Spinal cord diseases
- Understanding the Spinal Cord and its Diseases
- Neurological Symptoms Related to Spinal Cord Anatomy
- Key Neurological Syndromes in Spinal Cord Diseases
- Etiologies of Spinal Cord Diseases
- Treatment Principles for Spinal Cord Diseases
- Differential Diagnosis of Myelopathy
- Complications and Prognosis
- When to Seek Urgent Medical Attention
- References
Understanding the Spinal Cord and its Diseases
Anatomy and Function of the Spinal Cord
The spinal cord is a vital component of the central nervous system (CNS), acting as the main pathway for transmitting information between the brain and the rest of the body. It is a long, cylindrical structure composed of nervous tissue, extending from the base of the brain (medulla oblongata) down through the vertebral canal (spinal canal) formed by the vertebrae. The spinal cord contains ascending nerve pathways that carry sensory information from peripheral organs to the brain, and descending pathways that transmit motor commands from the brain to muscles. It also houses its own neurons and reflex arcs that mediate rapid, involuntary responses.
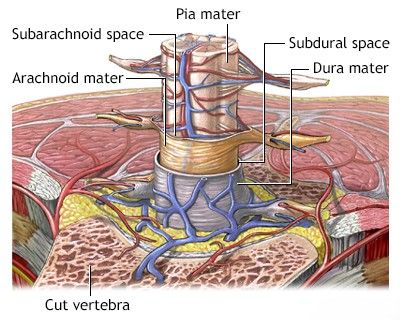
The spinal cord and the spinal nerve roots that emerge from it are protected within the bony spinal canal. In adults, the spinal cord typically begins at the foramen magnum (the opening at the base of the skull) and terminates at the level of the upper lumbar spine, usually around the L1 or L2 vertebra, tapering to a point called the conus medullaris. Below this, a bundle of nerve roots, known as the cauda equina ("horse's tail"), continues downwards through the lumbar and sacral spinal canal.
Impact of Spinal Cord Lesions
Diseases or injuries affecting the spinal cord (myelopathies) can lead to severe and often irreversible neurological damage, frequently resulting in subsequent disability. Even relatively small lesions can cause significant deficits due to the compact and critical nature of the neural pathways within the spinal cord. For instance, injuries can lead to paralysis of both arms and legs (tetraplegia or quadriplegia) if the cervical cord is affected, or paralysis of the legs (paraplegia) if the thoracic or lumbar cord is involved. These motor deficits are often accompanied by a complete absence or decrease in sensation below the level of the injury. The spinal cord's structure, akin to a tightly packed, multi-stranded cable, means that even minor damage can disrupt numerous crucial pathways, leading to permanent neurological symptoms and disability.
In diseases that cause compression of the spinal cord (either from extrinsic pressure or an intrinsic lesion), the resulting neurological deficit may be reversible if the compression is relieved promptly. Timely intervention to eliminate the compression is vital to prevent permanent death (necrosis) of nerve structures.
Diagnostic Approaches
The diagnosis of spinal cord diseases relies on a combination of clinical evaluation and specialized investigations:
- Neurological Examination: A thorough neurological examination is fundamental. The segmental anatomical structure of the spinal cord, with 31 pairs of spinal nerves innervating specific regions of the body (dermatomes for sensation, myotomes for muscles), allows clinicians to localize the level of a spinal cord lesion by identifying the border of sensory loss, the pattern of muscle weakness (paraplegia, tetraplegia, paraparesis, tetraparesis), reflex changes, and other typical neurological syndromes.
- Neuroimaging:
- Magnetic Resonance Imaging (MRI): MRI is the gold standard for imaging the spinal cord and surrounding structures. It provides excellent detail of the cord parenchyma, nerve roots, intervertebral discs, ligaments, and can detect lesions such as tumors, inflammation, demyelination, infarction, hemorrhage, or compression.
- Computed Tomography (CT) Scan: CT is superior for visualizing bony structures of the spine and can be useful for detecting fractures, bony tumors, or calcified lesions. CT myelography (CT scan after injection of contrast into the spinal canal) may be used if MRI is contraindicated or inconclusive, though it is less common now.
- Myelography (Historical): This invasive procedure involving contrast injection into the spinal canal followed by X-rays has largely been superseded by MRI and CT myelography due to its risks and lower resolution.
