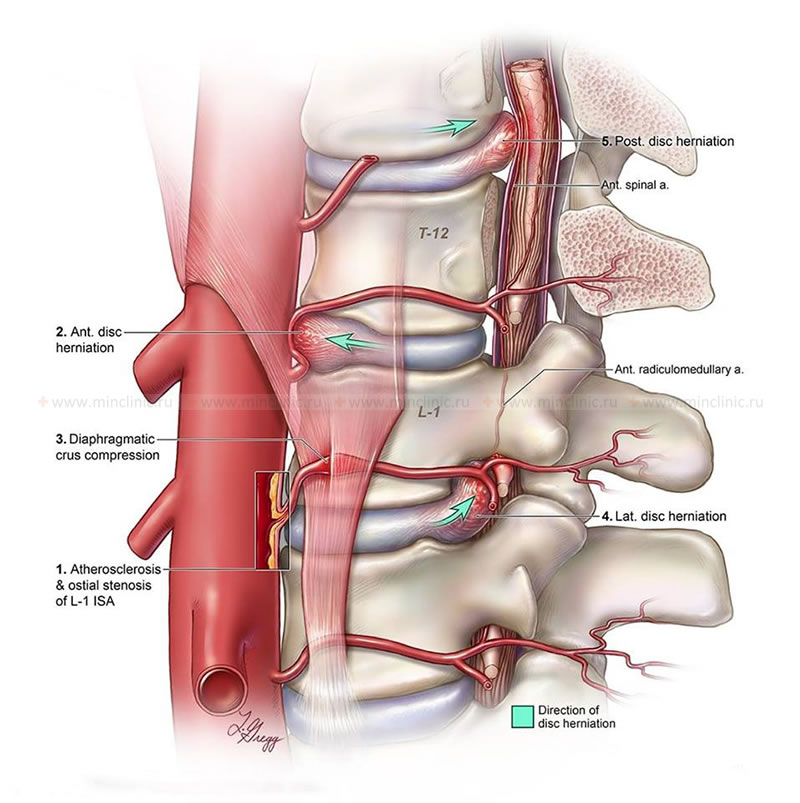
- Occlusion or Stenosis of Supplying Arteries:
- Stenosis of the ostia (openings) of segmental arteries as they arise from the aorta or other parent vessels.
- Compression of a segmental artery or its branches by an anterior, lateral, or posterior herniated intervertebral disc.
- Diaphragmatic crus syndrome (compression of celiac artery or aorta by diaphragmatic crura, rarely implicated directly in spinal cord ischemia unless major radicular arteries are involved).
- Arteritis: Vasculitis (inflammation of blood vessel walls) affecting the spinal arteries or their feeders, seen in conditions like systemic lupus erythematosus, polyarteritis nodosa, or giant cell arteritis.
- Embolism:
- Cardiogenic emboli (e.g., from atrial fibrillation, valvular heart disease).
- Atheroemboli from aortic plaques.
- Fibrocartilaginous embolism: Microscopic fragments of intervertebral disc nucleus pulposus can embolize into small intramedullary vessels or bone marrow of an adjacent vertebral body, causing spinal cord infarction. This can occur after minor trauma, often during sports or physical exertion. Patients typically experience acute local pain, followed by rapidly progressive paraplegia and a transverse myelopathy syndrome, developing within minutes to an hour. This condition should be suspected in young individuals presenting with acute transverse myelopathy after an accident or exertion. The exact pathway of pulp tissue penetration remains unclear.
- Systemic Hypotension: Severe drops in blood pressure (e.g., during surgery, cardiac arrest, shock) can lead to ischemia in watershed areas of the spinal cord.
- Iatrogenic Causes: Complications from spinal surgery, epidural injections, or intravascular procedures. For example, spinal cord infarction can occur after intravascular administration of a contrast agent if it causes vasospasm or direct toxicity; a harbinger of this can be severe back pain occurring during the contrast injection.
- Venous Infarction: Though rarer, occlusion of spinal veins can lead to hemorrhagic infarction.
- Decompression Sickness ("The Bends"): Nitrogen bubbles can cause embolic occlusion of spinal vessels in divers.
Various factors can lead to ischemia and stroke of the spinal cord, including stenosis or compression of a segmental artery (e.g., by a herniated intervertebral disc), or, less commonly, conditions like diaphragmatic crus syndrome affecting major vascular supply.
