The internal carotid artery and its branches occlusion syndromes
Middle cerebral artery occlusion syndromes
Branches of the Middle Cerebral Artery (MCA) supply arterial blood to the majority of the lateral surface of the cerebral hemispheres [1, 2]. However, this territory excludes certain areas: a strip of cortex along the superomedial margin (primarily of the frontal and parietal lobes) and the frontal pole, which are supplied by the Anterior Cerebral Artery (ACA) [1, 2]. Additionally, the inferior temporal gyrus is typically supplied by the Posterior Cerebral Artery (PCA) [1, 2].
The Middle Cerebral Artery (MCA) supplies arterial blood to the following brain regions [1, 2]:
- The cortex and underlying white matter of the lateral aspect of the frontal lobe.
- The primary motor cortex (Brodmann area 4) and premotor cortex (Brodmann area 6).
- The motor speech center (Broca's area), typically in the dominant hemisphere.
- The cortex and underlying white matter of the lateral aspect of the parietal lobe, including the primary somatosensory cortex, angular gyrus, and supramarginal gyrus.
- The superior and lateral portions of the temporal lobe.
- The Insula (Island of Reil).
The perforating branches of the Middle Cerebral Artery (MCA), often called the lenticulostriate arteries, supply arterial blood to the following deep brain structures [1, 2]:
- Putamen
- Lateral segment of the Globus Pallidus
- Superior portions of the Posterior Limb of the Internal Capsule
- Adjacent Corona Radiata
- Body of the Caudate Nucleus
- Head of the Caudate Nucleus (primarily superior and lateral aspects)
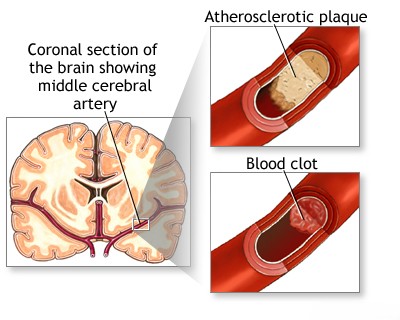
The Middle Cerebral Artery (MCA) territory is the area most frequently affected by embolism and thrombosis, events which lead to ischemic stroke [1, 3]. A complete blockage (occlusion) of the main MCA trunk obstructs blood flow not only to the deep perforating branches supplying subcortical structures (gray and white matter) but also to the large cortical branches supplying the surface of the cerebral hemisphere [1]. The classic clinical presentation resulting from such a major MCA occlusion is characterized by contralateral hemiplegia (paralysis of the opposite side of the body) and contralateral hemianesthesia (loss of sensation on the opposite side of the body) [1, 3].
If the stroke involves the dominant hemisphere (typically the left side for most individuals), patients often develop severe language impairment, commonly presenting as global aphasia (a profound deficit in both understanding and producing speech) [1, 3]. Conversely, strokes affecting the non-dominant hemisphere (typically the right) often result in different neurological deficits, such as hemispatial neglect (inattention to the contralateral side of space), anosognosia (unawareness or denial of one's deficits), and potentially constructional apraxia or dressing apraxia [1, 3]. Dysarthria (difficulty with the motor control of speech articulation) can occur with strokes in either hemisphere if motor pathways are affected, and should be distinguished from aphasia, which is a language disorder [1].
Partial neurological deficits within the Middle Cerebral Artery (MCA) territory are often seen when a branch of the MCA is occluded, typically by an embolus [1, 3]. While collateral circulation can sometimes mitigate the effects of a blockage, partial syndromes can also arise from incomplete occlusion (stenosis) of the MCA trunk or its branches, often due to thrombosis developing on top of underlying atherosclerosis [1, 3].
Partial neurological syndromes are common presentations of embolic MCA strokes [1, 3]. An embolus might initially lodge in the main trunk or a major branch, then potentially migrate more distally (further along the vessel) or undergo lysis (breakdown), leading to fluctuating or evolving neurological symptoms [1]. Specific, limited neurological deficits can result from embolic occlusion of individual MCA branches [1, 3]. Examples include:
- Isolated weakness of the hand and/or face.
- Predominant weakness of the upper extremity (brachial syndrome).
- Weakness of facial muscles (affecting contralateral lower face) often combined with motor aphasia (Broca's aphasia) if in the dominant hemisphere, with or without associated arm weakness (Opercular syndrome).
