Atherothrombotic occlusion of internal carotid artery
- Development of Carotid Artery Atherosclerosis with Thrombosis
- Proximal Internal Carotid Artery Atherosclerosis
- Cerebral Ischemia Caused by Reduced Blood Flow
- Embolization from the Carotid Artery
- Intracranial Internal Carotid Artery (Carotid Siphon) Atherosclerosis
- Atherosclerosis with Thrombosis of the Middle Cerebral Artery (MCA)
- Atherosclerosis with Thrombosis of the Anterior Cerebral Artery (ACA)
- Differential Diagnosis of Carotid Territory Stroke Symptoms
Development of Carotid Artery Atherosclerosis with Thrombosis
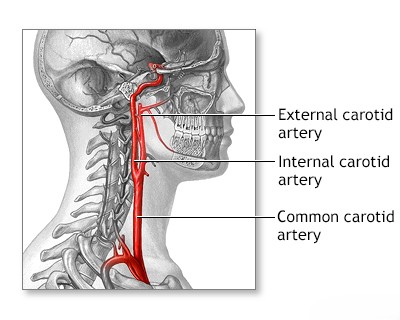
In the carotid arterial system, atherosclerotic thrombosis leading to a transient ischemic attack (TIA, or 'mini-stroke') or a full stroke commonly develops at the carotid bifurcation (where the main carotid artery splits into the internal and external branches). Less frequently, it occurs in the carotid siphon (the S-shaped segment of the internal carotid artery within the cavernous sinus), the proximal (initial) segment of the middle cerebral artery (MCA), or the anterior cerebral artery (ACA). Thrombosis related to atherosclerosis is least commonly observed at the origin of the common carotid artery itself [1, 2].
The exact timing of when stenosis (narrowing) or ulcerative changes (plaque surface breakdown) occur in the arterial walls at these locations relative to the onset of clinical symptoms (like TIA or stroke) remains unclear. However, it is generally accepted that carotid atherosclerosis associated with thrombosis is a progressive process, meaning it tends to worsen over time [3].
Proximal Internal Carotid Artery Atherosclerosis
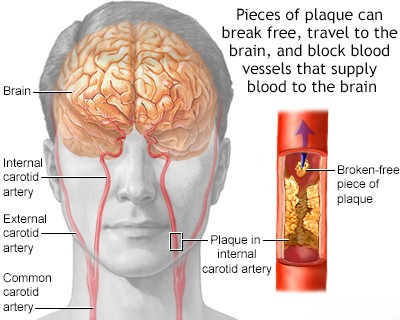
Atherosclerosis of the proximal internal carotid artery (the segment just after the carotid bifurcation) is typically most severe within the first 2 cm distal to the bifurcation (further from the heart) and is predominantly located on its posterior (back) wall. It often extends downwards (distally) into the common carotid artery [1, 2]. In 50–80% of stroke cases involving carotid disease, this atherosclerotic lesion contributes to small strokes (sometimes called lacunar strokes or microstrokes) or transient ischemic attacks (TIAs) [5, approximate range]. These events result either from critically reduced cerebral blood flow (hemodynamic insufficiency) due to severe narrowing (stenosis) or, more commonly, from embolism (plaque fragments or clots breaking off and traveling) from the carotid artery into its intracerebral branches (like the MCA or ACA) [1, 4].
Clinical experience and autopsy data suggest that strokes associated with carotid artery lesions are more frequently caused by emboli originating from the plaque rather than by reduced blood flow (low flow state) alone [1, 6]. An embolus from an atherosclerotic plaque at the origin of the internal carotid artery can certainly trigger a transient ischemic attack (TIA). However, if TIAs are recurrent, very brief (seconds to minutes), and consistently produce the same symptoms (stereotyped), they are more likely attributable to hemodynamic disturbances (temporary drops in blood flow past a severe stenosis) rather than repeated emboli [1, 7].
Cerebral Ischemia Caused by Reduced Blood Flow (Hemodynamic Insufficiency)
Insufficient arterial blood flow (ischemia) can lead to a brain stroke (infarction) or trigger a transient ischemic attack (TIA), particularly in "watershed" or border zones – areas located between the territories supplied by major cerebral arteries, which are most vulnerable to drops in perfusion pressure [1, 8]. The development of strokes and TIAs due to cerebral blood flow deficits (hemodynamic causes) is primarily linked to two conditions:
- A significant drop in blood pressure distal to (downstream from) a severe carotid artery stenosis, typically where the lumen diameter is reduced by more than 80% (leaving a residual lumen of less than 1.5–2 mm) [1, 9].
