Inflammatory myelopathies
- Understanding Inflammatory Myelopathies (Myelitis)
- Angiodysgenetic Necrotizing Myelopathy (Foix-Alajouanine Disease)
- Specific Infectious Myelopathies
- Toxic Myelopathy
- Spinal Arachnoiditis
- Diagnosis of Inflammatory Myelopathies
- Treatment Principles for Inflammatory Myelopathies
- Differential Diagnosis of Myelopathy
- Prognosis and Complications
- When to Consult a Neurologist
- References
Understanding Inflammatory Myelopathies (Myelitis)
Inflammatory myelopathies, collectively referred to as myelitis, encompass a diverse group of spinal cord diseases characterized by inflammation of the spinal cord. This inflammation can result from various causes, including infections, autoimmune disorders, or post-infectious immune responses. The clinical syndrome associated with myelitis typically develops acutely or subacutely in patients, over a period of a few days to weeks.
Acute Myelitis, Transverse Myelitis, and Necrotizing Myelopathy
Myelopathy (spinal cord dysfunction) due to myelitis can manifest with varying degrees of neurological deficit. Common patterns include:
- Complete Transverse Lesion Syndrome (Transverse Myelitis): This involves inflammation affecting a horizontal segment of the spinal cord, leading to bilateral motor weakness or paralysis (paraparesis or plegia), bilateral sensory loss below the level of the lesion, and autonomic dysfunction (e.g., bladder, bowel, and sexual dysfunction).
- Partial Transverse Lesion Syndromes (Partial Myelitis):
- Posterior Columnar Myelitis: Predominantly affects the posterior columns of the spinal cord, leading to ascending paresthesias (abnormal sensations like tingling or numbness) and loss of vibration sense and proprioception (joint position sense).
- Ascending Myelitis with Predominantly Spinothalamic Disorders: Inflammation primarily affecting the spinothalamic tracts, causing loss of pain and temperature sensation, often ascending up the body.
- Brown-Séquard Syndrome (Hemicord Syndrome): Results from a lesion affecting one half of the spinal cord. Characterized by ipsilateral (same side as the lesion) motor weakness (paresis) and loss of proprioception/vibration sense, combined with contralateral (opposite side) loss of pain and temperature sensation below the level of the lesion.
In many instances, the underlying cause of acute myelitis in patients is a viral infection (e.g., enteroviruses, herpesviruses, West Nile virus, influenza) or a post-infectious autoimmune response. Most often, transverse myelitis manifests with symptoms such as back pain at the level of inflammation, progressive muscle weakness (paraparesis, often affecting the legs), and asymmetric ascending paresthesias in the legs. If the inflammation extends to higher levels, the hands and arms can also become involved in the process. Due to these features, viral myelitis can sometimes be mistaken for Guillain-Barré syndrome (an acute inflammatory demyelinating polyneuropathy affecting peripheral nerves).
To exclude a compressive cause of spinal cord injury (e.g., tumor, herniated disc, epidural abscess), it is essential to conduct neuroimaging studies, primarily magnetic resonance imaging (MRI) of the spinal cord.
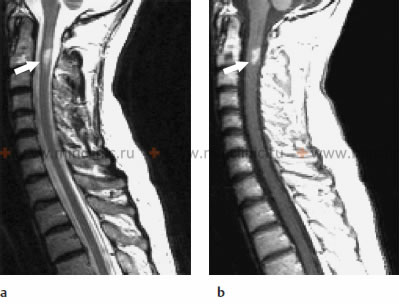
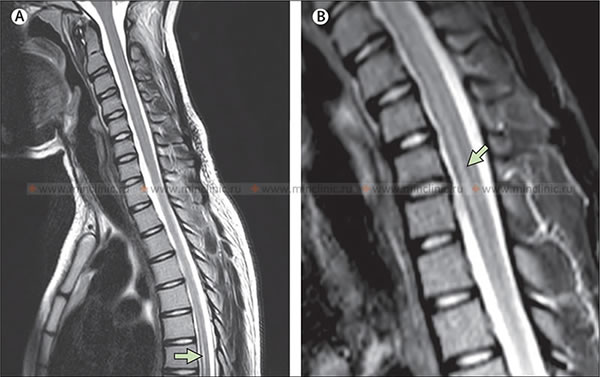
MRI of the cervical spinal cord in a case of infectious myelitis: (a) The sagittal T2-weighted image shows a lesion with a hyperintense signal (appears bright) within the spinal cord at the C2 vertebral body level, indicative of edema or inflammation. (b) On the T1-weighted image after the administration of gadolinium contrast agent, enhancement of the lesion pattern is noted, signifying disruption of the blood-spinal cord barrier and active inflammation.