Specific Mechanisms and Vulnerable Zones
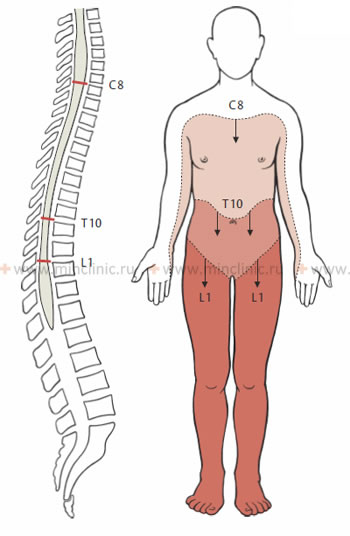
A spinal cord infarction usually develops in "watershed" areas, which are regions of adjacent blood supply between major feeding arteries. The thoracic spinal cord, particularly the mid-thoracic region (T4-T8), is considered a vascular watershed zone because it lies between the territory supplied by cervical radicular arteries (via the anterior spinal artery from above) and the major supply from the Artery of Adamkiewicz from below. This makes it particularly vulnerable to ischemia if systemic blood flow is compromised or if a critical radicular artery is occluded.
Clinical Syndromes Caused by Spinal Vessel Lesions
The clinical manifestations depend on which spinal artery is affected and the extent of the resulting infarction.
Anterior Spinal Artery (ASA) Syndrome (Occlusion)
Blockage (occlusion) of the anterior spinal artery leads to infarction of the anterior two-thirds of the spinal cord. Clinical manifestations usually appear suddenly (apoplectic onset), often due to a blood clot (thrombus or embolus). In some patients, the symptoms of ASA occlusion may develop or progress over 1-3 days, which can make an accurate initial diagnosis more challenging.
Key features of ASA syndrome include:
- Acute Onset of Pain: Often severe pain in the back at the level of infarction, or radicular pain. Paresthesias (tingling, numbness) may also occur.
- Motor Deficits (Bilateral):
- Flaccid paralysis (due to spinal shock initially) of muscles below the level of the lesion, progressing to spastic paraplegia or quadriplegia (central type paralysis) as spinal shock resolves.
- If the cervical ASA is occluded, this may cause flaccid paralysis of the arms (peripheral type, due to anterior horn cell damage at the lesion level) and spastic paraparesis of the leg muscles (central type, due to involvement of descending corticospinal tracts).
- Sensory Deficits (Bilateral):
- Loss of pain and temperature sensation below the level of the lesion (due to spinothalamic tract damage).
- Preservation of proprioception (joint position sense) and vibration sense (as these are carried in the posterior columns, supplied by the posterior spinal arteries). This dissociated sensory loss is characteristic.
- Tactile sensitivity may be variably affected or preserved.
- Autonomic Dysfunction:
- Impairment of bladder and rectal sphincter function (urinary retention, fecal incontinence) is common.
- Anhidrosis (lack of perspiration) on the paralyzed parts of the body can occur. This can lead to an increase in body temperature (hyperthermia), especially at high ambient temperatures, which might mistakenly simulate an infectious process.
This MRI of the spine demonstrates acute ischemia of the spinal cord within the territory supplied by the anterior spinal artery. This resulted from a compression fracture with displacement of vertebral bodies in a patient with severe osteoporosis.
Posterior Spinal Artery (PSA) Syndrome (Occlusion)
Blockage (occlusion) of one or both posterior spinal arteries is extremely rare in clinical practice due to their smaller size and more robust collateral circulation. If it occurs, the resulting infarction involves the posterior columns (gracile and cuneate fasciculi) and posterior horns of the spinal cord, and potentially partially the lateral corticospinal tracts due to supply from the vasocorona.
Clinical features include:
- Sensory Deficits (Bilateral or Unilateral): Loss of proprioception, vibration sense, and discriminative touch below the level of the lesion. Pain and temperature sensation are typically preserved. This can lead to sensory ataxia.
- Motor Deficits: Usually minimal or absent unless the infarction extends to involve the corticospinal tracts significantly, in which case spastic muscle paresis may occur.
- Reflex Disorders: May be present depending on the extent of corticospinal tract involvement.
- Anesthesia (loss of all sensation) and analgesia (loss of pain sensation) are not typical of pure PSA syndrome but would imply more extensive infarction or involvement of other vascular territories.
Other Vascular Syndromes
- Central Cord Infarction (Sulcocommissural Artery Syndrome): Occlusion of a sulcocommissural artery can cause a small, central infarction affecting gray matter and crossing spinothalamic fibers, leading to dissociated sensory loss and segmental weakness.
- Brown-Séquard Syndrome (Vascular Origin): A unilateral spinal cord infarction can produce a Brown-Séquard-like syndrome.
- Watershed Infarcts: Occur at the border zones between major arterial territories, often in the mid-thoracic region, due to systemic hypotension.
Diagnosis of Spinal Cord Infarction
Clinical Evaluation and Neurological Examination
A high index of suspicion for spinal cord infarction is necessary in patients presenting with acute onset of myelopathy, especially if there are known vascular risk factors, a history of aortic surgery or dissection, or recent trauma.