- Inadequate collateral blood flow (alternative circulatory pathways) to compensate for the reduced supply to the ischemic brain regions [1, 8].
Cerebral blood flow deficits often arise when the Circle of Willis (a ring of arteries at the base of the brain that connects the carotid and vertebrobasilar systems) is incomplete. This incompleteness is commonly due to congenital absence or underdevelopment (atresia/hypoplasia) of key segments, such as the A1 segment of the anterior cerebral artery or the anterior or posterior communicating arteries [8, 10]. Less frequently, significant brain damage occurs when occlusion of the *contralateral* (opposite side) carotid artery or the basilar artery further restricts blood flow entering the Circle of Willis. In some individuals, adequate compensatory blood supply can be maintained via orbital collaterals (connections through the eye socket arteries) originating from the external carotid artery system, or via superficial cortical collaterals (leptomeningeal anastomoses) connecting branches of major cerebral arteries over the brain surface [8, 10]. Even with an incomplete Circle of Willis, robust collateral circulation can sometimes limit the extent of ischemic damage. This inherent variability in collateral anatomy and efficiency helps explain the diverse locations and severity of lesions seen in strokes and transient ischemic attacks (TIAs) associated with carotid artery insufficiency [1].
Other mechanisms can also contribute to transient ischemic attacks (TIAs) linked to reduced cerebral blood flow. Severe stenosis at the common carotid artery bifurcation might theoretically lead to transient vessel occlusion due to spasm, though this is considered rare [1]. More commonly, systemic circulatory problems (like a sudden drop in overall blood pressure) can reduce blood flow through a critically narrowed vessel lumen to dangerously low levels. Additionally, regional blood flow within a brain hemisphere might fluctuate due to the compromised carotid artery flow, and temporary failure of these compensatory collateral mechanisms can precipitate a TIA [1, 7]. Other contributing factors – such as conditions causing thicker blood like polycythemia vera (excess red blood cells) or thrombocythemia (excess platelets), or cardiac arrhythmias affecting blood output – can also trigger recurrent TIAs, especially when both cerebral and systemic blood flow are already compromised [1].
Embolization from the Carotid Artery (Arterio-Arterial Embolism)
Emboli (mobile clots or plaque fragments) originating from a narrowed (stenotic) or ulcerated atherosclerotic lesion in the proximal internal carotid artery are a common cause of stroke and TIA [1, 4]. This process is known as arterio-arterial embolism. These emboli typically travel downstream and cause symptoms by occluding (blocking) arteries such as the ophthalmic artery (supplying the eye), the main trunk or branches of the middle cerebral artery (MCA), and occasionally the anterior cerebral artery (ACA) or its branches [1, 4, 6]. Generally, the size of the embolus dictates the caliber (size) of the vessel it ultimately occludes [1].
Small emboli (microemboli) might obstruct only small distal branches of the middle cerebral artery or the ophthalmic artery. Occlusion of the ophthalmic artery can lead to transient monocular blindness (amaurosis fugax) [1, 11]. Obstruction of small cerebral artery branches may result in minor, sometimes clinically silent (asymptomatic), infarctions, particularly in brain regions supplied by adjacent cerebral arteries (border zones or watershed areas) [1].
Larger platelet-fibrin emboli (composed mainly of platelets and fibrin protein) can occlude primary (major) and secondary branches of the middle cerebral artery. The specific neurological syndromes that result depend directly on the brain regions supplied by the blocked vessel [1, 6].
Very large emboli can completely obstruct the proximal (origin) segment of the middle cerebral artery (M1 segment). This typically leads to severe ischemia and infarction affecting its entire territory, including deep structures like the basal ganglia (lenticular nucleus) and the cortical surface supplied by the MCA [1, 6].
Even when large emboli occlude the proximal middle cerebral artery, the resulting infarct (stroke) might primarily affect deep brain structures if sufficient collateral flow through superficial cortical arteries (leptomeningeal collaterals) can partially compensate and protect the cortical surface [1, 8].