In most patients with acute myelitis, cerebrospinal fluid (CSF) analysis reveals inflammatory changes: typically 5-50 lymphocytes per mm³ (pleocytosis); sometimes more than 200 cells per mm³ are found, and occasionally polymorphonuclear cells (neutrophils) may predominate in very acute or bacterial infections. CSF protein may also be elevated. The inflammatory process in myelitis is more often localized in the middle and lower thoracic segments of the spinal cord, although any level of the spinal cord can be affected.
Progressive Necrotizing Myelopathy and Devic's Disease (Neuromyelitis Optica)
In some severe cases of myelitis, the necrosis (tissue death) of the spinal cord parenchyma can be quite deep and extensive. This necrotizing process in a patient with myelitis can periodically worsen or spread over several months, progressively capturing adjacent, previously intact areas of the spinal cord. As a result of this destruction, the spinal cord at the level of myelitis can be reduced in size to a thin, atrophic, cicatricial (scarred) cord, primarily composed of glial cells (gliosis). This severe condition is often denoted in clinical practice by the term **progressive necrotizing myelopathy**. Sometimes, the entire spinal cord can be involved in this pathological process, termed "necrotic panmyelopathy."
If a transverse necrotic lesion of the spinal cord (acute transverse myelitis) occurs in a patient before, concurrently with, or shortly after an episode of optic neuritis (inflammation of the optic nerve), this condition is referred to as **Neuromyelitis Optica Spectrum Disorder (NMOSD)**, historically known as Devic's disease or opticomyelitis. NMOSD is an autoimmune inflammatory disorder distinct from multiple sclerosis, often associated with antibodies against aquaporin-4 (AQP4-IgG). While some inflammatory myelopathies may be associated with multiple sclerosis, and many are considered variants or atypical presentations of MS (especially chronic progressive cervical myelitis), NMOSD is now recognized as a separate entity.
Myelitis in Systemic Autoimmune Diseases and Sarcoidosis
Systemic lupus erythematosus (SLE) and other autoimmune connective tissue diseases (e.g., Sjögren's syndrome, antiphospholipid syndrome) can also be accompanied by myelitis as a neurological manifestation. Post-infectious demyelinating processes, such as acute disseminated encephalomyelitis (ADEM) which can involve the spinal cord, usually have a monophasic (single episode) course, though relapses can occasionally occur. Patients often experience various symptoms indicative of damage at the affected level of the spinal cord.
Systemic sarcoidosis, a multisystem granulomatous disorder of unknown etiology, can cause damage to the nerve tissue of the spinal cord (neurosarcoidosis), leading to symptoms such as paresthesias and weakness in the upper and lower extremities. Spinal cord involvement with sarcoidosis is diagnosed using MRI, which may show enhancing lesions or cord swelling. Treatment with corticosteroids (e.g., methylprednisolone) and sometimes other immunosuppressants (e.g., azathioprine) can lead to regression of neurological symptoms and improvement in the morphological picture of the spinal cord on MRI.
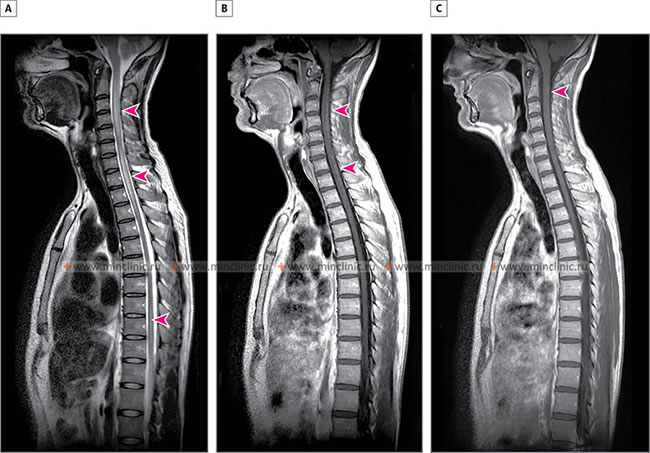
Imaging in Spinal Sarcoidosis: A - MRI of the spinal cord in the sagittal plane (T2-weighted mode) reveals a hyperintense lesion of the spinal cord with associated edema, distributed across the C2-C5 and C7-Th3 segments (indicated by arrows). B - MRI of the spinal cord with contrast (T1-weighted mode) demonstrates uneven, punctate accumulation of contrast material within the spinal cord (indicated by arrows), signifying active inflammation. C - After treatment with corticosteroids, there is an almost complete restoration of the normal morphological appearance of the spinal cord previously affected by sarcoidosis (residual change indicated by an arrow).
