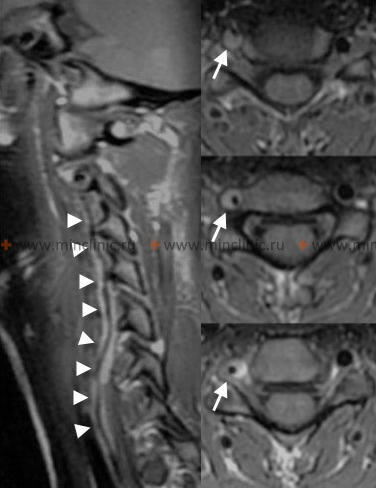
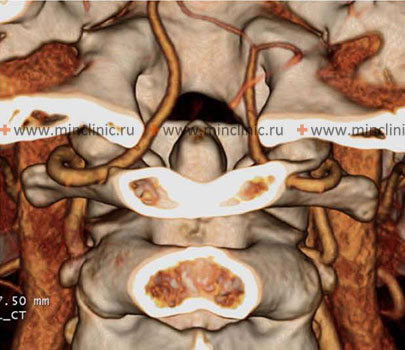
Atherothrombotic occlusion of vertebral and posterior inferior cerebellar arteries (PICA)
VA/PICA Ischemic Stroke: Causes & Anatomy
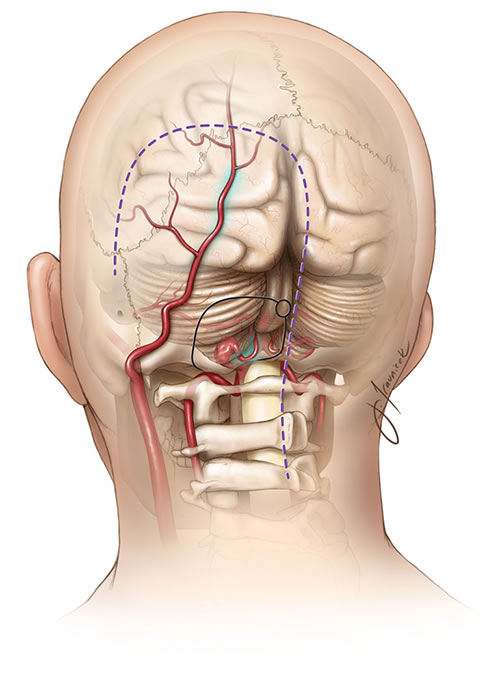
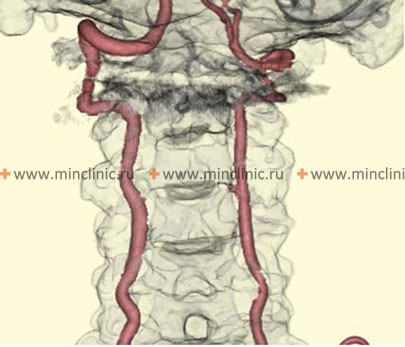
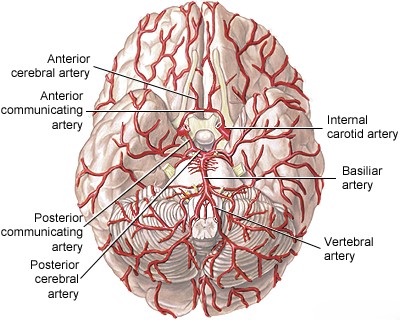
The vertebral artery (VA), arising typically from the subclavian artery (right from brachiocephalic trunk, left directly from subclavian), is divided into four anatomical segments [1, 2]:
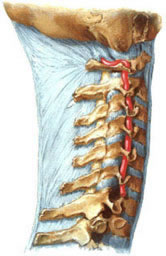
- V1 (Pre-foraminal segment): From its origin to entering the transverse foramen, usually at the C6 cervical vertebra level (sometimes C5 or C7).
- V2 (Foraminal segment): Ascends vertically through the transverse foramina of the C6 up to C2 vertebrae.
- V3 (Atlantic or Extradural segment): Exits the C2 transverse foramen, curves posteriorly and medially around the lateral mass of C1 (atlas), passes through the C1 transverse foramen, and courses horizontally in a groove on the posterior arch of C1 before piercing the posterior atlanto-occipital membrane and dura mater near the foramen magnum.
- V4 (Intradural segment): Starts after dural penetration, ascends anterior to the medulla oblongata, and joins the contralateral VA at the pontomedullary junction to form the basilar artery.