- Cerebrospinal Fluid (CSF) Analysis: Examination of CSF obtained via lumbar puncture can provide crucial diagnostic information for certain spinal cord diseases, such as infections (e.g., meningitis, myelitis), inflammatory conditions (e.g., multiple sclerosis), or subarachnoid hemorrhage. CSF analysis includes cell count, protein and glucose levels, cytology, and specific tests for antibodies or pathogens.
- Electrophysiological Studies: Somatosensory Evoked Potentials (SSEPs) and Motor Evoked Potentials (MEPs) can assess the integrity of sensory and motor pathways within the spinal cord. Electromyography (EMG) and Nerve Conduction Studies (NCS) can help differentiate spinal cord lesions from peripheral nerve or muscle disorders.
Neurological Symptoms Related to Spinal Cord Anatomy
Segmental Organization and Clinical Localization
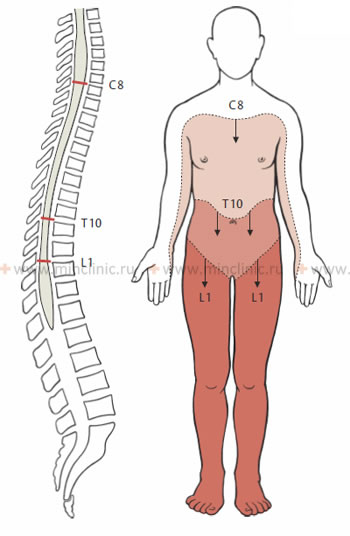
A fundamental understanding of the spinal cord's segmental anatomy and the distribution of the spinal nerves is essential for neuropathologists and neurosurgeons to accurately determine the level and extent of a lesion. During a neurological examination, the clinician systematically assesses sensory and motor functions, often proceeding from superior to inferior regions of the body, to identify the upper limit of sensory loss and the pattern of muscle weakness or paralysis. This helps to pinpoint the affected spinal cord segment(s).
It is crucial to remember that the spinal cord segments do not directly correspond to the vertebral bodies at the same level, especially in the lower spinal cord. This discrepancy arises because, during fetal development and childhood growth, the vertebral column elongates more than the spinal cord. As a result, the spinal cord "lags behind" the growth of the surrounding spine. In adults, the spinal cord typically ends at the level of the L1 or L2 vertebral body. The nerve roots originating from the lower spinal cord segments must then travel downwards within the spinal canal (as the cauda equina) to exit at their respective intervertebral foramina, innervating the lower limbs and pelvic organs.
The following clinical rules are used to approximate the relationship between spinal cord segments and vertebral levels:
- Cervical nerve roots C1-C7 exit the spinal canal *above* their correspondingly numbered vertebral bodies. The C8 nerve root exits *below* the C7 vertebra (between C7 and T1).
- Thoracic and lumbar nerve roots exit the spinal canal *below* their correspondingly named vertebrae.
- Upper cervical spinal cord segments (e.g., C1-C4) generally lie posterior to the vertebral bodies with the same numbers.
- Lower cervical spinal cord segments (e.g., C5-C8) lie approximately one vertebral level *higher* than their corresponding vertebrae (e.g., C7 cord segment is behind C6 vertebra).
- Upper thoracic spinal cord segments lie approximately two vertebral levels *higher* than their corresponding vertebrae.
- Lower thoracic spinal cord segments (e.g., T9-T12) lie approximately three vertebral levels *higher* than their corresponding vertebrae.
- The lumbar and sacral spinal cord segments, which form the conus medullaris, are localized posterior to the Th9 to L1 (or L2) vertebrae.
To further aid in localizing pathological processes, especially compressive ones like those seen in spondylosis, careful measurement of the sagittal diameters (anteroposterior lumen) of the spinal canal is important. Normal sagittal diameters of the spinal canal in an adult are approximately:
- Cervical spine: 16-22 mm
- Thoracic spine: 16-22 mm (can be narrower in mid-thoracic region)
- Lumbar spine (L1-L3): approximately 15-23 mm
- Lumbar spine (L3-L5 and below): 16-27 mm
Significant narrowing of these diameters indicates spinal stenosis, which can predispose to spinal cord or nerve root compression.