Angiodysgenetic Necrotizing Myelopathy (Foix-Alajouanine Disease, Subacute Necrotizing Myelitis)
Angiodysgenetic necrotizing myelopathy, also historically known as Foix-Alajouanine disease or subacute necrotizing myelitis, is a rare disorder. It encompasses lesions caused by vascular atrophy (degeneration of blood vessels) in combination with spinal cord infarctions (ischemic damage), localized most often in the lower thoracic and lumbar regions of the spinal cord. The spinal cord injuries (myelopathy) are caused by progressive changes (such as vasodilation, hypertrophy of vascular walls, symptomatic inflammation) in initially abnormal intra- and extraspinal arteries and veins, often related to spinal dural arteriovenous fistulas (SDAVF) which cause venous congestion and hypertension within the spinal cord.
In the clinical presentation of this subacute or chronic disease, progressive paraplegia (paralysis) of the legs typically dominates. Initially, the weakness may be mild and spastic (associated with increased muscle tone and hyperreflexia), but it then often transitions to a flaccid (limp) paralysis, combined with muscle atrophy. Initial disorders of pain and temperature sensitivity are frequently replaced by a more global loss of all types of sensation below the level of the lesion.
Specific Infectious Myelopathies
Viral Myelitis (e.g., Herpes Zoster, Polio)
Various viral infections of the spinal cord are accompanied by specific types of myelitis.
- Poliomyelitis: In the past, poliomyelitis (caused by poliovirus) was the most common viral infection causing spinal cord damage, primarily affecting anterior horn cells (motor neurons) leading to flaccid paralysis. Due to widespread vaccination, it is now rare in many parts of the world.
- Herpes Zoster Myelitis: Herpes zoster (shingles), caused by reactivation of the varicella-zoster virus (VZV), typically produces radicular symptoms (pain and vesicular rash in a dermatomal distribution). However, VZV can also cause myelitis, often affecting motor neurons (anterior horn cells) leading to segmental weakness, but it can also involve other parts of the spinal cord, unlike the more selective gray matter involvement in poliomyelitis. In patients with herpes zoster myelitis, CSF analysis almost always reveals lymphocytes (lymphocytic pleocytosis).
- Other Viral Agents: Other viruses that can cause myelitis include enteroviruses (e.g., Coxsackie, Echovirus), herpes simplex virus (HSV), cytomegalovirus (CMV, especially in immunocompromised individuals), Epstein-Barr virus (EBV), HIV (vacuolar myelopathy), HTLV-1 (tropical spastic paraparesis/HTLV-1 associated myelopathy - HAM), West Nile virus, and Zika virus.
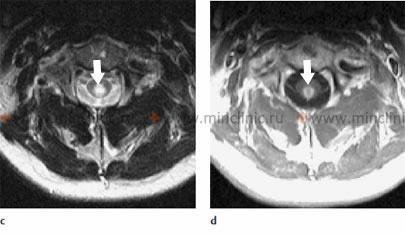
MRI of the cervical spinal cord in a case of infectious myelitis: (c) The axial T2-weighted image shows that the lesion within the spinal cord predominantly occupies its central part. (d) Enhancement of the spinal cord lesion pattern is visible on the axial T1-weighted image after the administration of gadolinium contrast medium, indicating active inflammation.
Magnetic Resonance Imaging (MRI) of the spinal cord in a patient with myelitis due to Zika virus infection. A - Sagittal T2-weighted MRI of the spine shows an area of increased signal intensity (hyperintensity) in the thoracic spinal cord at the T5–T8 region (indicated by an arrow) and also an increase in signal within the cervical spinal cord. B - Sagittal STIR (Short Tau Inversion Recovery) mode MRI of the spine demonstrates increased signal intensity in the cervical spinal cord from C4–C7 (indicated by an arrow).