- History: Onset of symptoms (sudden vs. gradual), nature of pain, specific neurological deficits, preceding events (trauma, surgery, systemic illness, contrast administration).
- Neurological Examination: To precisely define the level and extent of motor, sensory, and autonomic dysfunction, and to identify specific spinal cord syndromes (e.g., ASA syndrome, PSA syndrome).
Instrumental and Laboratory Diagnostics
- Magnetic Resonance Imaging (MRI) of the Spine: This is the most important diagnostic tool.
- Diffusion-Weighted Imaging (DWI): Can show restricted diffusion in the acutely infarcted spinal cord tissue within hours of onset, similar to cerebral stroke.
- T2-Weighted Images (T2WI): Typically show hyperintense signal (edema) in the affected area of the spinal cord, often in a characteristic pattern (e.g., "owl's eyes" or "snake eyes" appearance in ASA syndrome due to anterior horn cell involvement). These changes may take several hours to days to develop.
- Contrast-enhanced MRI may show enhancement in subacute stages.
- MRI also helps to rule out other causes of acute myelopathy (e.g., compressive lesions, transverse myelitis).
- Cerebrospinal Fluid (CSF) Analysis: May show a mild increase in protein and/or white blood cells, but is often normal or non-specific. Primarily useful to exclude inflammatory or infectious causes of myelopathy.
- Spinal Angiography (Catheter-based or MRA/CTA): May be considered in select cases to identify underlying vascular pathology (e.g., AVM, dissection, stenosis of segmental arteries), but it is often not performed in the acute setting unless an intervention is planned or the diagnosis is very unclear.
- Laboratory Tests: To investigate underlying causes or risk factors, including tests for vasculitis, hypercoagulable states, diabetes, and hyperlipidemia.
- Evoked Potentials (SSEPs, MEPs): Can demonstrate conduction abnormalities in spinal cord pathways but are non-specific for infarction.
Treatment of Spinal Cord Ischemic Stroke
Currently, there are no proven acute reperfusion therapies (like thrombolysis for cerebral stroke) specifically established for acute spinal cord infarction. Management is largely supportive and aimed at optimizing spinal cord perfusion, preventing complications, and rehabilitation.
- Supportive Care:
- Blood Pressure Management: Avoid hypotension; some advocate for permissive hypertension in the acute phase to improve spinal cord perfusion, but this is not universally established.
- Oxygenation and Ventilation: Ensure adequate oxygenation. Respiratory support may be needed for high cervical lesions.
- Management of Bladder and Bowel Dysfunction.
- Prevention of Complications: Such as deep vein thrombosis (DVT), pressure ulcers, infections (urinary, respiratory).
- Addressing Underlying Cause: If a specific cause is identified (e.g., aortic dissection, vasculitis, cardiac embolism), treatment should target that condition.
- Corticosteroids (Controversial): High-dose corticosteroids (e.g., methylprednisolone) have been used anecdotally or in the context of acute spinal cord injury, but their benefit in pure ischemic spinal cord stroke is unproven and not routinely recommended.
- Neuroprotection (Investigational): Various neuroprotective agents are under investigation but none are currently standard therapy.
- Rehabilitation: Early and intensive multidisciplinary rehabilitation (physical therapy, occupational therapy) is crucial to maximize functional recovery and improve quality of life.
Differential Diagnosis of Acute Myelopathy
Acute spinal cord infarction must be differentiated from other causes of acute myelopathy:
| Condition | Key Differentiating Features |
|---|---|
| Spinal Cord Infarction | Sudden onset of pain and neurological deficits (often ASA syndrome). MRI (DWI, T2WI) shows characteristic ischemic changes. Vascular risk factors or specific cause (e.g., aortic surgery). |
| Acute Transverse Myelitis | Subacute onset (hours to days). Bilateral sensory, motor, autonomic dysfunction. Sensory level. MRI shows intrinsic cord inflammation/swelling, often enhancing. CSF often shows pleocytosis/elevated IgG. |
| Acute Spinal Cord Compression (e.g., from disc herniation, tumor, epidural abscess/hematoma) | Can be acute or subacute. Pain often prominent. Progressive neurological deficits. MRI shows compressive lesion. Fever/infection signs with abscess. |
| Spinal Cord Hemorrhage (Hematomyelia) | Sudden onset severe pain and neurological deficits. Often associated with trauma, AVM, cavernoma, or coagulopathy. MRI shows blood products within the cord. |
| Guillain-Barré Syndrome (Acute Inflammatory Demyelinating Polyneuropathy) | Ascending symmetrical weakness, areflexia, sensory disturbances. Often follows infection. Primarily affects peripheral nerves, not spinal cord directly, but can mimic myelopathy. CSF shows albuminocytologic dissociation. |
| Conversion Disorder / Functional Neurological Disorder | Inconsistent neurological signs, non-anatomical sensory loss, normal imaging and CSF. Requires careful exclusion of organic causes. |
Prognosis and Rehabilitation
The prognosis after spinal cord infarction is variable and depends on the severity and level of the initial neurological deficit, the extent of the infarction, and the underlying cause. Recovery can be partial or limited, and many patients are left with significant permanent neurological impairments. Factors associated with a poorer prognosis include complete plegia at onset, advanced age, and extensive infarction on MRI.