The specific combination of symptoms helps localize the blockage [1]. For example, contralateral sensory loss, weakness (especially affecting the face and arm more than the leg), and motor aphasia (Broca's) typically suggest an occlusion involving the superior division of the MCA in the dominant hemisphere [1]. Conversely, receptive aphasia (Wernicke's aphasia) without significant motor weakness points towards a lesion in the territory of the inferior division of the MCA in the dominant hemisphere (supplying posterior language areas) [1]. The sudden onset of contralateral hemispatial neglect, anosognosia (unawareness of deficit), and visuospatial difficulties, without significant paralysis, is characteristic of damage to the territory supplied by the inferior division of the MCA in the non-dominant hemisphere [1].
Middle cerebral artery syndrome [1, 3]:
Signs and symptoms |
Structures involved |
| Paralysis of the contralateral face, arm, and leg; sensory impairment over the same area (pinprick, cotton touch, vibration, position, two-point discrimination, stereognosis, tactile localization, barognosis, cutaneographia) | Somatic motor area for face and arm and the fibers descending from the leg area to enter the corona radiata and corresponding somatic sensory system |
| Motor aphasia | Motor speech area of the dominant hemisphere |
| Central aphasia, word deafness, anomia, jargon speech, sensory agraphia, acalculia, alexia, finger agnosia, right-left confusion (the last four comprise the Gerstmann syndrome) | Central, suprasylvian speech area and parietooccipital cortex of the dominant hemisphere |
| Conduction aphasia | Central speech area (parietal operculum) |
| Apractognosia of the nondominant hemisphere, anosognosia, hemiasomatognosia, unilateral neglect, agnosia for the left half of external space, dressing “apraxia”, constructional “apraxia,” distortion of visual coordinates, inaccurate localization in the half field, impaired ability to judge distance, upside-down reading, visual illusions (e.g., it may appear that another person walks through a table) | Nondominant parietal lobe (area corresponding to speech area in dominant hemisphere); loss of topographic memory is usually due to a nondominant lesion, occasionally to a dominant one |
| Homonymous hemianopia (often homonymous inferior quadrantanopia) | Optic radiation deep to second temporal convolution |
| Paralysis of conjugate gaze to the opposite side | Frontal contraversive eye field or projecting fibers |
Anterior cerebral artery occlusion syndromes
The Anterior Cerebral Artery (ACA) is typically divided into segments based on its relationship to the Anterior Communicating Artery (AComm) [1, 2]:
- The pre-communicating segment (A1) extends from the termination of the Internal Carotid Artery (ICA) to the junction with the Anterior Communicating Artery.
- The post-communicating segment (A2) begins at the AComm junction and extends distally, arching over the corpus callosum.
Cortical branches, arising primarily from the A2 segment, supply blood to: the medial surfaces of the frontal and parietal lobes (approximately the superior two-thirds), the frontal pole, the medial aspect of the orbital surface of the frontal lobe, and the anterior portions (approximately two-thirds to four-fifths) of the corpus callosum [1, 2]. Additionally, the A1 segment and the AComm junction give rise to deep penetrating branches (including the medial lenticulostriate arteries and the recurrent artery of Heubner) [2]. These supply the anterior limb of the internal capsule, the inferior part of the head of the caudate nucleus, anterior portions of the putamen and globus pallidus, the anterior perforated substance, parts of the hypothalamus, and the septal nuclei [1, 2].
Ischemic strokes (infarcts) solely within the Anterior Cerebral Artery (ACA) territory are relatively uncommon [1]. Occlusion of the A1 segment is often well-compensated by collateral blood flow from the contralateral ACA via the Anterior Communicating Artery [1, 5]. However, severe neurological deficits occur when collateral circulation is insufficient or absent, or in cases of anatomical variants (e.g., a hypoplastic contralateral A1, or an azygos ACA where both A2 segments arise from a single trunk) [1]. Occlusion in these situations can lead to a massive infarct affecting the ACA territories of both cerebral hemispheres [1].
Clinical manifestations of an infarct involving both ACA territories typically include: bilateral lower extremity weakness (paraparesis or paraplegia) that is usually worse than any upper extremity weakness, associated sensory loss in the lower extremities, and significant cognitive and behavioral changes [1, 3]. These cognitive/behavioral changes, resulting from bilateral medial frontal lobe damage, can include abulia (lack of will or initiative), apathy, confusion, memory impairment, executive dysfunction, and sometimes urinary incontinence [1].