Important branches arise from the V4 segment, including small penetrating arteries supplying the medulla and, crucially, the Posterior Inferior Cerebellar Artery (PICA) [1, 2]. PICA typically originates from the distal V4 segment, loops around the medulla (supplying its lateral aspect), and then supplies the inferior surface of the cerebellum and the choroid plexus of the fourth ventricle [1, 2]. Significant anatomical variation exists in PICA's origin and territory [1, 2].
Collateral blood supply can occur via anastomoses between muscular branches of the V2 segment and branches of the external carotid artery (e.g., occipital artery) or thyrocervical trunk (e.g., ascending cervical artery) [1]. Vertebral artery hypoplasia (underdevelopment) is common (around 10%), making the contralateral VA and collateral pathways critical if the dominant VA becomes occluded [1].
The craniovertebral junction (where the skull meets the cervical spine) is anatomically complex [2]. Variations like ponticulus posticus (an bony arch over the VA groove on C1), abnormal PICA origin, or fenestrations can occur, sometimes associated with conditions like cervicocranial instability, Klippel-Feil syndrome, or rheumatoid arthritis [2].
Primary Causes of Ischemic Stroke in the VA/PICA territory [1, 3]:
- Atherosclerosis: The most common cause, particularly affecting the V1 segment (origin) and the V4 segment (intracranial portion, near PICA origin or VA confluence). Stenosis at V1 rarely causes brainstem strokes due to good collaterals unless the contralateral VA is diseased or hypoplastic. V4 atherosclerosis is more likely to cause direct medullary/cerebellar infarcts or artery-to-artery embolism into the basilar system.
- Vertebral Artery Dissection: A tear in the artery wall, often affecting the mobile V2 and especially V3 segments, sometimes related to trauma (even minor neck movements) or underlying connective tissue disorders. Can cause stroke via thromboembolism or hemodynamic failure.
- Embolism: Can originate from the heart (cardioembolism, e.g., atrial fibrillation), aortic arch, or proximal VA/subclavian atherosclerosis.
- Less Common Causes: Fibromuscular dysplasia (FMD), vasculitis, extrinsic compression (e.g., by osteophytes, tumors), subclavian steal syndrome (where arm exercise "steals" blood from the VA due to proximal subclavian stenosis, rarely causing stroke but often TIAs).
Atherosclerotic plaque location relative to the PICA origin is critical [1]. Plaque proximal to PICA origin can compromise flow to both PICA territory (lateral medulla, inferior cerebellum) and potentially the distal VA territory if collaterals are poor [1]. Occlusion of penetrating medullary branches from VA or PICA causes smaller, partial medullary syndromes [1].
VA/PICA Stroke: Clinical Manifestations
Transient Ischemic Attacks (TIAs): TIAs in the VA/PICA distribution often precede a stroke and typically involve symptoms related to lateral medullary or cerebellar dysfunction [1, 3]. Common transient symptoms include:
- Vertigo/Dizziness: Often described as spinning or unsteadiness.
- Ipsilateral facial numbness/paresthesia.
- Contralateral body numbness/paresthesia.
- Diplopia (double vision).
- Dysphonia (hoarseness).
- Dysphagia (difficulty swallowing).
- Dysarthria (slurred speech).
- Ataxia (imbalance or limb incoordination).
Hemiparesis (weakness) is rare in isolated PICA/lateral medullary TIAs [1]. These TIAs are often brief (minutes) and can be recurrent, suggesting hemodynamic compromise from stenosis or microemboli [1].
Completed Stroke Syndromes [1, 3]:
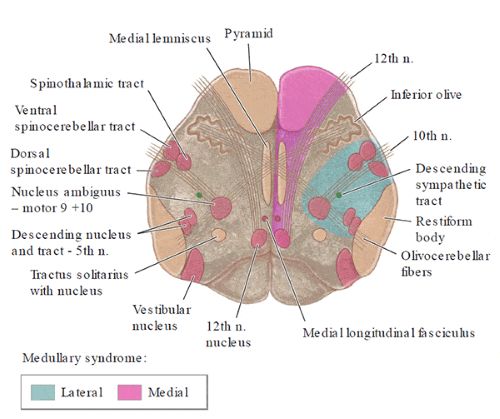
- Lateral Medullary Syndrome (Wallenberg Syndrome): This is the most common and classic syndrome resulting from occlusion affecting the PICA territory (caused by VA occlusion in ~80% of cases, direct PICA occlusion in ~20%). It involves infarction of the lateral portion of the medulla oblongata. Key features include:
- Ipsilateral:
- Loss of pain and temperature sensation on the face (CN V spinal tract/nucleus).
