Aneurysmal rerupture
Aneurysmal Rerupture Overview
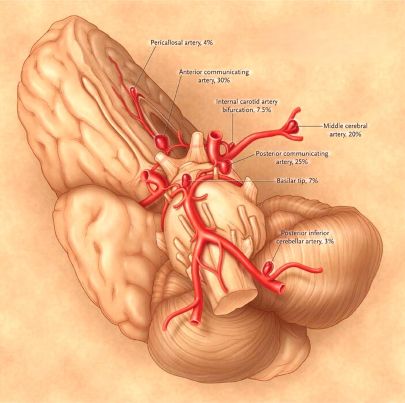
Types of brain aneurysms [1, 2]:
- Saccular ("berry") aneurysm (can be giant)
- Fusiform aneurysm (can be giant)
- Dissecting aneurysm
- Mycotic (infectious) aneurysm
Classification based on aneurysm size [1]:
- ≤6 mm – Small aneurysm
- 7 to 12 mm – Medium aneurysm
- 13 to 24 mm – Large aneurysm
- ≥25 mm – Giant aneurysm
What is Aneurysmal Rerupture?
Aneurysmal rerupture is one of the most feared and life-threatening complications following an initial aneurysmal subarachnoid hemorrhage (SAH) [1, 3]. It refers to a second bleed from the same aneurysm before it has been definitively treated (secured) [1, 3]. The unstable clot that forms at the rupture site can dislodge, or the weakened aneurysm wall can give way again, leading to another, often more severe, hemorrhage into the subarachnoid space or brain parenchyma [1].
The risk of rerupture is highest shortly after the initial SAH, peaking within the first 24 hours, and remaining significant, particularly within the first 7-14 days, until the aneurysm is secured by surgical clipping or endovascular coiling/flow diversion [1, 3]. Historically, rerupture rates in unsecured aneurysms were reported between 10-30% within the first month, with extremely high mortality (often exceeding 50-70%) associated with the rebleed event itself [1].
Risk Factors for Rerupture
Several factors increase the likelihood of an aneurysm rerupturing before treatment [1, 3]:
- Time Since Initial Rupture: The risk is highest immediately after the first bleed and decreases over time, but remains substantial until the aneurysm is secured.
- Poor Initial Clinical Grade: Patients presenting with worse neurological status (e.g., higher Hunt and Hess or WFNS grade) are at higher risk.
- Amount of Subarachnoid Blood: A large volume of blood seen on the initial CT scan (e.g., higher Fisher grade) is associated with increased risk.
- Elevated Blood Pressure: Uncontrolled hypertension significantly increases stress on the weakened aneurysm wall.
- Aneurysm Characteristics: Larger aneurysm size and specific locations may carry a higher rerupture risk, although this is complex.
- Delay in Treatment: The longer the delay between the initial SAH and definitive aneurysm securing, the greater the cumulative risk of rerupture.
Clinical Presentation and Diagnosis
Aneurysmal rerupture typically presents as a sudden, dramatic neurological deterioration in a patient being managed for SAH [1]. Key signs include:
- Sudden increase in headache severity.
- Abrupt decrease in level of consciousness (drowsiness, stupor, or coma).
- New focal neurological deficits (e.g., weakness, paralysis, pupil changes).
- Seizures.
- Cardiopulmonary instability (e.g., sudden hypertension, bradycardia, respiratory arrest).
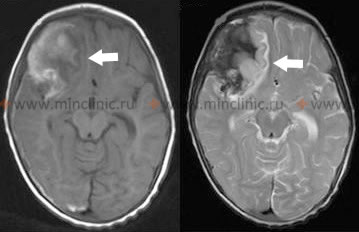
Diagnosis is usually confirmed urgently with a repeat computed tomography (CT) scan of the brain, which will typically show new or increased amounts of blood in the subarachnoid space, ventricles, or brain parenchyma compared to previous scans [1, 4].