Bacterial and Parasitic Myelitis
- Bacterial Myelitis: Direct bacterial infection of the spinal cord parenchyma (intramedullary abscess) is rare but can occur with systemic infections of bacterial or mycobacterial (e.g., tuberculosis - intramedullary tuberculoma) etiology, often in immunocompromised individuals or via hematogenous spread. Chronic infections of the spinal cord membranes (meninges) in conditions like syphilis can lead to a sluggish, secondary subpial myelitis and radiculitis (inflammation of nerve roots).
- Parasitic Myelitis: Certain parasitic infections can cause severe granulomatous, necrotic, and inflammatory myelitis. A notable example is schistosomiasis, particularly due to *Schistosoma mansoni* or *S. haematobium* invasion. The myelitis is often caused by a local inflammatory reaction to parasite eggs deposited in spinal cord tissue and the tissue-breaking enzymes they produce. Other parasites like *Toxoplasma gondii* (in immunocompromised hosts) or cysticercosis can also affect the spinal cord.
Toxic Myelopathy
Toxic non-inflammatory myelopathy, sometimes occurring concurrently with optic nerve atrophy, is a rare condition. It has been notably reported more often in Japan and has been attributed to the ingestion or exposure to certain antibacterial and antifungal drugs, such as clioquinol (iodochlorohydroxyquinoline). Clioquinol was historically part of some ointments for external use and oral medications for intestinal amebiasis (a condition known as subacute myelo-optic neuropathy - SMON). Most patients with toxic myelopathy may experience some recovery, but many can be left with persistent sensory disturbances (paresthesias) or other neurological deficits. Other toxins, heavy metals, and certain chemotherapeutic agents can also cause toxic myelopathies.
Spinal Arachnoiditis
Etiology and Clinical Features
Spinal arachnoiditis is an inflammatory condition characterized by scar formation and fibrous thickening of the arachnoid mater, one of the membranes covering the spinal cord. This process can lead to clumping of nerve roots, adhesions, and sometimes cyst formation, potentially causing compression of the nerve roots and, occasionally, the spinal cord itself. Causes of spinal arachnoiditis include:
- Post-Surgical Complication: Following spinal surgery.
- Post-Procedural: After myelography (especially with older oil-based contrast agents), spinal anesthesia, or the intrathecal (into the subarachnoid space) introduction of radiopaque substances, antibiotics, anesthetics, or other chemicals.
- Infections: Previous meningitis (bacterial, fungal, tuberculous) or viral infections affecting the spinal meninges.
- Trauma: Spinal cord injury or hemorrhage into the subarachnoid space.
- Chronic Inflammatory Conditions.
Soon after an adverse inciting event, CSF analysis may show a large number of cells and a high protein concentration, but the acute inflammatory process typically subsides, leaving behind chronic adhesive changes. During the acute period of spinal arachnoiditis, the patient might experience a slight increase in body temperature. Bilateral, often asymmetric, radicular pain in the extremities is a common symptom. Signs of nerve root compression, such as loss of tendon reflexes, may be determined. Back pain and symptoms of root irritation are associated with lumbar arachnoiditis more frequently than perhaps accurately attributed. Spinal arachnoiditis is not a common primary cause of direct spinal cord compression, but rather nerve root tethering and dysfunction, though severe cases with cyst formation can compress the cord.
Treatment Considerations for Spinal Arachnoiditis
The optimal approaches to the treatment of spinal arachnoiditis are often controversial among neurologists and neurosurgeons, as outcomes can be variable and often unsatisfactory. Management is primarily focused on symptomatic relief:
- Pain Management: Neuropathic pain medications (gabapentin, pregabalin, antidepressants), NSAIDs, and sometimes opioids.
- Physical Therapy: To maintain mobility and function.
- Spinal Cord Stimulation: May be considered for intractable chronic pain.
- Surgical Intervention: In some selected patients, surgical procedures like laminectomy with lysis of adhesions or drainage of arachnoid cysts may provide some improvement, but surgery can also sometimes worsen scarring.
Multiple meningeal arachnoid cysts found along nerve roots can also be a congenital anomaly. As these cysts grow in volume, they can cause deformation or stretching of the spinal nerve roots and ganglia, leading to severe radicular pain in adult patients. Surgical fenestration or removal of symptomatic cysts may be considered.
Diagnosis of Inflammatory Myelopathies
A comprehensive diagnostic workup is essential:
- Detailed Neurological History and Examination: To characterize the onset, progression, and pattern of neurological deficits.