- Ataxia (limb and gait) (Inferior cerebellar peduncle/cerebellum).
- Vertigo, nausea, vomiting, nystagmus (Vestibular nuclei).
- Dysphagia, hoarseness, decreased gag reflex (Nucleus ambiguus - CN IX, X).
- Horner's syndrome (ptosis, miosis, anhidrosis) (Descending sympathetic fibers).
- Contralateral:
- Loss of pain and temperature sensation on the body (Spinothalamic tract).
- Motor function (corticospinal tract) and proprioception/touch (medial lemniscus) are typically spared. Severe dysphagia requiring feeding tube placement can occur.
- Ipsilateral:
- Medial Medullary Syndrome (Dejerine Syndrome): Less common, caused by occlusion of paramedian branches of the VA or anterior spinal artery. Features include:
- Ipsilateral:
- Tongue paralysis and atrophy (CN XII nucleus/fascicles).
- Contralateral:
- Hemiparesis (sparing the face) (Pyramidal tract).
- Loss of proprioception and discriminative touch (Medial lemniscus).
- Ipsilateral:
- Combined/Partial Syndromes: Occlusion of smaller penetrating medullary branches can cause incomplete versions of these syndromes. Total unilateral medullary infarction (both medial and lateral) can occur with proximal VA occlusion.
- Cerebellar Infarction: Occlusion of PICA distal to its medullary branches, or sometimes VA occlusion, can cause isolated infarction of the inferior cerebellum. Symptoms primarily include vertigo, nausea, vomiting, severe ataxia, and nystagmus. Crucially, large cerebellar infarcts can swell (edema), compressing the fourth ventricle and brainstem, leading to obstructive hydrocephalus, brainstem compression, and potentially sudden respiratory arrest. Warning signs like drowsiness, worsening headache, or new bilateral signs require urgent attention.
Medullary Syndromes Table (Summary) [1]:
| Signs and Symptoms | Structures Typically Involved |
|---|---|
| 1. Medial Medullary Syndrome (Dejerine) (Occlusion of VA paramedian branch or anterior spinal artery) |
|
| Ipsilateral: | |
| Paralysis/atrophy of half the tongue | Hypoglossal nerve (CN XII) fibers/nucleus |
| Contralateral: | |
| Hemiplegia (arm/leg, sparing face) | Pyramidal tract |
| Impaired proprioception/discriminative touch | Medial lemniscus |
| 2. Lateral Medullary Syndrome (Wallenberg) (Occlusion commonly of PICA or VA branch) |
|
| Ipsilateral: | |
| Vertigo, nausea, vomiting, nystagmus | Vestibular nuclei |
| Dysphagia, hoarseness, decreased gag reflex | Nucleus ambiguus (CN IX, X) |
| Ataxia (limb/gait) | Inferior cerebellar peduncle/cerebellum |
| Facial pain/temperature loss | Spinal trigeminal nucleus/tract (CN V) |
| Horner’s syndrome | Descending sympathetic pathway |
| Loss of taste (less common) | Nucleus solitarius |
| Contralateral: | |
| Impaired pain/temperature sensation (body) | Spinothalamic tract |
VA/PICA Stroke: Diagnosis & Imaging
Diagnosing ischemic stroke in the VA/PICA territory involves clinical assessment and targeted imaging [1, 5].
- Clinical Evaluation: History taking focuses on symptom onset, nature of symptoms (vertigo, ataxia, sensory changes, swallowing difficulty, etc.), and risk factors (hypertension, smoking, diabetes, atrial fibrillation, history of neck trauma/manipulation). Neurological examination aims to identify patterns consistent with medullary or cerebellar dysfunction (e.g., Wallenberg syndrome features).
- Non-Contrast CT Head: Essential initial step primarily to exclude hemorrhage. CT is often normal in the early hours of ischemic stroke and has poor sensitivity for detecting small infarcts in the posterior fossa (brainstem/cerebellum) due to bony artifacts.
- MRI Brain: The imaging modality of choice. Diffusion-Weighted Imaging (DWI) sequences are highly sensitive for detecting acute ischemia within minutes to hours, clearly visualizing infarcts in the medulla and cerebellum. FLAIR sequences help assess infarct age, and GRE/SWI sequences can detect subtle hemorrhage or dissection signs.