Key Neurological Syndromes in Spinal Cord Diseases
Damage to the spinal cord at different levels or in specific patterns results in characteristic neurological syndromes:
Transverse Myelopathy Syndrome
This syndrome results from a lesion affecting the entire cross-section of the spinal cord at a particular level. Key features include:
- Sensory Loss: Loss of all sensory modalities (pain, temperature, touch, vibration, proprioception) below the level of the spinal cord injury. This defines the "sensory level."
- Motor Weakness/Paralysis: Weakness or paralysis in the limbs innervated by descending motor pathways (corticospinal tracts) originating from levels below the spinal cord injury.
- If the lesion is above C5, tetraplegia (quadriplegia) results.
- If the lesion is in the thoracic cord, paraplegia (paralysis of the legs) results.
- Signs of Upper Motor Neuron (UMN) Lesion (below the level):
- Increased muscle tone (spasticity).
- Hyperreflexia (exaggerated deep tendon reflexes).
- Pathological reflexes, such as Babinski's sign (upgoing great toe on plantar stimulation).
- Segmental Signs (at the level of the lesion):
- A band of altered sensation (hyperalgesia or hyperpathia - increased or abnormal pain perception) near the upper border of the sensory level.
- Signs of Lower Motor Neuron (LMN) lesion at the affected segment(s) due to damage to anterior horn cells: muscle weakness/atrophy (hypotonia initially, later atrophy), and isolated loss or diminution of deep tendon reflexes corresponding to the damaged segments.
- Autonomic Dysfunction: Dysfunction of the pelvic organs (bladder and bowel incontinence or retention) is a common and early symptom of transverse spinal cord injury. Sexual dysfunction and disturbances in sweating can also occur.
The sensory level and segmental neurological signs provide a rough indication of the transverse localization of the spinal cord lesion. Pain felt along the midline of the back, especially at the thoracic level, can be an accurate localizing sign. Interscapular pain may be an early symptom of spinal cord compression. Radicular pain (shooting down a limb or around the trunk) suggests primary involvement of nerve roots or the outer aspects of the spinal cord. Pain in the lower back is often noted with lesions affecting the conus medullaris.
In the early stages of an acute transverse spinal cord lesion (e.g., spinal cord infarction or severe trauma), there may be a period of **spinal shock**, characterized by flaccid paralysis (decreased muscle tone/hypotension) and areflexia below the level of the lesion, rather than spasticity. Spinal shock can last for several days to weeks and can sometimes be mistaken for an extensive segmental LMN lesion. Later, as spinal shock resolves, UMN signs like spasticity and hyperreflexia typically emerge. In transverse lesions, particularly those caused by infarction, the paralysis is often preceded by short clonic or myoclonic convulsions in the affected limbs.
Intramedullary vs. Extramedullary Lesion Syndromes
Pathological processes compressing the spinal cord can be located either within the substance of the cord (intramedullary) or outside the cord but within the spinal canal (extramedullary – which can be further subdivided into intradural-extramedullary and extradural). While they can manifest similarly, certain neurological signs may favor one localization over the other, though definitive localization often requires imaging.
Neurological signs suggesting an extramedullary process (compressing the cord from the outside):
- Prominent radicular pain (due to nerve root compression at the level of the lesion).
- Early and often asymmetrical signs of corticospinal tract involvement (UMN signs like spasticity, weakness).
- Brown-Séquard syndrome (see below) is more common with extramedullary lesions.
- Sensory loss often ascends (starts distally and moves upwards).
- Significant decrease in sensation in the sacral segments (sacral anesthesia) may occur relatively early.
- Early and pronounced changes in cerebrospinal fluid (CSF), such as increased protein (Froin's syndrome if there's a complete block).
- Segmental LMN signs (weakness, atrophy, hyporeflexia) at the level of compression, often asymmetric.
Neurological signs suggesting an intramedullary process (lesion within the spinal cord):
- Poorly localized, often burning or dysesthetic pain, rather than clear radicular pain.
- Dissociated sensory loss: loss of pain and temperature sensation (spinothalamic tracts, which cross near the center of the cord) with relative preservation of light touch, vibration, and proprioception (posterior columns). This pattern often occurs in a "cape-like" distribution over the shoulders and arms if the cervical central cord is involved.