Anterior cerebral artery syndrome [1, 3]:
Signs and symptoms |
Structures involved |
| Paralysis of opposite foot and leg | Motor leg area |
| A lesser degree of paresis of opposite arm | Arm area of cortex or fibers descending to corona radiata |
| Cortical sensory loss over toes, foot, and leg | Sensory area for foot and leg |
| Urinary incontinence | Sensorimotor area in paracentral lobule |
| Contralateral grasp reflex, sucking reflex, gegenhalten (paratonic rigidity) | Medial surface of the posterior frontal lobe; likely supplemental motor area |
| Abulia (akinetic mutism), slowness, delay, intermittent interruption, lack of spontaneity, whispering, reflex distraction to sights and sounds | Uncertain localization — probably cingulate gyrus and medial inferior portion of frontal, parietal, and temporal lobes |
| Impairment of gait and stance (gait apraxia) | Frontal cortex near leg motor area |
| Dyspraxia of left limbs, tactile aphasia in left limbs | Corpus callosum |
The vascular plexus of anterior artery occlusion syndromes (Anterior Choroidal Artery)
The Anterior Choroidal Artery (AChA) typically originates from the Internal Carotid Artery (ICA) [1, 2]. It supplies several deep brain structures, including the posterior limb of the internal capsule, the optic tract and the origin of the optic radiation (fibers traveling from the Lateral Geniculate Body towards the visual cortex), parts of the globus pallidus (particularly the internal segment), portions of the thalamus, the hippocampus, the amygdala, and the choroid plexus in the temporal horn of the lateral ventricle [1, 2]. Blood supply to some regions near the AChA territory is often shared or can be supplemented by collateral flow from [1]:
- Penetrating branches (lateral lenticulostriate arteries) from the Middle Cerebral Artery (MCA).
- Penetrating branches from the Posterior Communicating Artery (PCommA).
- Branches of the Posterior Choroidal Arteries (PChA), which arise from the Posterior Cerebral Artery (PCA).
Because of this potential for collateral supply and the specific territory involved, isolated occlusion of the AChA does not typically produce the full classic triad of contralateral hemiplegia (paralysis), hemianesthesia (sensory loss), and homonymous hemianopia (visual field loss) often seen with larger MCA strokes [1]. Instead, AChA occlusion usually results in a more limited pattern of neurological deficits, though these can still be significant (often referred to as the "AChA syndrome," which classically includes contralateral hemiparesis, hemisensory loss, and homonymous hemianopia, but the extent can vary) [1].
Historically, surgical ligation (occlusion) of the Anterior Choroidal Artery was sometimes performed as a treatment for movement disorders like Parkinson's disease [1]. Observations from these procedures provided insights: some patients experienced minimal or no neurological deficits post-ligation, likely due to robust collateral circulation [1]. Other patients who initially developed neurological symptoms often showed significant partial or complete recovery over time, further highlighting the potential role of collateral blood flow in mitigating the effects of AChA occlusion [1].
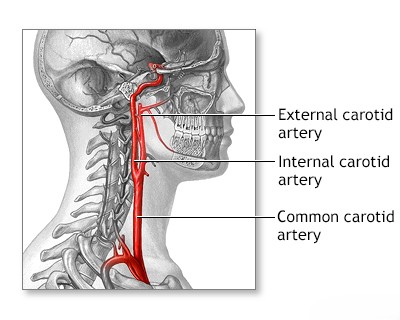
Internal carotid artery occlusion syndromes
The clinical presentation of Internal Carotid Artery (ICA) occlusion varies significantly depending on the underlying cause of cerebral ischemia (e.g., thrombus propagation from the ICA into its branches, artery-to-artery embolism, or hemodynamic compromise/hypoperfusion) and the adequacy of collateral circulation [1, 3]. Occlusion of the ICA itself can be asymptomatic if collateral pathways, primarily through the Circle of Willis, adequately compensate for the reduced blood flow [1, 5]. However, an extensive cerebral infarction involving both deep structures (gray and white matter) and the cortical surface can occur [1]. This typically happens if the thrombus completely blocking the ICA lumen propagates distally into the origins of the Middle Cerebral Artery (MCA) and Anterior Cerebral Artery (ACA), or if fragments of the thrombus break off (embolize) and lodge within the MCA or ACA, or if collateral circulation is insufficient [1].