Differential Diagnosis of Lateral Medullary Syndrome (Wallenberg) / PICA Stroke Symptoms [1, 6]
| Condition | Key Features / Distinguishing Points | Typical Investigations / Findings |
|---|---|---|
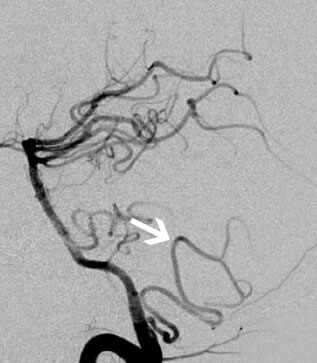
| Lateral Medullary Syndrome (Ischemic Stroke) | Classic constellation: vertigo, nystagmus, ipsilateral ataxia, dysphagia/hoarseness, ipsilateral facial & contralateral body pain/temp loss, Horner's syndrome. Sudden onset. | MRI (DWI) confirms acute infarct in lateral medulla +/- inferior cerebellum. Vascular imaging (CTA/MRA/DSA) identifies VA/PICA occlusion/stenosis/dissection. |
| Cerebellar Hemorrhage | Sudden onset ataxia, vomiting, inability to stand, headache (often occipital). May have CN palsies, gaze abnormalities. LOC uncommon initially but can decline rapidly. | Non-contrast CT head shows cerebellar hemorrhage. MRI confirms location/size. |
| Cerebellar Ischemic Stroke (non-PICA territory, e.g., SCA, AICA) | Ataxia, vertigo, nausea/vomiting. SCA: often involves superior cerebellum/peduncle, contralateral pain/temp loss. AICA: often involves lateral pons, hearing loss, facial palsy. | MRI (DWI) shows infarct in SCA or AICA territory. Vascular imaging identifies causative vessel. |
| Multiple Sclerosis (MS) Plaque | Acute/subacute onset of brainstem/cerebellar symptoms (vertigo, ataxia, diplopia, sensory changes). Usually younger adults, may have prior episodes. | MRI shows demyelinating lesion(s) in medulla, pons, or cerebellum, +/- enhancement. May have other typical MS lesions. CSF may show oligoclonal bands. |
| Posterior Fossa Tumor (e.g., Vestibular Schwannoma, Meningioma, Metastasis) | Usually progressive symptoms (hearing loss, tinnitus, vertigo, ataxia, CN palsies, headache). Can present acutely with hemorrhage into tumor. | MRI with contrast shows characteristic mass lesion. |
| Peripheral Vestibulopathy (Labyrinthitis, Vestibular Neuronitis) | Acute, severe vertigo, nausea/vomiting, nystagmus. Usually NO other brainstem signs (no ataxia beyond gait instability, no dysphagia, no crossed sensory loss, no Horner's). Labyrinthitis includes hearing loss. | Clinical exam crucial (HINTS exam negative for central cause). Normal brain imaging. Audiometry. |
| Migraine with Brainstem Aura | Transient vertigo, ataxia, dysarthria, diplopia, bilateral sensory/visual symptoms followed by headache. History of migraine. Fully reversible. | Clinical diagnosis. Normal imaging. Normal exam between attacks. |
| Brainstem Encephalitis | Subacute onset of multiple brainstem signs, often fever, altered mental status. | MRI shows inflammatory changes in brainstem. CSF shows pleocytosis. Infectious/autoimmune workup positive. |
- Vascular Imaging: Crucial to identify the underlying cause (occlusion, stenosis, dissection) [4, 5].
- CTA (CT Angiography) Head and Neck: Rapidly acquired, provides good visualization of VAs and proximal PICA, useful for detecting significant stenosis, occlusion, or dissection features.
- MRA (MR Angiography) Head and Neck: Non-invasive alternative, good for screening but may be less accurate than CTA/DSA for stenosis grading or subtle dissection. High-field MRA (e.g., 3 Tesla) offers improved resolution.
- DSA (Digital Subtraction Angiography): Remains the gold standard for detailed vessel anatomy, particularly for distal vessels, dissection flaps, or subtle lesions, but is invasive and reserved for specific indications or when endovascular treatment is considered.
- Transcranial Doppler (TCD): Can assess flow dynamics in V4 and basilar artery but provides limited anatomical detail.
- Cardiac Evaluation: ECG and Echocardiography (TTE/TEE) are performed to rule out cardioembolic sources, especially if no clear vessel pathology is identified.
VA/PICA Stroke: Treatment Strategies
Treatment focuses on acute management, preventing complications, and long-term secondary prevention [1, 5].