- Preservation of sensation in the perineum and sacral segments ("sacral sparing") often occurs until late in the course, as these fibers are located most peripherally in the spinothalamic tracts.
- Pyramidal (UMN) symptoms may arise later and be less pronounced initially compared to extramedullary lesions.
- CSF composition may be normal or only slightly altered, especially early on.
An intramedullary lesion involving the central part of the cord primarily damages gray matter neurons and decussating (crossing) segmental fibers (like the spinothalamic tract fibers). If the lesion affects the most internal fibers of the spinothalamic pathways but spares the outermost fibers that convey sensation from the sacral dermatomes (S3-S5), pain and temperature perception in these sacral areas will be preserved, which is a key feature of sacral sparing.
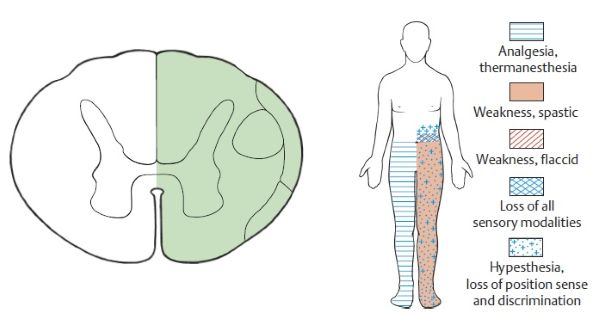
Brown-Séquard Syndrome (Hemicord Lesion)
Brown-Séquard syndrome results from a lesion affecting approximately one half (hemisection) of the spinal cord's diameter. It is clinically characterized by:
- Ipsilateral (same side as the lesion) deficits below the level of the lesion:
- Paralysis or weakness of muscles (monoplegia or hemiplegia) due to corticospinal tract damage.
- Loss of proprioception (joint position sense), vibration sense, and discriminative touch (deep sensation) due to posterior column damage.
- Contralateral (opposite side to the lesion) deficits below the level of the lesion:
- Loss of pain and temperature sensation (superficial sensation) due to spinothalamic tract damage. This loss typically begins one or two segments below the actual level of the lesion because the spinothalamic tract fibers ascend for a few segments on the same side before crossing to the contralateral side.
- At the level of the lesion (ipsilateral):
- Segmental LMN signs (flaccid paralysis, atrophy, areflexia in muscles supplied by the damaged segment).
- A band of complete sensory loss (all modalities) for the dermatomes corresponding to the damaged segment.
- Radicular pain may be present if nerve roots are involved.
A complete Brown-Séquard syndrome is rare; partial forms are more common. It is often caused by trauma (e.g., stab wound), extramedullary tumors, or demyelinating lesions.
Central Cord Syndrome
This syndrome typically results from lesions predominantly affecting the central part of the spinal cord, often in the cervical region. It is commonly seen with hyperextension injuries in individuals with pre-existing cervical spondylosis, or with intramedullary lesions like syringomyelia, tumors, or contusions. Clinical features include:
- Greater weakness in the arms (especially hands and distal arms) compared to the legs. This is because the corticospinal tract fibers innervating the arms are located more medially (centrally) within the cord than those for the legs.
- Dissociated sensory loss: Loss of pain and temperature sensation (spinothalamic tracts crossing near the central canal are damaged) with relative preservation of light touch, vibration, and proprioception (posterior columns are often spared). This sensory loss often occurs in a "cape-like" distribution over the shoulders, upper chest, and arms if the cervical central cord is affected.
- Bladder dysfunction (urinary retention) may be present.
- Varying degrees of sensory loss below the level of the lesion.
Conus Medullaris and Cauda Equina Syndromes
Lesions affecting the terminal end of the spinal cord (conus medullaris, typically at L1-L2 vertebral level) or the bundle of nerve roots (cauda equina) below this level cause distinct syndromes:
- Conus Medullaris Syndrome: Results from lesions at the L1-L2 vertebral level affecting spinal cord segments S3-S5 and sometimes L4-S2.
- Early and prominent bowel, bladder, and sexual dysfunction (often symmetrical).
- Saddle anesthesia (sensory loss in the perineum, buttocks, and posterior thighs).
- Symmetrical, often mild, leg weakness (if lumbar segments involved).