Large emboli obstructing large vessels are not always permanent; they can sometimes be dissolved (lysed) by the body's natural fibrinolytic system or fragment and migrate distally [1]. Rapid dissolution or fragmentation of emboli can result in transient neurological deficits or even complete resolution of symptoms [1, 7].
In patients presenting with neurological symptoms potentially related to carotid disease, the underlying vascular lesion can range from a single, non-stenotic but potentially ulcerated plaque at the carotid bifurcation to a high-grade stenosis with a residual lumen diameter of less than 2 mm [1, 9].
The exact frequency of massive embolic strokes (cerebral infarctions) caused *solely* by ulcerated, non-stenotic atherosclerotic lesions remains unclear. Some evidence suggests that the incidence of embolic stroke from such lesions might be relatively low and primarily associated with large ulcerated plaques (e.g., ≥4 mm in size) [1, 13]. The occurrence of a stroke or a transient ischemic attack (TIA) with prolonged symptoms, especially when diagnostic imaging shows minimal or absent carotid artery stenosis, should prompt consideration of alternative sources, particularly a cardiac source of emboli (e.g., atrial fibrillation) [1, 14]. Atherosclerotic lesions at the origin of the major aortic arch branches (like the brachiocephalic, common carotid, or subclavian arteries) can also lead to cerebral embolism, resulting in transient ischemia or infarction, although the precise incidence of this mechanism is also uncertain [1, 6].
Complete occlusion of the proximal internal carotid artery may remain asymptomatic if there is robust collateral blood flow through the Circle of Willis from the contralateral carotid artery and the posterior (vertebrobasilar) circulation [1, 8]. Conversely, insufficient collateral circulation can lead to hemodynamic stroke or transient ischemic attack (TIA) shortly after the occlusion occurs. Furthermore, a thrombus (clot) can propagate upwards from the site of occlusion in the neck, extending through the carotid siphon intracranially to the origins of the middle and anterior cerebral arteries, resulting in a major stroke [1]. More frequently after occlusion, however, fresh thrombotic emboli may detach from the top of the clot column within the occluded ICA and lodge distally in the middle or anterior cerebral arteries or their branches [1, 4]. Some authors have suggested that emboli might originate from the residual stump of the occluded internal carotid artery and travel retrograde into the external carotid artery, then re-enter the intracranial circulation via ECA-ICA collaterals to reach the internal carotid artery branches. However, such stump emboli are generally considered rare [1].
The etiology (cause) of delayed stroke, occurring months or even years after a documented complete carotid artery occlusion, often remains unclear, and its true incidence is unknown [1]. One older study reported a 5% annual incidence of delayed stroke following ICA occlusion; however, this figure is generally considered high based on contemporary clinical experience [1, citation needed for specific study if available]. It is thought that most embolic events related to carotid artery occlusion occur within the first year after the occlusion, although they can potentially occur up to two years later [1]. Hemodynamic strokes (due to low flow) typically develop much earlier, usually within days or weeks of the acute carotid artery occlusion [1].
Intracranial Internal Carotid Artery (Carotid Siphon) Atherosclerosis
Atherosclerosis and subsequent thrombosis can also affect the carotid siphon – the S-shaped portion of the internal carotid artery located intracranially within the cavernous sinus at the base of the skull [1, 17]. These lesions can sometimes represent an upward extension of disease from the proximal internal carotid artery in the neck, or they can develop independently within the siphon itself. Lesions within the carotid siphon can lead to strokes and transient ischemic attacks (TIAs), with pathophysiological mechanisms (embolism or low flow) and clinical manifestations similar to those described for proximal carotid disease, as they affect the same downstream arterial territories (MCA, ACA, ophthalmic artery) [1, 17]. However, the clinical presentation specifically attributable to carotid siphon stenosis is often nonspecific and difficult to distinguish from more proximal lesions based on symptoms alone [1].
Carotid siphon stenosis may remain asymptomatic for a long time, often until the narrowing becomes critical and the residual lumen diameter is reduced to approximately 1.5 mm or less, potentially compromising blood flow or increasing the risk of local thrombus formation [1]. Due to its deep location within the skull base, surrounded by bone, diagnosing carotid siphon stenosis accurately often requires invasive catheter angiography or high-resolution non-invasive imaging like CT angiography (CTA) or MR angiography (MRA); standard carotid ultrasound is generally unable to visualize this segment adequately [15, 17]. The status of collateral blood flow through the Circle of Willis significantly influences the clinical consequences (pathogenesis) of these lesions and plays a crucial role in determining patient outcomes and the potential efficacy of medical or surgical/endovascular treatment strategies [1, 8].
