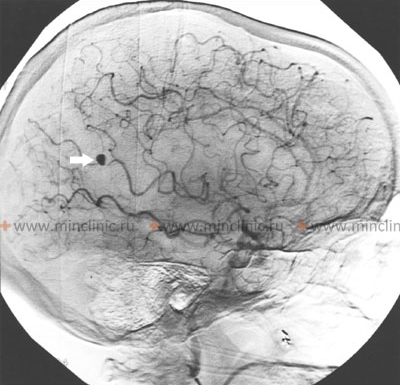
Mycotic intracranial aneurysms
Mycotic Intracranial Aneurysms: Overview and Causes
Brain aneurysms represent localized weaknesses and dilations in the walls of cerebral arteries. They can be classified based on their shape, cause, and size:
Types of Brain Aneurysms by Shape/Cause [1, 2]:
- Saccular ("berry") aneurysm: The most common type, often occurring at vessel bifurcations. Can become giant.
- Fusiform aneurysm: A spindle-shaped dilation involving a longer segment of the artery. Can also become giant.
- Dissecting aneurysm: Occurs when blood enters the vessel wall, separating its layers.
- Mycotic (Infectious) aneurysm: Caused by infection weakening the arterial wall. This is the focus here.
Classification based on Aneurysm Size [1]:
- ≤6 mm – Small aneurysm
- 7 to 12 mm – Medium aneurysm
- 13 to 24 mm – Large aneurysm
- ≥25 mm – Giant aneurysm
Understanding Mycotic Aneurysms
A mycotic intracranial aneurysm should be strongly suspected if an aneurysm is located distally (further along the vessel pathway) from the major bifurcations of the Circle of Willis, particularly on peripheral cortical branches [1, 3]. The primary underlying cause is typically a systemic infection, most commonly infective endocarditis (an infection of the heart valves or lining), leading to septic emboli [1, 3, 4]. Therefore, obtaining blood cultures is crucial for diagnosis and guiding treatment [4]. Mycotic aneurysms located in these more distal arterial branches (e.g., within cerebellar hemispheres or on the cortical surface) are less likely than proximal saccular aneurysms to cause massive subarachnoid hemorrhage filling the basal cisterns, although intracerebral or localized subarachnoid bleeding is common upon rupture [1].
Unlike ruptured saccular aneurysms, pronounced cerebral vasospasm is less frequently observed following the rupture of a mycotic aneurysm, though it can still occur [1]. However, mycotic aneurysms possess a significant risk of re-rupture [1, 3]. While appropriate, long-term antibiotic therapy targeting the identified pathogen is fundamental and can sometimes lead to aneurysm shrinkage or resolution, it doesn't eliminate the risk entirely [3, 4]. Definitive treatment often requires direct obliteration of the aneurysm, either through neurosurgical clipping or endovascular techniques (like coiling or vessel occlusion) [1, 3]. This intervention is generally pursued even while the patient is receiving intensive antibiotic treatment for the underlying infective endocarditis or other sepsis source [3].
Pathogenesis and Epidemiology
The development of mycotic cerebral aneurysms stems from a systemic infection, most frequently bacterial endocarditis [1, 3, 4]. Infectious emboli (clumps of bacteria, fibrin, and inflammatory cells) originating from the primary infection site (e.g., heart valve vegetation) travel through the bloodstream, eventually lodging within the cerebral arterial tree [1, 3]. These emboli can directly damage the vessel wall or obstruct the tiny vessels supplying the artery wall itself (the vasa vasorum), initiating an infectious and inflammatory process (vasculitis) [1, 3]. This inflammation progressively weakens the structural layers of the artery (intima, media, adventitia), leading to dilation and the formation of a fragile mycotic aneurysm [1, 3].
The term "mycotic" was first coined by Sir William Osler in 1885 while describing an aortic aneurysm in a patient with bacterial endocarditis [1]. Although "mycotic" implies a fungal origin, the term persists historically and is now broadly applied to any aneurysm arising from microbial infection (bacterial, fungal, or rarely other pathogens) of the vessel wall, including those affecting cerebral arteries [1, 3].
The precise mechanism of wall destruction is debated: some theories suggest direct damage from septic emboli lodging within the main artery lumen, while others propose infection spreading from the vasa vasorum outwards [1].
Mycotic aneurysms of cerebral arteries are detected in approximately 2-10% of patients diagnosed with infective endocarditis [3, 4]. They account for roughly 2.5-6% of all diagnosed intracranial aneurysms [1, 3]. About 75% of these infectious aneurysms affect the distal branches within the middle cerebral artery (MCA) territory, with the remainder distributed among other cerebral arteries (anterior cerebral, posterior cerebral, vertebrobasilar system) [1, 3]. Their characteristic distal location contrasts sharply with typical saccular aneurysms, which favor proximal locations and major bifurcations near the Circle of Willis [1, 3].
Mycotic aneurysms can form relatively rapidly, sometimes within weeks following the initial septic embolization event [1]. Streptococcus and Staphylococcus species are the most commonly implicated bacteria, reflecting their prevalence as causes of infective endocarditis [3, 4]. However, various other bacteria and, less commonly, fungi can also be responsible [3, 4].
