Ischemic stroke, transient ischemic attack (TIA), cerebral ischemia
- Cerebrovascular Diseases: Ischemic Stroke, Transient Ischemic Attack (TIA), and Cerebral Ischemia Overview
- Pathophysiology of Ischemic Stroke
- Ischemic Stroke and Cerebral Metabolic Impairment
- Ischemic Stroke and Pathological Changes in Neural Tissues
- Ischemic Stroke Syndromes
- Symptoms of Transient Ischemic Attack (TIA)
- Diagnosis of Ischemic Stroke, Transient Ischemic Attack (TIA), and Cerebral Ischemia
- Differential Diagnosis of Cerebrovascular Diseases and Stroke Mimics
- Treatment of Ischemic Stroke, Transient Ischemic Attack (TIA), and Cerebral Ischemia
Cerebrovascular Diseases: Ischemic Stroke, Transient Ischemic Attack (TIA), and Cerebral Ischemia Overview
In developed countries, cerebrovascular diseases (including ischemic stroke, transient ischemic attack [TIA], and cerebral ischemia) are the third leading cause of death, following cardiovascular diseases and cancer [1]. Moreover, among neurological disorders, vascular brain lesions represent a primary cause of long-term disability in adults [1]. The prevalence of these conditions is estimated at approximately 800 cases per 100,000 population, with about 5% of individuals over the age of 65 having experienced a stroke [1, 2 - Note: Specific prevalence figures vary, cite source used].
Therefore, the treatment of these diseases is a key area of specialization for our clinic.
Current medical advancements allow for the prevention of up to 80% of ischemic strokes in individuals identified as being at risk [4 - Note: This is a general statement, specific figures depend on risk factor control]. With timely and appropriate intervention, a significant proportion of ischemic stroke patients can achieve substantial functional recovery [5].
Pathophysiology of Ischemic Stroke
How does a cerebral stroke develop? Typically, the underlying pathological process involves one or more cerebral blood vessels [6]. This can affect the carotid or vertebral arteries and their intracranial branches, which are responsible for supplying arterial blood to various regions of the brain [6]. This pathology can arise from [6, 7]:
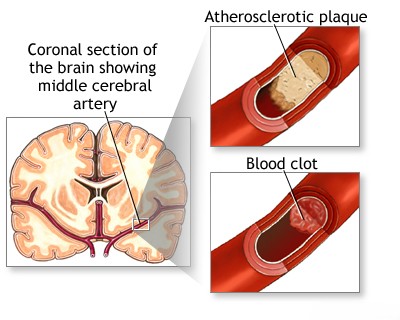
- Direct damage to the vessel wall, such as occurs in atherosclerosis, lipohyalinosis, inflammation (vasculitis), amyloidosis, dissection (either traumatic or spontaneous), congenital malformations, or aneurysms.
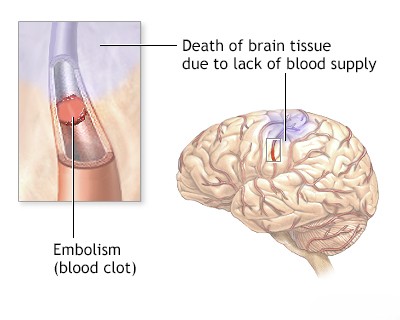
- A pathological process originating elsewhere in the body, such as an embolism from the heart or extracranial arteries, or reduced cerebral perfusion due to systemic hypotension or blood hyperviscosity.
Vascular lesions may remain asymptomatic until they cause significant narrowing (stenosis) leading to cerebral ischemia, or until the vessel undergoes embolism, complete blockage (occlusion), or rupture [6].
Stroke is defined as a neurological deficit resulting from these pathological processes affecting cerebral vessels [6]. The narrowing or occlusion of a cerebral vessel's lumen, leading to brain tissue ischemia and subsequently stroke, can be caused by [6, 7]:
- Thrombus (a blood clot forming locally)
- Atheroma (plaque buildup) or embolus (a clot or debris traveling from elsewhere)
- Vessel rupture leading to intracerebral or subarachnoid hemorrhage
Secondary neurological symptoms related to vascular lesions can include [6]:
- Cranial nerve compression by an expanding aneurysm
- Vascular headache (such as migraine or headaches related to severe hypertension)
- Increased intracranial pressure (ICP) resulting from cerebral venous thrombosis
Vascular disease can affect various parts of the body, leading to a wide range of symptoms. The most severe consequences include [6]:
- Stroke (cerebral infarction or hemorrhage)
- Myocardial infarction (heart attack)
Strokes are broadly classified as either hemorrhagic (caused by bleeding) or ischemic (caused by blockage) [6]. Ischemic stroke is significantly more common, particularly in patients with hypertension and atherosclerosis, although other vascular diseases are less frequent causes [6, 7].
The pathogenesis of stroke is complex. Key factors contributing to vascular brain disease include [6]:
- Changes in vascular reactivity, such as excessive vasoconstriction, loss of vascular tone (angioparalysis), blood flow stagnation (stasis), and venous congestion.
- Morphological changes in blood vessels, like atherosclerosis, arteriosclerosis (hardening of arteries), and aneurysm formation.
- Alterations in blood composition, including increased clotting tendency (hypercoagulability) and increased viscosity due to conditions like erythrocytosis (high red blood cell count) or thrombocythemia (high platelet count).
- Hemodynamic factors, such as abrupt fluctuations in blood pressure or overall decreased cerebral blood flow.