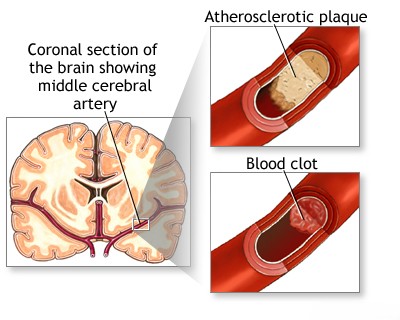
Atherosclerosis with Thrombosis of the Middle Cerebral Artery (MCA)
Atherosclerotic thrombosis affecting the main trunk (M1 segment) of the middle cerebral artery can cause cerebral ischemia. This can occur either through severe arterial stenosis reducing blood flow directly, or by occlusion of the origins of the proximal lenticulostriate arteries. These small penetrating vessels arise from the M1 segment and supply crucial deep brain structures, including parts of the basal ganglia and internal capsule [1, 18]. Atherosclerotic plaque causing clinical symptoms in the MCA most often develops in the M1 segment, proximal to (before) the main bifurcation where the MCA typically splits into its major branches [1, 18]. Because the Circle of Willis provides collateral flow *proximal* to the MCA origin (at the end of the internal carotid artery), collateral blood supply to the MCA territory, should the M1 segment become occluded, relies primarily on superficial cortical anastomoses (also called leptomeningeal collaterals). These are connections over the brain surface between distal branches of the MCA and adjacent branches of the anterior and posterior cerebral arteries [1, 8].
Available data suggest that preceding a completed cerebral infarction (stroke) in the MCA territory, transient ischemic attacks (TIAs) often serve as a warning sign, potentially indicating progressive narrowing (stenosis) of the vessel lumen [1]. These TIAs can present with symptoms similar to those associated with reduced blood flow (hemodynamic insufficiency) due to severe internal carotid artery stenosis. However, unlike internal carotid artery disease where both hemodynamic insufficiency and embolism are major factors, occlusion of the MCA trunk and its major branches is statistically more commonly caused by emboli originating from elsewhere (such as arterio-arterial emboli from the carotid bifurcation or aorta, cardiac emboli from the heart, or sometimes of unknown origin) rather than by primary *in situ* atherosclerotic thrombosis of the MCA itself [1, 6, 18].
Atherosclerosis with Thrombosis of the Anterior Cerebral Artery (ACA)
Atherosclerotic deposits (plaques) developing in the proximal anterior cerebral artery (typically the A1 segment, between the internal carotid artery terminus and the anterior communicating artery) rarely produce significant clinical neurological deficits on their own [1, 19]. This is because occlusion of one A1 segment is often well compensated for by collateral flow from the contralateral (opposite side) A1 segment via the anterior communicating artery (AComm), assuming the AComm is present and patent [1, 8]. The risk of transient ischemic attack (TIA) and stroke related to ACA disease increases significantly when this collateral pathway is compromised. Such compromise can occur due to congenital absence or severe underdevelopment (atresia/hypoplasia) of the anterior communicating artery, or if there are concurrent atherosclerotic changes affecting the contralateral A1 segment or the distal anterior cerebral artery (A2 segment and beyond) [1, 8, 19].