- Achilles reflexes (S1-S2) are often absent or diminished. Knee reflexes (L2-L4) may be preserved or even brisk if upper motor neuron pathways above the conus are also affected.
- Pain is often less severe and poorly localized compared to cauda equina syndrome.
- A mix of UMN signs (from tracts passing through the conus) and LMN signs (from sacral anterior horn cells) can occur.
- Cauda Equina Syndrome: Results from compression or damage to the lumbosacral nerve roots within the spinal canal below the termination of the spinal cord (below L1-L2 vertebra).
- Severe radicular pain, often asymmetrical and radiating down one or both legs (sciatica).
- Asymmetrical, flaccid (LMN type) weakness or paralysis of the legs (paraparesis/paraplegia), often more pronounced distally.
- Areflexia or hyporeflexia in the lower limbs (e.g., absent Achilles and knee reflexes).
- Saddle anesthesia.
- Bowel and bladder dysfunction (urinary retention, overflow incontinence, constipation) often occurs later than in conus medullaris syndrome, and may be asymmetrical.
- Sensory loss in a radicular (dermatomal) pattern in the lower limbs.
Compressive processes can simultaneously involve both the conus medullaris and the cauda equina, leading to a combined syndrome with mixed UMN and LMN signs (e.g., LMN signs at the level of root compression, with UMN signs like increased reflexes and Babinski's sign below if the conus itself is also compressed).
Foramen Magnum Syndrome
Lesions at the level of the foramen magnum (the opening at the base of the skull where the spinal cord connects to the brainstem) can compress the cervicomedullary junction. Symptoms include:
- Neck pain and occipital headache, often radiating to the head and shoulders.
- Weakness in the shoulder girdle and arms, often starting unilaterally and then potentially progressing to involve the leg on the opposite side, followed by the ipsilateral leg (a "round-the-clock" or "clockwise" pattern of weakness).
- UMN signs (spasticity, hyperreflexia, Babinski's sign) in all four limbs.
- Sensory disturbances, including impaired proprioception.
- Lower cranial nerve signs (e.g., dysphagia, dysarthria) may occur.
- Horner's syndrome (miosis, ptosis, anhydrosis) can be another sign of a high cervical lesion (up to the T1 segment) affecting sympathetic pathways.
- Respiratory difficulties if phrenic nerve outflow (C3-C5) is compromised.
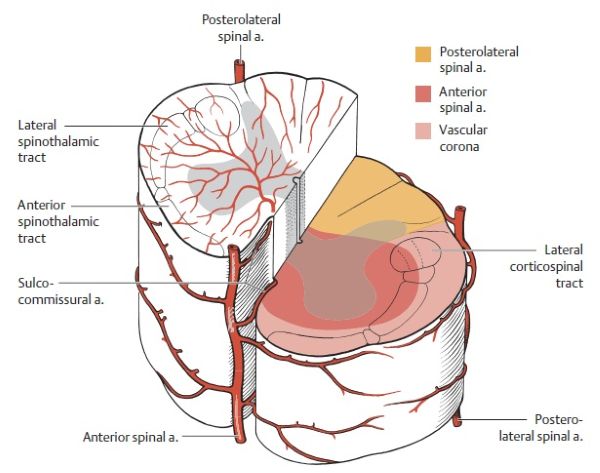
The spinal cord receives its blood supply primarily from one anterior spinal artery and two posterior spinal arteries, along with contributions from segmental radicular arteries. Disruption to this supply can lead to spinal cord infarction.
Etiologies of Spinal Cord Diseases (Myelopathies)
Spinal cord diseases can be broadly categorized by their mode of onset (acute vs. chronic) and underlying cause.
Acute Myelopathies
Some diseases of the spine or spinal cord can cause sudden onset myelopathy, often without preceding symptoms. These include:
- Traumatic Spinal Cord Injury (SCI): From accidents, falls, or violence.
- Spinal Cord Infarction (Spinal Stroke): Due to occlusion of spinal arteries (e.g., anterior spinal artery syndrome).
- Epidural or Subdural Hemorrhage: Bleeding into the spaces around the spinal cord, often related to trauma, anticoagulation, or vascular malformations.
- Hematomyelia: Hemorrhage within the substance of the spinal cord.