Differential Diagnosis of Sudden Neurological Deterioration After SAH [1, 4]
| Condition | Key Features / Distinguishing Points | Typical Investigations / Findings |
|---|---|---|
| Aneurysmal Rerupture | Sudden, catastrophic decline in LOC, often severe headache increase, new deficits. Highest risk early before aneurysm secured. | Repeat Head CT shows new/increased hemorrhage (SAH, IVH, ICH). Urgent angiography needed if unsecured. |
| Delayed Cerebral Ischemia (DCI) due to Vasospasm | Typically occurs 4-14 days post-SAH. Gradual or fluctuating onset of new focal deficits (hemiparesis, aphasia) or decreased LOC. May have preceding subtle changes on TCD. | CT head often initially normal (no new bleed). CT Perfusion/MRI may show ischemia. Angiography confirms vasospasm (vessel narrowing). TCD shows increased flow velocities. |
| Acute Hydrocephalus | Can occur early (due to blood obstructing CSF pathways) or later. Decreased LOC, headache, vomiting, +/- gaze palsies. | CT head shows enlarged ventricles disproportionate to sulcal size. May require EVD placement. |
| Seizures | Can cause transient neurological deficits (Todd's paralysis) or prolonged altered LOC (non-convulsive status epilepticus). May occur early or late after SAH. | Clinical seizure activity or subtle signs. EEG confirms seizure activity or post-ictal slowing. Imaging excludes new structural lesion. |
| Systemic Complications (e.g., Sepsis, Hypoxia, Hyponatremia, Cardiac event) | Deterioration often associated with signs of systemic illness (fever, hypotension, respiratory distress, metabolic disturbance). Neurological changes may be more diffuse initially. | Relevant systemic workup (blood cultures, ABG, electrolytes, ECG, chest X-ray). Head CT usually shows no acute intracranial change explaining deterioration. |
| Medication Effects (e.g., Sedation) | Decreased LOC related to administration or accumulation of sedative/analgesic medications. | Review medication record. Response to decreased sedation or reversal agents (if applicable). Normal imaging. |
Prevention and Management
Preventing rerupture is a primary goal in the management of aneurysmal SAH [1, 3]. The cornerstone of prevention is early and definitive securing of the ruptured aneurysm [1, 3].
- Urgent Aneurysm Treatment: Most centers aim to treat ruptured aneurysms within 24-72 hours of the initial SAH, either by [1, 3]:
- Surgical Clipping: Open brain surgery to place a metal clip across the neck of the aneurysm, excluding it from circulation [1, 5].
- Endovascular Coiling/Flow Diversion: Minimally invasive procedures using catheters inserted via blood vessels to place platinum coils inside the aneurysm sac (promoting thrombosis) or deploy flow-diverting stents in the parent artery [1, 5].
- Blood Pressure Control: Careful management to maintain systolic blood pressure below a certain target (e.g., <160 mmHg, but varies) before the aneurysm is secured helps reduce stress on the aneurysm wall without compromising cerebral perfusion [1, 3].
- Minimizing Stress: Keeping the patient calm, managing pain and agitation, and preventing straining (e.g., constipation) are also important supportive measures [1].
If rerupture occurs, management involves immediate resuscitation, control of intracranial pressure (ICP), and urgent consideration for securing the aneurysm if the patient's condition permits, though the prognosis is often very poor [1].
Role of Antifibrinolytic Therapy
Antifibrinolytic drugs, such as aminocaproic acid (Amicar) or tranexamic acid (TXA), work by inhibiting the breakdown of blood clots [1]. They have been studied for their potential to stabilize the clot at the aneurysm rupture site and thereby reduce the risk of rerupture before definitive treatment can be performed [1, 6].
Current evidence and practice suggest a limited role for these agents [1, 3, 6]:
- They may be considered for short-term use (e.g., up to 72 hours) as a bridge therapy only in patients with a confirmed SAH where there is an unavoidable delay in securing the aneurysm [3, 6].
- Their use is controversial because while they might decrease rerupture rates, clinical trials have shown they may increase the risk of delayed cerebral ischemia (ischemic stroke), possibly by promoting microthrombosis or worsening the effects of cerebral vasospasm (another major SAH complication) [1, 6].
- Overall impact on long-term functional outcome remains uncertain, with some meta-analyses showing no net benefit or potential harm [6].
- If used, they are typically administered intravenously (e.g., aminocaproic acid or tranexamic acid infusion) from admission until the time of aneurysm repair, but discontinued promptly afterwards or if signs of significant vasospasm or ischemia develop [1, 3].