Differential Diagnosis of Acute Focal Neurological Deficits (Carotid Territory Stroke Symptoms) [20]
| Condition | Key Features / Distinguishing Points | Typical Investigations / Findings |
|---|---|---|
| Ischemic Stroke (Carotid Territory - Thrombotic/Embolic) | Sudden onset hemiparesis/hemisensory loss (face/arm > leg), aphasia (dominant hemisphere), neglect (non-dominant), gaze deviation, +/- amaurosis fugax (transient monocular blindness - a symptom of TIA). Vascular risk factors. | Non-contrast CT head (initially to exclude hemorrhage). MRI (esp. DWI) confirms ischemia. Carotid imaging (Ultrasound, CTA, MRA) identifies stenosis/occlusion or plaque. Cardiac workup (ECG, Echo) for embolism source. |
| Intracerebral Hemorrhage (ICH) | Sudden onset focal neurological deficit. Often severe headache, vomiting, decreased consciousness. Symptoms depend on location (e.g., basal ganglia hemorrhage causes contralateral hemiparesis). Often linked to hypertension. | Non-contrast CT head definitively shows hemorrhage. MRI later for detail. Control BP. |
| Subarachnoid Hemorrhage (SAH) | Sudden "thunderclap" headache. Altered consciousness, neck stiffness. Focal signs may occur due to vasospasm or associated ICH. | Non-contrast CT head shows subarachnoid blood. LP if CT negative but high suspicion. CTA/DSA identifies aneurysm. |
| Seizure with Todd's Paralysis | Post-ictal focal weakness mimicking stroke. History of seizure. Usually resolves within minutes to 48 hours. | History of seizure. EEG may show abnormalities. Brain imaging often normal or shows seizure focus. Transient nature. |
| Migraine with Aura (Hemiplegic) | Transient hemiparesis/hemisensory symptoms precede or accompany migraine headache. Often history of similar episodes. Full recovery between attacks. | Clinical diagnosis. Normal neurological exam between attacks. Imaging usually normal. |
| Hypoglycemia | Can cause focal neurological deficits mimicking stroke, confusion, seizures. History of diabetes important. | Low blood glucose. Symptoms resolve with glucose administration. |
| Brain Tumor | Progressive focal deficits, headache, seizures. Symptoms depend on location. | MRI with contrast shows mass lesion. |
| Brain Abscess | Focal deficits, headache, fever, seizures. History of infection source often present. | MRI shows ring-enhancing lesion with restricted diffusion. Elevated inflammatory markers. |
| Multiple Sclerosis (MS) Relapse | Acute/subacute onset focal deficits (weakness, sensory, visual). History of prior episodes possible. | MRI shows characteristic demyelinating lesions. |
| Subdural Hematoma (SDH) | Can cause focal signs due to compression. Headache, altered mental status. History of trauma (may be trivial in chronic SDH). | CT/MRI shows crescent-shaped subdural collection. |
| Peripheral Nerve Lesion (e.g., Bell's Palsy) | Presents with isolated peripheral nerve deficit (e.g., facial weakness) without central signs (no associated weakness/sensory loss in limb, no aphasia). | Clinical diagnosis. Brain imaging normal. EMG/NCS may help. |
| Functional Neurological Disorder | Neurological symptoms inconsistent with organic disease. Positive clinical signs (e.g., Hoover's sign). | Diagnosis of exclusion after thorough workup. Normal imaging/labs. |
References
- Ropper AH, Samuels MA, Klein JP, Prasad S. Adams and Victor's Principles of Neurology. 11th ed. McGraw Hill; 2019. Chapter 34: Cerebrovascular Diseases.
- Caplan LR. Caplan's Stroke: A Clinical Approach. 5th ed. Cambridge University Press; 2016. Chapter on Large Artery Atherosclerosis.
- Libby P, Buring JE, Badimon L, Hansson GK, Deanfield J, Bittencourt MS, et al. Atherosclerosis. Nat Rev Dis Primers. 2019 Aug 29;5(1):56.
- Grotta JC, Albers GW, Broderick JP, et al. Stroke: Pathophysiology, Diagnosis, and Management. 7th ed. Elsevier; 2021. Chapter on Mechanisms of Ischemic Stroke.
- Kernan WN, Ovbiagele B, Black HR, et al; American Heart Association Stroke Council, Council on Cardiovascular and Stroke Nursing, Council on Clinical Cardiology, and Council on Peripheral Vascular Disease. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014 Jul;45(7):2160-236. (Provides context on stroke burden related to TIA/prior stroke).
- Caplan LR. Caplan's Stroke: A Clinical Approach. 5th ed. Cambridge University Press; 2016. Chapter on Embolic Stroke.
- Caplan LR. Caplan's Stroke: A Clinical Approach. 5th ed. Cambridge University Press; 2016. Chapter on Transient Ischemic Attacks.
- Ropper AH, Samuels MA, Klein JP, Prasad S. Adams and Victor's Principles of Neurology. 11th ed. McGraw Hill; 2019. Chapter 34: Cerebrovascular Diseases, sections on Collateral Circulation and Watershed Infarction.
- Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2019 Dec;50(12):e344-e418. (Discusses hemodynamic factors).
- Blumenfeld H. Neuroanatomy through Clinical Cases. 2nd ed. Sinauer Associates; 2010. Chapter 4: Blood Supply, Meninges, and Venous Drainage.
- Ropper AH, Samuels MA, Klein JP, Prasad S. Adams and Victor's Principles of Neurology. 11th ed. McGraw Hill; 2019. Chapter 13: Disorders of Ocular Movement and Pupillary Function (Section on Amaurosis Fugax).
- Brott TG, Halperin JL, Abbara S, et al. 2011 ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/SAIP/SCAI/SIR/SNIS/SVM/SVS guideline on the management of patients with extracranial carotid and vertebral artery disease. Stroke. 2011 Aug;42(8):e464-540. (Or more recent update).
- Grotta JC, Albers GW, Broderick JP, et al. Stroke: Pathophysiology, Diagnosis, and Management. 7th ed. Elsevier; 2021. Chapter on Atherosclerosis and Stroke.
- January CT, Wann LS, Calkins H, et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation. 2019 Jul 9;140(2):e125-e151. (Discusses AFib as embolic source).
- Osborn AG, Hedlund GL, Salzman KL. Osborn's Brain: Imaging, Pathology, and Anatomy. 2nd ed. Elsevier; 2017. Section on Vascular Diseases, Carotid Artery.
- Grant EG, Benson CB, Moneta GL, et al. Carotid artery stenosis: gray-scale and Doppler US diagnosis--Society of Radiologists in Ultrasound Consensus Conference. Radiology. 2003 Nov;229(2):340-6. (Standard for ultrasound assessment).
- Grotta JC, Albers GW, Broderick JP, et al. Stroke: Pathophysiology, Diagnosis, and Management. 7th ed. Elsevier; 2021. Chapter on Intracranial Atherosclerosis.
- Ropper AH, Samuels MA, Klein JP, Prasad S. Adams and Victor's Principles of Neurology. 11th ed. McGraw Hill; 2019. Chapter 34: Cerebrovascular Diseases, section on Middle Cerebral Artery Occlusion.
- Ropper AH, Samuels MA, Klein JP, Prasad S. Adams and Victor's Principles of Neurology. 11th ed. McGraw Hill; 2019. Chapter 34: Cerebrovascular Diseases, section on Anterior Cerebral Artery Occlusion.
- Ropper AH, Samuels MA, Klein JP, Prasad S. Adams and Victor's Principles of Neurology. 11th ed. McGraw Hill; 2019. Chapter 34: Cerebrovascular Diseases, section on Differential Diagnosis of Stroke.
See also
- Ischemic stroke, cerebral ischemia
- Vertebrobasilar insufficiency (VBI) with vertigo symptom
- Somatoform autonomic dysfunction
- Dizziness, stuffiness in ear and tinnitus
- Ischemic brain disease:
- Atherosclerotic thrombosis
- Atherothrombotic occlusion of internal carotid artery
- Asymptomatic carotid bifurcation stenosis with noise
- Atherothrombotic occlusion of vertebrobasilar and posterior cerebral arteries
- Atherothrombotic occlusion of posterior cerebral artery
- Atherothrombotic occlusion of vertebral and posterior inferior cerebellar arteries (PICA)
- Atherothrombotic occlusion of basilar artery
- Small-vessel stroke (lacunar infarction)
- Other causes of ischemic stroke (cerebral infarction)
- Cerebral embolism
- Spontaneous intracranial (subarachnoid) and intracerebral hemorrhage:
- Arteriovenous malformations of the brain
- Hypertensive intracerebral hemorrhage
- Cerebral arteries inflammatory diseases (cerebral arteritis)
- Giant intracranial aneurysms
- Other causes of intracerebral hemorrhage
- Lobar intracerebral hemorrhage
- Saccular aneurysm and subarachnoid hemorrhage
- Mycotic intracranial aneurysms
- Repeated cerebral artery aneurysm rupture
- Communicating hydrocephalus after intracerebral hemorrhage with ruptured aneurysm
- Cerebral vasospasm
- Cerebrovascular diseases - ischemic stroke, transient ischemic attack (TIA):
- Transient ischemic attack (TIA)
- Sigmoid sinus suppurative thrombophlebitis with thrombosis