When ICA occlusion leads to significant infarction, the resulting symptoms often resemble those of a major MCA occlusion, as this is the largest territory supplied [1]. If the infarction involves both the ACA and MCA territories (due to thrombus propagation, embolism, or collateral failure), the deficits are usually severe, potentially including contralateral hemiplegia, hemianesthesia, global aphasia (if the dominant hemisphere is affected), or hemispatial neglect and anosognosia (if the non-dominant hemisphere is affected) [1, 3]. Such large strokes can also impair consciousness, leading to stupor or coma [1]. In cases where the Posterior Cerebral Artery (PCA) arises directly from the ICA (an anatomical variant known as a fetal PCA), an ICA occlusion can also cause infarction in the PCA territory, adding symptoms like contralateral homonymous hemianopia or other deficits related to occipital or thalamic ischemia [1].
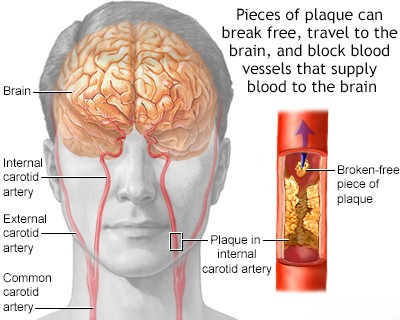
When Internal Carotid Artery (ICA) atherosclerosis, often complicated by thrombosis, becomes symptomatic, the Middle Cerebral Artery (MCA) territory is the most frequently affected downstream vascular basin, regardless of whether ischemia is caused by hypoperfusion (low blood flow) or artery-to-artery embolism [1, 3]. Cerebral infarctions resulting purely from hypoperfusion secondary to severe ICA stenosis or occlusion are often located in watershed (or border zone) areas, typically between the distal territories of the major cerebral arteries (e.g., between ACA and MCA, or MCA and PCA) [1]. These watershed strokes can manifest as transient or progressively worsening weakness, sometimes preferentially affecting the proximal muscles of the limbs [1]. Patients with symptomatic ICA disease commonly experience Transient Ischemic Attacks (TIAs) [1, 6]. These episodes, often lasting minutes (e.g., 10-15 minutes) before resolving completely, may present as transient contralateral hemiparesis (weakness on one side of the body) or aphasia/dysphasia (difficulty with producing or understanding speech, if the dominant hemisphere is involved) [1, 6]. Some patients may experience multiple, frequent TIAs over a short period (crescendo TIAs), which indicate a high risk of impending stroke [1].
The specific symptoms during a TIA depend on the affected brain region [1]. Involvement of the dominant hemisphere (usually left) can cause transient aphasia or dyscalculia/acalculia (difficulty with calculations) [1]. Involvement of the non-dominant hemisphere (usually right) may lead to transient hemispatial neglect (unawareness of the contralateral side of space) [1]. If the territory of the inferior division of the MCA in the dominant hemisphere is affected, perhaps by an embolus, the patient might exhibit symptoms of Wernicke's aphasia: fluent but nonsensical speech (jargon aphasia) with markedly impaired comprehension of spoken and written language [1]. Neurological symptoms caused by artery-to-artery embolism from the ICA can often be transient, either because the embolus causes only partial occlusion of a vessel, undergoes spontaneous lysis (breakdown), or migrates more distally into smaller branches, restoring flow to the initially affected area [1].
Generally, neurological symptoms of cerebral ischemia lasting for a significant duration (minutes to hours) but resolving within 24 hours (consistent with a Transient Ischemic Attack or TIA) are frequently attributed to embolism [1, 6]. However, when neurological symptoms are extremely brief, lasting only seconds or a few minutes, it can be challenging to definitively distinguish between an embolic cause and a hemodynamic cause (related to temporary low blood flow) based solely on the duration of the event [1, 6].
In addition to supplying the brain, the Internal Carotid Artery (ICA) also supplies the optic nerve and retina via its branch, the Ophthalmic Artery [1, 2]. Consequently, symptomatic ICA disease can manifest with visual symptoms [1]. Approximately 25% of patients experiencing TIAs related to ICA disease report episodes of transient monocular blindness (amaurosis fugax) [1]. These episodes represent a significant risk factor for subsequent stroke and potential permanent vision loss due to central or branch retinal artery occlusion (CRAO/BRAO) [1]. Patients often describe amaurosis fugax as a painless "curtain" or "shade" descending or ascending across the field of vision in one eye, or sometimes as blurred vision or loss of a portion (e.g., upper or lower half) of the visual field [1]. These visual symptoms typically last for only a few minutes before resolving completely [1]. Less commonly, a patient may suffer a concurrent cerebral stroke and an occlusion of the ophthalmic or central retinal artery, resulting in both neurological deficits and permanent vision loss in the affected eye [1].