Intracranial hemorrhage (both intracerebral and subarachnoid) occurs in about 2.7-7% of patients with infective endocarditis, often resulting from the rupture of an underlying mycotic aneurysm [3, 4]. Historically, the mortality rate for patients with untreated, unruptured mycotic aneurysms has been reported as high as 30%, often due to the severity of the underlying sepsis or neurological complications [3]. Once a mycotic aneurysm ruptures, mortality rates tragically increase, historically approaching 80%, although outcomes are improving with prompt diagnosis and modern multidisciplinary management [1, 3].
Mycotic Intracranial Aneurysm Diagnosis and Imaging Studies
Diagnosing a mycotic intracranial aneurysm requires a high index of suspicion, particularly in patients with known or suspected systemic infection (especially endocarditis) presenting with neurological symptoms [1, 3]. Key diagnostic steps include:
- Clinical Evaluation: Assessing for symptoms like severe headache (often sudden), fever, focal neurological deficits (e.g., weakness, numbness, speech problems), seizures, or altered mental status [1]. A history of IV drug use, recent dental procedures, or known heart conditions are important risk factors [3, 4].
- Blood Tests:
- Blood Cultures: Essential for identifying the causative microorganism and guiding antibiotic therapy [4]. Multiple sets should be drawn.
- Inflammatory Markers: Elevated C-reactive protein (CRP) and erythrocyte sedimentation rate (ESR) are common but non-specific [3].
- Neuroimaging:
- CT Scan (Computed Tomography): Often the initial imaging test, primarily to detect hemorrhage (subarachnoid or intracerebral) or ischemic stroke resulting from septic emboli [1, 5].
- CTA (CT Angiography): Can often visualize larger aneurysms and provides vascular mapping [5]. May be performed alongside the initial CT.
- MRI (Magnetic Resonance Imaging) & MRA (MR Angiography): MRI is more sensitive for detecting small infarcts, surrounding brain inflammation (cerebritis), or abscesses associated with septic emboli [5]. MRA provides non-invasive vascular imaging, though its sensitivity for small, distal aneurysms might be lower than DSA [5].
- DSA (Digital Subtraction Angiography): Considered the gold standard for definitively diagnosing, characterizing (size, shape, location), and identifying multiple mycotic aneurysms, which can occur [1, 3, 5]. It's an invasive procedure but offers the highest resolution imaging of the cerebral vasculature.
- Echocardiography: Transthoracic (TTE) and often Transesophageal (TEE) echocardiograms are crucial to identify vegetations on heart valves or other signs of infective endocarditis, the most common source of septic emboli [3, 4].
Differential Diagnosis of Mycotic Aneurysm [1, 5]
| Condition | Key Features / Distinguishing Points | Typical Investigations / Findings |
|---|---|---|
| Mycotic (Infectious) Aneurysm | History of systemic infection (esp. endocarditis). Distal location (peripheral branches). Often multiple. May change/grow/resolve with antibiotics. Risk of rupture (ICH/SAH). | DSA is gold standard. CTA/MRA may detect. MRI brain shows associated infarcts/inflammation. Positive blood cultures. Echocardiogram shows endocarditis. |
| Saccular (Berry) Aneurysm | Most common type. Typically at major bifurcations (Circle of Willis). Usually no infectious history. Rupture causes SAH typically. | CTA/MRA/DSA confirm aneurysm at typical location. Stable size unless enlarging (less rapid than mycotic). |
| Septic Embolism with Hemorrhagic Transformation | History of endocarditis/infection. Acute neurological deficit (stroke). Hemorrhage occurs within an area of ischemic infarct. No distinct aneurysm seen. | MRI shows infarct (DWI positive early) with superimposed hemorrhage. Angiography is negative for aneurysm. Positive blood cultures/Echo. |
| Vasculitis (Primary CNS or Systemic) | Inflammation of vessel walls. Can cause aneurysms (often fusiform or irregular), stenosis, occlusion leading to stroke or hemorrhage. May have systemic inflammatory signs. | Angiography shows characteristic vessel wall irregularities ("beading", stenosis, occlusion). MRI may show infarcts/hemorrhages. Inflammatory markers elevated. Biopsy (if feasible) confirms. |
| Blood Blister-like Aneurysm (BBA) | Shallow, fragile outpouching, often on non-branching segments of ICA. High risk of rupture/re-rupture. Usually presents with SAH. | Difficult to see on CTA/MRA. DSA best modality, shows small, broad-based bulge. Fragile wall. |
| Dissecting Aneurysm / Pseudoaneurysm | Often post-traumatic or spontaneous. Headache/neck pain common. Can cause stroke or SAH. Variable shape/location. | CTA/MRA/DSA shows intimal flap, double lumen, pseudoaneurysm formation. |
Mycotic Intracranial Aneurysm Treatment
Management of mycotic intracranial aneurysms is complex and requires a multidisciplinary approach involving neurologists, neurosurgeons, infectious disease specialists, and interventional neuroradiologists [1, 3]. Treatment focuses on two main goals: eradicating the underlying infection and securing the aneurysm to prevent rupture or re-rupture [1, 3].