- Acute Intervertebral Disc Prolapse (Herniation): Sudden extrusion of disc material causing acute spinal cord or cauda equina compression.
- Acute Vertebral Subluxation or Dislocation: Often traumatic.
- Acute Transverse Myelitis: An inflammatory myelopathy that can be idiopathic, post-infectious, or associated with autoimmune diseases like multiple sclerosis or neuromyelitis optica.
- Infectious Myelitis: Direct infection of the spinal cord by viruses (e.g., polio, herpes zoster, West Nile virus), bacteria (e.g., tuberculous myelitis, pyogenic spinal epidural abscess leading to cord compression).
Chronic Myelopathies
Chronic myelopathies develop more gradually and can be caused by a variety of conditions affecting the spine or spinal cord:
- Degenerative Spinal Conditions:
- Cervical or Thoracic Spondylosis (Spondylotic Myelopathy): Degenerative changes in the vertebrae and intervertebral discs leading to spinal canal narrowing and chronic spinal cord compression. This is the most common cause of non-traumatic myelopathy in older adults.
- Lumbar Spinal Stenosis: While primarily affecting the cauda equina, severe stenosis can sometimes involve the conus medullaris.
- Degenerative and Hereditary Myelopathies:
- Motor Neuron Diseases: Such as Amyotrophic Lateral Sclerosis (ALS), which affects both upper and lower motor neurons.
- Hereditary Spastic Paraplegia (HSP): A group of genetic disorders causing progressive spasticity and weakness in the legs.
- Friedreich's Ataxia: An inherited disease causing progressive damage to the nervous system, including the spinal cord (posterior columns, spinocerebellar tracts).
- Spinocerebellar Ataxias (SCAs): Various inherited disorders affecting the cerebellum and spinal cord.
- Syringomyelia: Formation of a fluid-filled cyst (syrinx) within the spinal cord, often associated with Chiari malformations or post-traumatic changes. Leads to a characteristic central cord syndrome.
- Chronic Inflammatory/Demyelinating Diseases:
- Multiple Sclerosis (MS): Can cause demyelinating plaques in the spinal cord.
- Neuromyelitis Optica Spectrum Disorder (NMOSD).
- Sarcoidosis: Can involve the spinal cord (neurosarcoidosis).
- Nutritional Deficiencies:
- Vitamin B12 Deficiency (Subacute Combined Degeneration): Affects posterior and lateral columns of the spinal cord.
- Copper Deficiency Myelopathy.
- Vitamin E Deficiency.
- Infectious (Chronic):
- Tabes Dorsalis (Syphilitic Myelopathy): A late manifestation of neurosyphilis affecting the posterior columns and dorsal roots.
- HIV-Associated Myelopathy.
- HTLV-1 Associated Myelopathy/Tropical Spastic Paraparesis (HAM/TSP).
- Tuberculous Spondylitis (Pott's Disease) with Cord Compression.
- Neoplastic:
- Primary Intramedullary Spinal Cord Tumors (e.g., ependymoma, astrocytoma).
- Extramedullary Tumors (intradural-extramedullary like meningioma, schwannoma; or extradural like metastatic tumors to vertebrae causing spinal cord compression).
- Hemangiomas of the spinal cord and epidural space.
- Non-compressive oncological myelopathies (e.g., paraneoplastic myelopathy, radiation myelopathy).
- Vascular Malformations: Arteriovenous malformations (AVMs) or dural arteriovenous fistulas (DAVFs) of the spinal cord.
- Radiation Myelopathy: Delayed complication of radiation therapy to the spine.
Treatment Principles for Spinal Cord Diseases
The treatment of spinal cord diseases is highly dependent on the underlying etiology, the severity and acuity of the lesion, and the patient's overall condition. General principles include:
- Addressing the Underlying Cause: This is paramount. For example:
- Surgical decompression for compressive lesions (tumors, disc herniations, hematomas, epidural abscesses).
- Antibiotics for infections.
- Immunomodulatory therapies for inflammatory/demyelinating conditions (e.g., corticosteroids, disease-modifying therapies for MS).
- Correction of nutritional deficiencies.
- Management of vascular risk factors for spinal cord infarction.
- Oncological treatment (surgery, radiation, chemotherapy) for tumors.
- Supportive Care:
- Pain management.