Acute Management [1, 5]:
- Supportive Care: Admission to a stroke unit, airway protection (especially with dysphagia/decreased consciousness), hydration, blood pressure management (avoiding hypotension, careful lowering of severe hypertension), glucose control, fever management.
- Reperfusion Therapy:
- IV Thrombolysis (tPA): May be considered within the time window (3-4.5 hours) if eligible and hemorrhage excluded, but data specifically for isolated PICA strokes are limited. May be more applicable for proximal VA occlusions causing the syndrome.
- Endovascular Therapy (EVT): Mechanical thrombectomy is primarily for large vessel occlusions (LVOs). While standard for basilar artery occlusion, its role for isolated VA or PICA occlusion is less established and considered on a case-by-case basis, especially if caused by VA occlusion near PICA origin or associated with significant clinical deficit.
- Management of Cerebellar Edema: This is a critical concern. If signs of brainstem compression or hydrocephalus develop due to cerebellar swelling:
- Medical management: Head elevation, osmotic therapy (mannitol, hypertonic saline), temporary hyperventilation.
- Surgical decompression: Urgent placement of an external ventricular drain (EVD) for hydrocephalus and/or suboccipital decompressive craniectomy (removing bone over the posterior fossa) may be life-saving.
- Dysphagia Management: Early swallowing assessment is vital. Patients with severe dysphagia may require nasogastric or percutaneous endoscopic gastrostomy (PEG) tube feeding to prevent aspiration pneumonia.
Secondary Prevention [1, 7]:
-
Antiplatelet Therapy: This is the cornerstone for preventing recurrence in non-cardioembolic strokes (typically due to atherosclerosis, small vessel disease, or cryptogenic causes).
- Common initial choices include Aspirin monotherapy or Clopidogrel monotherapy.
- Dual Antiplatelet Therapy (DAPT) with Aspirin plus Clopidogrel is often used for a limited duration (e.g., 21-90 days) following a minor stroke or high-risk TIA, or after endovascular stenting, before transitioning to monotherapy.
- Other options like Aspirin/extended-release Dipyridamole or Ticagrelor (in specific situations, sometimes with Aspirin initially) may also be considered based on guidelines and individual patient factors.
-
Anticoagulation: Used for specific stroke etiologies, not routinely for atherothrombosis.
- Vertebral Artery Dissection: Management is debated, with evidence supporting both anticoagulation (e.g., heparin followed by warfarin or a DOAC for 3-6 months) and antiplatelet therapy. The choice often depends on the presence of high-risk features (e.g., associated pseudoaneurysm, ongoing embolic signs) and local protocols. Anticoagulation is aimed at preventing thrombus formation/embolization from the dissection site.
- Cardioembolic Stroke: Long-term anticoagulation is indicated for strokes caused by sources like atrial fibrillation. Direct Oral Anticoagulants (DOACs) are generally preferred over warfarin for non-valvular atrial fibrillation due to ease of use and similar or better safety/efficacy profiles.
- Atherothrombotic VA/PICA Stroke: Anticoagulation is generally *not* indicated as first-line therapy. Antiplatelet agents are preferred. Anticoagulation might be considered in rare cases of documented thrombus propagation (e.g., into the basilar artery) or potentially recurrent embolic events despite adequate antiplatelet therapy, but this requires careful risk-benefit assessment due to bleeding risks.
- Statin Therapy: High-intensity statin therapy (e.g., Atorvastatin 80mg, Rosuvastatin 20-40mg) is strongly recommended for nearly all patients with ischemic stroke presumed to be atherosclerotic, regardless of their baseline LDL cholesterol level. The goal is plaque stabilization and potent LDL reduction (often targeting LDL < 70 mg/dL or >50% reduction).
-
Comprehensive Risk Factor Management: Aggressively addressing modifiable vascular risk factors is crucial. This includes:
- Hypertension Control: Achieving and maintaining target blood pressure levels (often <130/80 mmHg).
- Diabetes Management: Strict glycemic control (monitoring HbA1c).
- Hyperlipidemia Management: Primarily addressed with statins, potentially adding other agents if needed.
- Smoking Cessation: Counseling and pharmacological support.
- Lifestyle Modifications: Promoting a healthy diet (e.g., Mediterranean, DASH), regular aerobic exercise, weight management (achieving healthy BMI), and limiting alcohol intake.
-
Surgical/Endovascular Revascularization (Highly Selected Cases): These interventions are generally reserved for patients with recurrent symptoms directly attributable to severe stenosis despite optimal medical therapy.