It is crucial to understand that antifibrinolytic therapy is not a substitute for definitive aneurysm treatment (clipping or coiling), which remains the most effective way to prevent rerupture [1, 3].
Furthermore, cerebral vasospasm, a narrowing of cerebral arteries typically occurring 4-14 days after SAH due to irritation from blood breakdown products, is a separate major cause of morbidity and mortality after SAH, leading to ischemic stroke [1, 3]. Managing vasospasm involves therapies like calcium channel blockers (nimodipine) and maintaining adequate blood volume and pressure (triple-H therapy components, now often modified), and sometimes angioplasty [1, 3]. The risk of vasospasm exists independently of whether antifibrinolytics are used.
References
- Ropper AH, Samuels MA, Klein JP, Prasad S. Adams and Victor's Principles of Neurology. 11th ed. McGraw Hill; 2019. Chapter 34: Cerebrovascular Diseases (Section on Intracranial Aneurysms and Subarachnoid Hemorrhage, including rerupture and complications).
- Greenberg MS. Handbook of Neurosurgery. 9th ed. Thieme; 2019. Chapter 37: Intracranial Aneurysms.
- Connolly ES Jr, Rabinstein AA, Carhuapoma JR, et al; American Heart Association Stroke Council; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; Council on Cardiovascular Surgery and Anesthesia; Council on Clinical Cardiology. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2012 Jun;43(6):1711-37.
- Osborn AG, Hedlund GL, Salzman KL. Osborn's Brain: Imaging, Pathology, and Anatomy. 2nd ed. Elsevier; 2017. Section on Aneurysms and Subarachnoid Hemorrhage.
- Winn HR. Youmans and Winn Neurological Surgery. 7th ed. Elsevier; 2017. Volume 4, Chapter 369: Management of Patients with Aneurysmal Subarachnoid Hemorrhage.
- Bahra A, Matharu MS, Buchem MA, et al. Antifibrinolytic treatment for aneurysmal subarachnoid haemorrhage. Cochrane Database Syst Rev. 2006 Oct 18;(4):CD001245. Review. Update in: Cochrane Database Syst Rev. 2013;8:CD001245. (Or more recent Cochrane review/meta-analysis).
- Report of World Federation of Neurological Surgeons Committee on a Universal Subarachnoid Hemorrhage Grading Scale. J Neurosurg. 1988 Jun;68(6):985-6.
- Hunt WE, Hess RM. Surgical risk as related to time of intervention in the repair of intracranial aneurysms. J Neurosurg. 1968 Jan;28(1):14-20.
See also
- Ischemic stroke, cerebral ischemia
- Vertebrobasilar insufficiency (VBI) with vertigo symptom
- Somatoform autonomic dysfunction
- Dizziness, stuffiness in ear and tinnitus
- Ischemic brain disease:
- Atherosclerotic thrombosis
- Atherothrombotic occlusion of internal carotid artery
- Asymptomatic carotid bifurcation stenosis with noise
- Atherothrombotic occlusion of vertebrobasilar and posterior cerebral arteries
- Atherothrombotic occlusion of posterior cerebral artery
- Atherothrombotic occlusion of vertebral and posterior inferior cerebellar arteries (PICA)
- Atherothrombotic occlusion of basilar artery
- Small-vessel stroke (lacunar infarction)
- Other causes of ischemic stroke (cerebral infarction)
- Cerebral embolism
- Spontaneous intracranial (subarachnoid) and intracerebral hemorrhage:
- Arteriovenous malformations of the brain
- Hypertensive intracerebral hemorrhage
- Cerebral arteries inflammatory diseases (cerebral arteritis)
- Giant intracranial aneurysms
- Other causes of intracerebral hemorrhage
- Lobar intracerebral hemorrhage
- Saccular aneurysm and subarachnoid hemorrhage
- Mycotic intracranial aneurysms
- Repeated cerebral artery aneurysm rupture
- Communicating hydrocephalus after intracerebral hemorrhage with ruptured aneurysm
- Cerebral vasospasm
- Cerebrovascular diseases - ischemic stroke, transient ischemic attack (TIA):
- Transient ischemic attack (TIA)
- Sigmoid sinus suppurative thrombophlebitis with thrombosis