- Management of spasticity (medications, physical therapy).
- Bladder and bowel management (catheterization, bowel regimens).
- Prevention and treatment of complications like pressure sores, deep vein thrombosis (DVT), and respiratory issues.
- Rehabilitation: Comprehensive neurological rehabilitation involving physical therapy, occupational therapy, and sometimes speech therapy is crucial to maximize functional recovery, improve mobility, teach adaptive strategies, and enhance quality of life.
- Neuroprotection (Investigational): In acute SCI, high-dose methylprednisolone was historically used but is now controversial due to limited benefit and potential side effects. Research continues into neuroprotective agents.
- Symptomatic Treatment: Addressing specific symptoms like neuropathic pain, fatigue, or mood disorders.
For many chronic and degenerative myelopathies, treatment is often focused on slowing progression, managing symptoms, and maintaining function, as a cure may not be possible.
Differential Diagnosis of Myelopathy
Myelopathy refers to any neurological deficit related to the spinal cord. Differentiating the cause is critical for appropriate management.
| Category of Myelopathy | Key Causes / Examples | Typical Onset & Features |
|---|---|---|
| Compressive Myelopathy | Spondylosis, Disc Herniation, Tumors (metastatic, primary), Epidural Abscess/Hematoma, Fracture/Dislocation | Often gradual (spondylosis, tumors) or acute (trauma, disc herniation, abscess, hemorrhage). UMN signs below lesion, possible LMN signs at lesion level, sensory level, bowel/bladder dysfunction. |
| Inflammatory/Demyelinating Myelopathy | Multiple Sclerosis, Transverse Myelitis, Neuromyelitis Optica Spectrum Disorder (NMOSD), Sarcoidosis | Often acute or subacute onset. Sensory, motor, and autonomic dysfunction. MRI shows characteristic lesions. CSF may show inflammation. |
| Vascular Myelopathy | Spinal Cord Infarction, Arteriovenous Malformation (AVM), Dural Arteriovenous Fistula (DAVF), Hematomyelia | Sudden onset of pain and neurological deficits (infarction). AVM/DAVF may have progressive or stepwise course. Hematomyelia often traumatic or with vascular anomaly. |
| Infectious Myelopathy | Viral (HIV, HTLV-1, Polio, Herpes Zoster), Bacterial (Tuberculosis, Syphilis), Fungal, Parasitic | Variable onset. Associated with systemic signs of infection or specific pathogen characteristics. CSF analysis and serology crucial. |
| Hereditary & Degenerative Myelopathies | Motor Neuron Disease (ALS), Hereditary Spastic Paraplegia, Friedreich's Ataxia, Spinocerebellar Ataxias | Usually chronic, progressive. Specific patterns of neurological involvement. Genetic testing often required. |
| Metabolic/Nutritional Myelopathy | Vitamin B12 Deficiency, Copper Deficiency, Vitamin E Deficiency | Subacute or chronic onset. Posterior and lateral column signs common (e.g., B12 deficiency). Specific lab tests for deficiencies. |
| Syringomyelia | Formation of a syrinx (fluid-filled cavity) within the cord. Often associated with Chiari malformation or post-traumatic. | Gradual onset. "Cape-like" dissociated sensory loss, weakness/atrophy in arms, scoliosis. MRI diagnostic. |
| Neoplastic Myelopathy | Intramedullary tumors, Extramedullary tumors, Paraneoplastic syndromes, Radiation myelopathy | Often progressive. Pain common. Specific syndrome depends on tumor location and type. Radiation myelopathy is a delayed effect. |
Complications and Prognosis
Spinal cord diseases can lead to numerous complications, impacting multiple body systems:
- Motor Deficits: Weakness (paresis) or paralysis (plegia), spasticity, contractures.
- Sensory Deficits: Loss of sensation, chronic pain (neuropathic pain).
- Autonomic Dysfunction: Bladder, bowel, and sexual dysfunction; orthostatic hypotension; impaired temperature regulation.
- Respiratory Complications: Weakness of respiratory muscles (especially with high cervical lesions), recurrent pneumonia.
- Pressure Ulcers (Bedsores): Due to immobility and sensory loss.
- Deep Vein Thrombosis (DVT) and Pulmonary Embolism (PE): Due to immobility.