Common carotid artery occlusion syndromes
Occlusion of the Common Carotid Artery (CCA) can produce the same neurological symptoms as an Internal Carotid Artery (ICA) occlusion, as it interrupts flow to the ICA [1]. The severity depends heavily on the adequacy of collateral circulation, particularly retrograde flow from the External Carotid Artery (ECA) territory into the ICA via connections around the orbit, and cross-filling via the Circle of Willis [1, 5]. In conditions affecting the aortic arch, such as Takayasu's arteritis (sometimes referred to as "pulseless disease") or severe atherosclerosis, occlusion of one or both Common Carotid Arteries can occur, often near their origins from the brachiocephalic trunk (right side) or the aortic arch itself (left side) [1].
The diagnosis of conditions involving severe stenosis or occlusion of both Common Carotid Arteries (often as part of an aortic arch syndrome like Takayasu's arteritis or "pulseless disease") is suggested by a constellation of symptoms reflecting widespread hypoperfusion [1]. Key signs and symptoms may include [1]:
- Weak or absent pulses in the carotid arteries and often in the upper extremities (e.g., radial arteries, indicating associated subclavian artery involvement).
- Orthostatic hypotension or syncope (fainting, especially upon standing up).
- Recurrent episodes of syncope or pre-syncope (near-fainting).
- Headaches (can be non-specific).
- Neck pain.
- Transient visual loss, which can be monocular (amaurosis fugax) or bilateral.
- Visual blurring or graying out, particularly exacerbated by exercise (visual claudication).
- Premature development of cataracts.
- Evidence of chronic ocular ischemia: retinal atrophy, pigmentary changes, iris atrophy, corneal opacities (leucoma).
- Development of peripapillary arteriovenous anastomoses (optociliary shunt vessels) on funduscopic exam.
- Optic atrophy (late finding).
- Pain or fatigue in the jaw muscles with chewing (jaw claudication).
Partial syndromes involving various combinations of stenosis and occlusion affecting the major aortic arch branches – the brachiocephalic (innominate) artery, the common carotid arteries, and the subclavian arteries – are also frequently encountered [1].
Differential Diagnosis of Anterior Circulation Stroke Syndromes (Carotid/MCA/ACA Territory) [1, 8]
| Condition | Key Features / Distinguishing Points | Typical Investigations / Findings |
|---|---|---|
| Ischemic Stroke / TIA (Anterior Circulation) | Sudden onset focal deficit (hemiparesis face/arm > leg, hemisensory loss, aphasia, neglect, leg > arm weakness [ACA]). Amaurosis fugax possible (ICA). | CT head excludes hemorrhage. MRI (DWI) confirms ischemia. Carotid/Cerebral vascular imaging (US, CTA, MRA, DSA) identifies occlusion/stenosis/source (ICA, Embolism). |
| Intracerebral Hemorrhage (ICH) | Sudden onset focal deficit. Often headache, vomiting, altered LOC, severe HTN. Can occur in lobar white matter (Lobar ICH) mimicking cortical stroke. | Non-contrast CT head shows hemorrhage. |
| Subarachnoid Hemorrhage (SAH) | Sudden "thunderclap" headache. Altered LOC, neck stiffness. Focal signs may develop due to vasospasm or associated ICH. | Non-contrast CT head shows SAH. LP if needed. CTA/DSA identifies aneurysm. |
| Seizure with Todd's Paralysis | Post-ictal focal weakness. History of seizure. Transient. | History. EEG. Normal imaging usually. Transient. |
| Migraine with Aura (Hemiplegic/Sensory/Aphasic) | Transient focal deficits, often gradual spread, followed by/with headache. History of similar episodes. | Clinical diagnosis. Normal imaging usually. |
| Hypoglycemia | Can cause focal deficits. History of diabetes. | Low blood glucose. Resolves with glucose. |
| Brain Tumor | Can present acutely (hemorrhage/seizure) but often progressive deficits. | MRI with contrast shows mass. |
| Brain Abscess | Focal deficits, headache, fever, seizures. History of infection source. | MRI shows ring-enhancing lesion, restricted diffusion. Inflammatory markers elevated. |
| Subdural Hematoma (SDH) | Can cause focal signs (hemiparesis) due to compression. Headache, altered mental status. History of trauma. | CT/MRI shows subdural collection. |
| Multiple Sclerosis Relapse | Acute/subacute onset focal deficits (weakness, sensory, optic neuritis). | MRI shows demyelinating lesions. |
| Functional Neurological Disorder | Symptoms inconsistent with organic patterns. Positive clinical signs. | Diagnosis of exclusion. Normal imaging/labs. |
References
- Ropper AH, Samuels MA, Klein JP, Prasad S. Adams and Victor's Principles of Neurology. 11th ed. McGraw Hill; 2019. Chapter 34: Cerebrovascular Diseases (Sections on MCA, ACA, ICA, CCA syndromes).