Intensive and prolonged multidisciplinary rehabilitation is crucial for maximizing functional recovery. This includes:
- Physical Therapy: To improve strength, mobility, balance, and gait.
- Occupational Therapy: To assist with activities of daily living and adaptive strategies.
- Management of Complications: Such as spasticity, pain, bladder/bowel dysfunction, pressure ulcers.
- Psychosocial Support: To address the emotional and psychological impact of the disability.
When to Seek Urgent Medical Attention
Spinal cord infarction is a neurological emergency. Any individual experiencing sudden onset of:
- Weakness or paralysis in the legs or arms.
- Numbness or loss of sensation below a certain level of the body.
- Loss of bladder or bowel control.
- Severe, unexplained back pain accompanied by neurological symptoms.
should seek immediate medical evaluation at an emergency department. Prompt recognition and investigation are essential, even though specific acute treatments are limited, to rule out treatable mimics (like acute cord compression) and to initiate supportive care and rehabilitation as early as possible.
References
- Novy J, Carruzzo A, Maeder P, Bogousslavsky J. Spinal cord ischemia: clinical and imaging patterns, pathogenesis, and outcomes in 27 patients. Arch Neurol. 2006 Nov;63(11):1545-50.
- Salvador de la Barrera S, Barca-Buyo A, Montoto-Marqués A, et al. Spinal cord infarction: prognosis and recovery in a series of 36 patients. Spinal Cord. 2001 Aug;39(8):420-7.
- Weidauer S, Nichtweiss M, Lanfermann H, Zanella FE. Spinal cord infarction: MR imaging and clinical features in 16 cases. Neuroradiology. 2002 Oct;44(10):851-7.
- Sandson TA, Friedman JH. Spinal cord infarction. Report of 8 cases and review of the literature. Medicine (Baltimore). 1989 Sep;68(5):282-92.
- Cheshire WP, Santos CC, Massey EW, Howard JF Jr. Spinal cord infarction: etiology and outcome. Neurology. 1996 Aug;47(2):321-30.
- Robertson CE, Brown RD Jr, Wijdicks EF, Rabinstein AA. Recovery after spinal cord infarcts: long-term outcome in 115 patients. Neurology. 2012 Jan 10;78(2):114-21.
- Foo D, Rossier AB. Anterior spinal artery syndrome and its natural history. Paraplegia. 1983 Feb;21(1):1-10.
- Vargas MI, Gariani J, Sztajzel R, Barnaure-Nachbar I, Delattre BA, Lovblad KO, Dietemann JL. Spinal cord ischemia: practical imaging tips, pearls, and pitfalls. AJNR Am J Neuroradiol. 2015 May;36(5):825-30.
See also
- Anatomy of the spine
- Ankylosing spondylitis (Bechterew's disease)
- Back pain by the region of the spine:
- Back pain during pregnancy
- Coccygodynia (tailbone pain)
- Compression fracture of the spine
- Dislocation and subluxation of the vertebrae
- Herniated and bulging intervertebral disc
- Lumbago (low back pain) and sciatica
- Osteoarthritis of the sacroiliac joint
- Osteocondritis of the spine
- Osteoporosis of the spine
- Guidelines for Caregiving for Individuals with Paraplegia and Tetraplegia
- Sacrodinia (pain in the sacrum)
- Sacroiliitis (inflammation of the sacroiliac joint)
- Scheuermann-Mau disease (juvenile osteochondrosis)
- Scoliosis, poor posture
- Spinal bacterial (purulent) epiduritis
- Spinal cord diseases:
- Spinal spondylosis
- Spinal stenosis
- Spine abnormalities
- Spondylitis (osteomyelitic, tuberculous)
- Spondyloarthrosis (facet joint osteoarthritis)
- Spondylolisthesis (displacement and instability of the spine)
- Symptom of pain in the neck, head, and arm
- Pain in the thoracic spine, intercostal neuralgia
- Vertebral hemangiomas (spinal angiomas)
- Whiplash neck injury, cervico-cranial syndrome