- Intracranial Vertebral Artery Stenting: Considered for symptomatic high-grade (>70%) stenosis, but carries significant periprocedural risks (stroke/death). Major trials (e.g., VISSIT, SAMMPRIS findings for intracranial stenosis in general) have shown medical management is often superior or non-inferior, making stenting a less common option reserved for specific refractory cases treated at experienced centers.
- Extracranial Vertebral Artery Stenting (V1 segment): May be considered more readily for symptomatic high-grade stenosis at the VA origin, though evidence is less robust than for carotid stenting.
- Surgical Bypass (e.g., Occipital Artery to PICA): Rarely performed. Primarily considered in exceptional circumstances like planned therapeutic occlusion of the VA/PICA (e.g., for complex aneurysms) or possibly for highly refractory hemodynamic insufficiency not amenable to other treatments. Robust evidence supporting its efficacy for stroke prevention is lacking.
- Rehabilitation: Although not strictly secondary prevention *of stroke*, comprehensive rehabilitation (physical, occupational, speech therapy) is vital for maximizing functional recovery and quality of life after a VA/PICA stroke.
Prognosis varies greatly depending on the extent of the infarct, presence of complications like cerebellar edema, and underlying cause [1].
References
- Ropper AH, Samuels MA, Klein JP, Prasad S. Adams and Victor's Principles of Neurology. 11th ed. McGraw Hill; 2019. Chapter 34: Cerebrovascular Diseases (Sections on Posterior Circulation Stroke, Vertebral Artery, PICA).
- Blumenfeld H. Neuroanatomy through Clinical Cases. 2nd ed. Sinauer Associates; 2010. Chapter 18: Brainstem III: Vascular Supply.
- Caplan LR. Caplan's Stroke: A Clinical Approach. 5th ed. Cambridge University Press; 2016. Chapter on Posterior Circulation Stroke Syndromes.
- Osborn AG, Hedlund GL, Salzman KL. Osborn's Brain: Imaging, Pathology, and Anatomy. 2nd ed. Elsevier; 2017. Section on Stroke and Vascular Disease (Posterior Circulation).
- Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2019 Dec;50(12):e344-e418.
- Caplan LR. Stroke Mimics. Semin Neurol. 2016 Apr;36(2):203-12.
- Kernan WN, Ovbiagele B, Black HR, et al; American Heart Association Stroke Council, Council on Cardiovascular and Stroke Nursing, Council on Clinical Cardiology, and Council on Peripheral Vascular Disease. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014 Jul;45(7):2160-236. (Or more recent updates).
- Greenberg MS. Handbook of Neurosurgery. 9th ed. Thieme; 2019. Chapter 42: Extracranial-Intracranial Bypass.
See also
- Ischemic stroke, cerebral ischemia
- Vertebrobasilar insufficiency (VBI) with vertigo symptom
- Somatoform autonomic dysfunction
- Dizziness, stuffiness in ear and tinnitus
- Ischemic brain disease:
- Atherosclerotic thrombosis
- Atherothrombotic occlusion of internal carotid artery
- Asymptomatic carotid bifurcation stenosis with noise
- Atherothrombotic occlusion of vertebrobasilar and posterior cerebral arteries
- Atherothrombotic occlusion of posterior cerebral artery
- Atherothrombotic occlusion of vertebral and posterior inferior cerebellar arteries (PICA)
- Atherothrombotic occlusion of basilar artery
- Small-vessel stroke (lacunar infarction)
- Other causes of ischemic stroke (cerebral infarction)
- Cerebral embolism
- Spontaneous intracranial (subarachnoid) and intracerebral hemorrhage:
- Arteriovenous malformations of the brain
- Hypertensive intracerebral hemorrhage
- Cerebral arteries inflammatory diseases (cerebral arteritis)
- Giant intracranial aneurysms
- Other causes of intracerebral hemorrhage
- Lobar intracerebral hemorrhage
- Saccular aneurysm and subarachnoid hemorrhage
- Mycotic intracranial aneurysms
- Repeated cerebral artery aneurysm rupture
- Communicating hydrocephalus after intracerebral hemorrhage with ruptured aneurysm
- Cerebral vasospasm
- Cerebrovascular diseases - ischemic stroke, transient ischemic attack (TIA):
- Transient ischemic attack (TIA)
- Sigmoid sinus suppurative thrombophlebitis with thrombosis

 view.jpg)
.jpg)
.jpg)