- Antibiotic Therapy: This is the cornerstone of treatment [3, 4]. Prolonged courses (typically 4-6 weeks or longer) of high-dose, intravenous antibiotics targeted against the specific pathogen identified in blood cultures (or based on likely pathogens if cultures are negative) are essential [4]. Successful antibiotic therapy can sometimes lead to stabilization, shrinkage, or even complete resolution of the aneurysm, particularly if it is small and unruptured [3].
- Aneurysm Obliteration: The decision on whether and how to directly treat the aneurysm depends on its status (ruptured vs. unruptured), size, location, morphology, the patient's overall condition, and the response to antibiotic therapy [1, 3]. Options include:
- Conservative Management (Antibiotics Alone): May be considered for very small, unruptured aneurysms in patients responding well to antibiotics, but requires very close imaging follow-up (e.g., repeat angiography) due to the risk of growth or rupture [3].
- Neurosurgical Clipping: Involves open brain surgery to place a small metal clip across the neck of the aneurysm, excluding it from circulation [1]. May be preferred for accessible aneurysms, especially if there's associated intracerebral hematoma requiring evacuation [1].
- Endovascular Therapy: Minimally invasive techniques performed via catheters inserted through blood vessels (usually in the groin) [1, 3]. Options include:
- Coiling: Packing the aneurysm sac with platinum coils to induce thrombosis.
- Flow Diversion: Placing a stent-like device in the parent artery to redirect blood flow away from the aneurysm.
- Parent Vessel Occlusion: Sometimes necessary for distal aneurysms where selective treatment isn't feasible, potentially combined with bypass surgery. Endovascular options are often favored for surgically difficult-to-access or distal aneurysms.
- Management of the Infectious Source: Aggressively treating the underlying infective endocarditis (which may require heart valve surgery) or other source of infection is critical for overall success and preventing further embolization [3, 4].
- Monitoring: Close clinical and radiological follow-up is essential regardless of the treatment strategy chosen, to monitor for aneurysm changes, new aneurysm formation, or complications related to treatment or the underlying infection [1, 3].
The prognosis depends heavily on whether the aneurysm ruptured, the severity of the underlying infection, the patient's neurological condition at presentation, and the timeliness and effectiveness of treatment [1, 3].
References
- Ropper AH, Samuels MA, Klein JP, Prasad S. Adams and Victor's Principles of Neurology. 11th ed. McGraw Hill; 2019. Chapter 34: Cerebrovascular Diseases (Section on Mycotic Aneurysms).
- Greenberg MS. Handbook of Neurosurgery. 9th ed. Thieme; 2019. Chapter 37: Intracranial Aneurysms.
- Kannoth S, Thomas SV. Intracranial microbial aneurysm (infectious aneurysm). Neurosurg Focus. 2009 Dec;27(6):E13. (Or similar review article on mycotic aneurysms).
- Baddour LM, Wilson WR, Bayer AS, et al; American Heart Association Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease of the Council on Cardiovascular Disease in the Young, Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and Stroke Council. Infective Endocarditis in Adults: Diagnosis, Antimicrobial Therapy, and Management of Complications: A Scientific Statement for Healthcare Professionals From the American Heart Association. Circulation. 2015 Oct 13;132(15):1435-86.
- Osborn AG, Hedlund GL, Salzman KL. Osborn's Brain: Imaging, Pathology, and Anatomy. 2nd ed. Elsevier; 2017. Section on Infectious Aneurysms.
See also
- Ischemic stroke, cerebral ischemia
- Vertebrobasilar insufficiency (VBI) with vertigo symptom
- Somatoform autonomic dysfunction
- Dizziness, stuffiness in ear and tinnitus
- Ischemic brain disease:
- Atherosclerotic thrombosis
- Atherothrombotic occlusion of internal carotid artery
- Asymptomatic carotid bifurcation stenosis with noise
- Atherothrombotic occlusion of vertebrobasilar and posterior cerebral arteries
- Atherothrombotic occlusion of posterior cerebral artery
- Atherothrombotic occlusion of vertebral and posterior inferior cerebellar arteries (PICA)
- Atherothrombotic occlusion of basilar artery
- Small-vessel stroke (lacunar infarction)
- Other causes of ischemic stroke (cerebral infarction)
- Cerebral embolism
- Spontaneous intracranial (subarachnoid) and intracerebral hemorrhage:
- Arteriovenous malformations of the brain
- Hypertensive intracerebral hemorrhage
- Cerebral arteries inflammatory diseases (cerebral arteritis)
- Giant intracranial aneurysms
- Other causes of intracerebral hemorrhage
- Lobar intracerebral hemorrhage
- Saccular aneurysm and subarachnoid hemorrhage
- Mycotic intracranial aneurysms
- Repeated cerebral artery aneurysm rupture
- Communicating hydrocephalus after intracerebral hemorrhage with ruptured aneurysm
- Cerebral vasospasm
- Cerebrovascular diseases - ischemic stroke, transient ischemic attack (TIA):
- Transient ischemic attack (TIA)
- Sigmoid sinus suppurative thrombophlebitis with thrombosis