- Chronic Pain Syndromes.
- Psychosocial Issues: Depression, anxiety, social isolation.
- Skeletal Deformities: Scoliosis, kyphosis.
- Contractures.
The prognosis for spinal cord diseases varies enormously depending on the underlying cause, severity of the initial injury or disease process, timeliness of treatment, and the potential for recovery or regeneration of neural tissue. Some conditions are reversible with prompt treatment (e.g., some compressive myelopathies, some inflammatory myelopathies), while others lead to permanent deficits (e.g., severe traumatic SCI, many neurodegenerative myelopathies).
When to Seek Urgent Medical Attention
Urgent medical evaluation is critical if an individual experiences any of the following, as they may indicate an acute or rapidly progressing spinal cord disorder:
- Sudden onset of weakness or paralysis in the limbs.
- Sudden onset of numbness or loss of sensation.
- Acute onset of severe back or neck pain, especially if radiating or associated with neurological symptoms.
- Sudden loss of bladder or bowel control (incontinence or retention).
- Rapidly progressive neurological symptoms.
- Symptoms following trauma to the spine.
- Fever associated with back pain and neurological symptoms (suggesting infection like epidural abscess or meningitis).
Timely diagnosis and intervention can be crucial for minimizing permanent neurological damage and improving outcomes in many spinal cord diseases.
References
- Ropper AH, Samuels MA, Klein JP, Prasad S. Adams and Victor's Principles of Neurology. 11th ed. McGraw-Hill Education; 2019. (Chapters on Spinal Cord Diseases)
- Waxman SG. Clinical Neuroanatomy. 29th ed. McGraw-Hill Education; 2020. (Section on Spinal Cord)
- Aminoff MJ, Greenberg DA, Simon RP. Clinical Neurology. 10th ed. McGraw-Hill Education; 2018. (Chapter on Diseases of the Spinal Cord)
- National Institute of Neurological Disorders and Stroke (NINDS). Spinal Cord Injury Information Page. Accessed [Current Date].
- Kirshblum SC, Burns SP, Biering-Sorensen F, et al. International standards for neurological classification of spinal cord injury (revised 2011). J Spinal Cord Med. 2011;34(6):535-546.
- Frohman EM, Wingerchuk DM. Transverse myelitis. N Engl J Med. 2010 Aug 5;363(6):564-72.
- Harrop JS, Hunt GE Jr, Vaccaro AR. Conus medullaris and cauda equina syndrome as a result of traumatic injuries: management principles. Neurosurg Focus. 2004 Jun 15;16(6):e4.
- Fehlings MG, Tetreault LA, Wilson JR, et al. A Clinical Practice Guideline for the Management of Patients With Degenerative Cervical Myelopathy: Recommendations for Patients With Mild, Moderate, and Severe Disease and Nonmyelopathic Patients With Evidence of Cord Compression. Global Spine J. 2017 Sep;7(3 Suppl):70S-83S.
See also
- Anatomy of the spine
- Ankylosing spondylitis (Bechterew's disease)
- Back pain by the region of the spine:
- Back pain during pregnancy
- Coccygodynia (tailbone pain)
- Compression fracture of the spine
- Dislocation and subluxation of the vertebrae
- Herniated and bulging intervertebral disc
- Lumbago (low back pain) and sciatica
- Osteoarthritis of the sacroiliac joint
- Osteocondritis of the spine
- Osteoporosis of the spine
- Guidelines for Caregiving for Individuals with Paraplegia and Tetraplegia
- Sacrodinia (pain in the sacrum)
- Sacroiliitis (inflammation of the sacroiliac joint)
- Scheuermann-Mau disease (juvenile osteochondrosis)
- Scoliosis, poor posture
- Spinal bacterial (purulent) epiduritis
- Spinal cord diseases:
- Spinal spondylosis
- Spinal stenosis
- Spine abnormalities
- Spondylitis (osteomyelitic, tuberculous)
- Spondyloarthrosis (facet joint osteoarthritis)
- Spondylolisthesis (displacement and instability of the spine)
- Symptom of pain in the neck, head, and arm
- Pain in the thoracic spine, intercostal neuralgia
- Vertebral hemangiomas (spinal angiomas)
- Whiplash neck injury, cervico-cranial syndrome