- Blumenfeld H. Neuroanatomy through Clinical Cases. 2nd ed. Sinauer Associates; 2010. Relevant chapters on vascular territories (e.g., Ch 4, Ch 17, Ch 18).
- Caplan LR. Caplan's Stroke: A Clinical Approach. 5th ed. Cambridge University Press; 2016. Chapters on specific artery territories and stroke syndromes.
- Osborn AG, Hedlund GL, Salzman KL. Osborn's Brain: Imaging, Pathology, and Anatomy. 2nd ed. Elsevier; 2017. Section on Stroke and Vascular Disease.
- Ropper AH, Samuels MA, Klein JP, Prasad S. Adams and Victor's Principles of Neurology. 11th ed. McGraw Hill; 2019. Chapter 34: Cerebrovascular Diseases, sections on Collateral Circulation.
- Easton JD, Saver JL, Albers GW, et al; American Heart Association; American Stroke Association Stroke Council; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; Interdisciplinary Council on Peripheral Vascular Disease. Definition and evaluation of transient ischemic attack: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association Stroke Council; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; and the Interdisciplinary Council on Peripheral Vascular Disease. The American Academy of Neurology affirms the value of this statement as an educational tool for neurologists. Stroke. 2009 Jun;40(6):2276-93.
- Brott TG, Halperin JL, Abbara S, et al. 2011 ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/SAIP/SCAI/SIR/SNIS/SVM/SVS guideline on the management of patients with extracranial carotid and vertebral artery disease. Stroke. 2011 Aug;42(8):e464-540. (Or more recent updates on carotid interventions).
- Caplan LR. Stroke Mimics. Semin Neurol. 2016 Apr;36(2):203-12. (Or relevant section in Adams/Victor or Grotta).
See also
- Ischemic stroke, cerebral ischemia
- Vertebrobasilar insufficiency (VBI) with vertigo symptom
- Somatoform autonomic dysfunction
- Dizziness, stuffiness in ear and tinnitus
- Ischemic brain disease:
- Atherosclerotic thrombosis
- Atherothrombotic occlusion of internal carotid artery
- Asymptomatic carotid bifurcation stenosis with noise
- Atherothrombotic occlusion of vertebrobasilar and posterior cerebral arteries
- Atherothrombotic occlusion of posterior cerebral artery
- Atherothrombotic occlusion of vertebral and posterior inferior cerebellar arteries (PICA)
- Atherothrombotic occlusion of basilar artery
- Small-vessel stroke (lacunar infarction)
- Other causes of ischemic stroke (cerebral infarction)
- Cerebral embolism
- Spontaneous intracranial (subarachnoid) and intracerebral hemorrhage:
- Arteriovenous malformations of the brain
- Hypertensive intracerebral hemorrhage
- Cerebral arteries inflammatory diseases (cerebral arteritis)
- Giant intracranial aneurysms
- Other causes of intracerebral hemorrhage
- Lobar intracerebral hemorrhage
- Saccular aneurysm and subarachnoid hemorrhage
- Mycotic intracranial aneurysms
- Repeated cerebral artery aneurysm rupture
- Communicating hydrocephalus after intracerebral hemorrhage with ruptured aneurysm
- Cerebral vasospasm
- Cerebrovascular diseases - ischemic stroke, transient ischemic attack (TIA):
- Transient ischemic attack (TIA)
- Sigmoid sinus suppurative thrombophlebitis with thrombosis