- Magnetic Resonance Imaging (MRI) of the Spine: The primary imaging modality. MRI with gadolinium contrast can show spinal cord lesions (areas of inflammation, swelling, demyelination, necrosis, or cavitation like a syrinx), enhancement patterns, and rule out compressive lesions. Longitudinally extensive transverse myelitis (LETM - lesions spanning three or more vertebral segments) is characteristic of NMOSD.
- Cerebrospinal Fluid (CSF) Analysis: Obtained via lumbar puncture. Typically shows pleocytosis (increased white blood cells, usually lymphocytes), elevated protein, and sometimes oligoclonal bands (more common in MS than some other myelitides). Specific tests for infectious agents (PCR, cultures, antibodies) or autoimmune markers (e.g., AQP4-IgG for NMOSD, MOG-IgG) are performed as indicated.
- Blood Tests: To screen for systemic infections, autoimmune markers (ANA, ENA, RF, antiphospholipid antibodies), vitamin deficiencies (B12, E), and inflammatory markers (ESR, CRP). Serology for specific infections (e.g., syphilis, Lyme, HIV, VZV).
- Evoked Potentials (Somatosensory, Motor, Visual): Can help assess the functional integrity of sensory and motor pathways in the spinal cord and brain.
- Exclusion of Other Causes: Differentiating inflammatory myelopathy from compressive myelopathies (tumors, disc herniation, spondylosis), vascular myelopathies (spinal cord infarction), metabolic or nutritional myelopathies, and hereditary degenerative conditions.
Treatment Principles for Inflammatory Myelopathies
Treatment depends on the underlying cause:
- Acute Idiopathic or Post-Infectious Transverse Myelitis: High-dose intravenous corticosteroids (e.g., methylprednisolone) are often the first-line treatment to reduce inflammation. If response is poor, plasma exchange (plasmapheresis) or intravenous immunoglobulin (IVIG) may be considered.
- Multiple Sclerosis (MS)-Related Myelitis: Acute attacks are treated with high-dose corticosteroids. Long-term disease-modifying therapies for MS are used to prevent relapses.
- Neuromyelitis Optica Spectrum Disorder (NMOSD): Acute attacks are treated similarly with high-dose corticosteroids, plasma exchange, or IVIG. Long-term immunosuppressive therapy (e.g., rituximab, azathioprine, mycophenolate mofetil, or newer biologics like eculizumab, inebilizumab, satralizumab) is crucial to prevent relapses, as standard MS therapies can worsen NMOSD.
- Infectious Myelitis: Specific antimicrobial therapy (antiviral, antibacterial, antifungal, antiparasitic) targeting the identified pathogen.
- Autoimmune/Connective Tissue Disease-Associated Myelitis: Treatment of the underlying systemic disease with corticosteroids and other immunosuppressants.
- Sarcoid Myelopathy: Corticosteroids are the mainstay; other immunosuppressants may be needed for refractory cases.
- Supportive Care and Rehabilitation: Essential for all types, including management of pain, spasticity, bladder/bowel dysfunction, and physical/occupational therapy to maximize functional recovery.
Differential Diagnosis of Myelopathy
Myelopathy (spinal cord dysfunction) from inflammatory causes must be distinguished from other conditions affecting the spinal cord:
| Condition | Key Differentiating Features |
|---|---|
| Inflammatory Myelopathies (e.g., Transverse Myelitis, NMOSD, MS-related) | Acute/subacute onset, often bilateral symptoms, sensory level, motor weakness, autonomic dysfunction. MRI shows intrinsic cord lesion(s) with enhancement. CSF often inflammatory. Specific antibodies (AQP4, MOG) in some. |
| Compressive Myelopathy (e.g., Disc Herniation, Tumor, Spondylosis, Epidural Abscess/Hematoma) | Symptoms depend on level and severity of compression. Often progressive. MRI clearly shows extrinsic compression of the spinal cord. |
| Vascular Myelopathies (Spinal Cord Infarction) | Sudden onset, often with pain. Deficits correspond to a vascular territory (e.g., anterior spinal artery syndrome). MRI (DWI) confirms ischemia. Risk factors for vascular disease. |
| Nutritional/Metabolic Myelopathies (e.g., B12 Deficiency, Copper Deficiency) | Subacute/chronic onset. Posterior column and corticospinal tract dysfunction common. Specific laboratory deficiencies. |
| Hereditary Spastic Paraparesis / Degenerative Myelopathies | Slowly progressive spastic weakness, often family history. Genetic testing. |
| Syringomyelia | Central cord syndrome with dissociated sensory loss, weakness, atrophy. MRI shows syrinx cavity. |
| Radiation Myelopathy | Delayed onset after radiation therapy to or near the spinal cord. Progressive. MRI may show cord changes. |
Prognosis and Complications
The prognosis of inflammatory myelopathies varies greatly depending on the specific etiology, severity of the initial attack, extent of spinal cord damage, and response to treatment. Some individuals may experience good recovery, while others may have persistent neurological deficits. Potential long-term complications include:
- Chronic pain (neuropathic pain).
- Spasticity.
- Motor weakness or paralysis.
- Sensory loss.
- Bladder, bowel, and sexual dysfunction.
- Complications of immobility (e.g., pressure sores, deep vein thrombosis).
- Psychological impact (depression, anxiety).
Relapses can occur in conditions like MS and NMOSD if not adequately managed with long-term immunomodulatory or immunosuppressive therapies.
When to Consult a Neurologist
Urgent consultation with a neurologist is essential if an individual develops acute or subacute symptoms suggestive of myelitis, such as:
- Rapidly progressive weakness or paralysis in the limbs.
- Sudden onset of numbness, tingling, or loss of sensation, particularly if there is a clear sensory level.
- New onset of bladder or bowel incontinence or retention.
- Severe back pain accompanied by neurological symptoms.
- Symptoms suggestive of Brown-Séquard syndrome.
Prompt diagnosis and initiation of appropriate treatment are critical to optimize the chances of recovery and minimize long-term disability.
References
- Transverse Myelitis Consortium Working Group. Proposed diagnostic criteria and nosology of acute transverse myelitis. Neurology. 2002 Dec 10;59(11):1748-53.
- Scott TF, Frohman EM, De Seze J, et al. Evidence-based guideline: clinical evaluation and treatment of transverse myelitis: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2011 Dec 13;77(24):2128-34.
- Wingerchuk DM, Banwell B, Bennett JL, et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology. 2015 Jul 14;85(2):177-89.
- Frohman EM, Wingerchuk DM. Clinical practice. Transverse myelitis. N Engl J Med. 2010 Aug 12;363(6):564-72.
- Jacob A, Weinshenker BG. An approach to the diagnosis of acute transverse myelitis. Lancet Neurol. 2008 Feb;7(2):164-75.
- Ropper AH, Samuels MA, Klein JP, Prasad S. Adams and Victor's Principles of Neurology. 11th ed. McGraw-Hill Education; 2019. (Chapter on Diseases of the Spinal Cord).
- National Institute of Neurological Disorders and Stroke (NINDS). Transverse Myelitis Fact Sheet. Accessed [Current Date]. (Refer to latest NINDS information).
- Pittock SJ, Lucchinetti CF. Neuromyelitis optica and the evolving spectrum of autoimmune aquaporin-4 channelopathy: a decade later. Ann N Y Acad Sci. 2016 Jan;1366(1):20-39.
See also
- Anatomy of the spine
- Ankylosing spondylitis (Bechterew's disease)
- Back pain by the region of the spine:
- Back pain during pregnancy
- Coccygodynia (tailbone pain)
- Compression fracture of the spine
- Dislocation and subluxation of the vertebrae
- Herniated and bulging intervertebral disc
- Lumbago (low back pain) and sciatica
- Osteoarthritis of the sacroiliac joint
- Osteocondritis of the spine
- Osteoporosis of the spine
- Guidelines for Caregiving for Individuals with Paraplegia and Tetraplegia
- Sacrodinia (pain in the sacrum)
- Sacroiliitis (inflammation of the sacroiliac joint)
- Scheuermann-Mau disease (juvenile osteochondrosis)
- Scoliosis, poor posture
- Spinal bacterial (purulent) epiduritis
- Spinal cord diseases:
- Spinal spondylosis
- Spinal stenosis
- Spine abnormalities
- Spondylitis (osteomyelitic, tuberculous)
- Spondyloarthrosis (facet joint osteoarthritis)
- Spondylolisthesis (displacement and instability of the spine)
- Symptom of pain in the neck, head, and arm
- Pain in the thoracic spine, intercostal neuralgia
- Vertebral hemangiomas (spinal angiomas)
- Whiplash neck injury, cervico-cranial syndrome