Hemorrhagic stroke often results from the leakage of red blood cells (erythrodiapedesis) due to neurogenically mediated vasomotor dysfunction (leading to spasm, paralysis, stasis, and increased vessel permeability) [6, 8]. Abrupt changes in blood pressure are frequently a contributing factor [8]. Bleeding commonly occurs in deep brain structures like the internal capsule and basal ganglia, and less often in the pons and cerebellum [8].
Hemorrhages in the cerebral hemispheres and subcortical areas often rupture into the lateral and third ventricles, while brainstem hemorrhages may extend into the fourth ventricle [8]. The center of the hemorrhage undergoes a process called red softening, eventually leading to the formation of a gliotic scar [8, 10].
Ischemic stroke results from thrombosis, embolism, cerebral vasospasm, or prolonged stasis due to angioparalysis [6, 7]. Ischemic necrosis (tissue death) typically occurs when blood flow is reduced by 40-50%, not necessarily requiring complete cessation [9]. In approximately 25% of cases, brain infarction is caused by thrombosis or stenosis (narrowing) of major extracranial vessels, namely the carotid and vertebral arteries [6, 7].
Ischemic Stroke and Cerebral Metabolic Impairment
Normal brain function demands a continuous supply of oxygenated blood [9]. However, even significantly reduced arterial blood flow might not immediately cause infarction, allowing tissue to remain viable for a certain period [9].
Loss of consciousness typically occurs within 10 seconds following cardiac arrest [6]. Animal studies demonstrate that complete cessation of cerebral blood flow for as little as 3 minutes can result in irreversible infarction [9]. While reduced blood flow leads to cerebral ischemia, the affected tissue may remain viable for some time before either infarction occurs or blood flow is successfully restored [9]. For instance, patients experiencing cerebral embolization or vasospasm after a subarachnoid hemorrhage often show partial or even full recovery [6]. This observation indicates that brain function in ischemic areas can potentially be restored within hours or days. This concept led to the definition of the ischemic penumbra: a region of potentially salvageable tissue surrounding the core infarct [9].
The full extent of potential brain function recovery after an ischemic event remains uncertain [6]. However, once brain infarction is established, neuronal cell membrane integrity is compromised, the blood-brain barrier (BBB) is disrupted, and crucial mitochondrial energy metabolism ceases [9].
Ischemic Stroke and Pathological Changes in Neural Tissues
Initially, an area of infarction appears pale [10]. Within hours to days, signs of congestion, hyperemia (increased blood flow), vascular dilation, and small hemorrhages known as petechiae (indicating hemorrhagic transformation) may develop, particularly within the gray matter [10].
The etiology of hemorrhagic transformation is complex, but it is frequently associated with embolic occlusion of major cerebral arteries, such as the middle cerebral artery [6, 10]. The natural dissolution (lysis) or fragmentation of the embolus can restore blood flow to the previously infarcted area [6]. This reperfusion, however, can lead to hemorrhagic transformation and worsen cerebral edema due to the disrupted blood-brain barrier [6, 10]. In contrast, primary intracerebral hemorrhage directly destroys brain tissue and compresses surrounding structures from the outset [8].
An accurate diagnosis is crucial for selecting the appropriate treatment for ischemic stroke, intracerebral hemorrhage, or transient ischemic attacks (TIAs) [5]. This diagnostic process involves determining the nature and precise location of the lesion, identifying the underlying vascular pathology, and assessing the status of collateral blood flow [5, 6].
Anatomical recovery following stroke is limited, typically resulting in the formation of a fibroglial scar at the site of the infarct or hemorrhage [10]. Therefore, treatment primarily focuses on prevention and minimizing damage: protecting viable and at-risk brain tissue from both primary and recurrent injury, and mitigating potential complications of stroke, such as secondary hemorrhage or edema [5, 6]. This approach has three key objectives [5]:
- Reducing modifiable risk factors to prevent initial strokes.
- Implementing strategies for primary and secondary stroke prevention, such as carotid endarterectomy in appropriate candidates.
- Minimizing secondary brain injury by maintaining adequate perfusion in potentially salvageable ischemic regions and actively managing cerebral edema.
Beyond modifying risk factors, the treatment of established stroke remains complex [5]. The evidence supporting various therapeutic approaches can sometimes be limited. Consequently, modern stroke management is often guided by empirical evidence and clinical experience, carefully balancing the potential risks and benefits associated with diagnostic procedures and therapeutic interventions [5, 6].
Strokes are often classified based on their presumed pathophysiological mechanisms [6, 7]. The clinical manifestations, diagnostic principles, and treatment strategies are typically defined for each subtype. In the diagnosis of stroke or TIA, the initial clinical features and their evolution over time are critical for differentiating between potential causes [6].
Advanced clinical assessment and neuroimaging techniques allow for accurate characterization of the stroke type and assessment of the underlying vascular lesion, thereby facilitating targeted treatment [5, 11]. Key diagnostic tools include [11]:
- MR angiography (Magnetic Resonance Angiography) of the brain vessels.
- CT (Computed Tomography) and spiral CT angiography of the neck and brain vessels, often requiring intravascular contrast agents.
Ischemic Stroke Syndromes
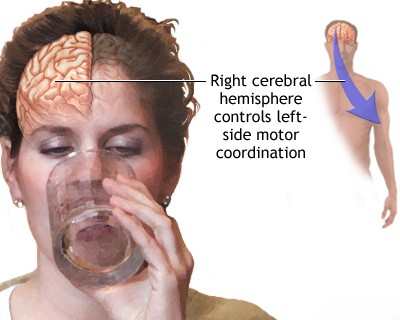
The characteristics of stroke onset, combined with specific neurological signs and symptoms, provide valuable clues regarding the location and etiology of the brain lesion [6]. An acute onset of focal neurological deficits strongly suggests a stroke, particularly if the symptoms correspond to the territory supplied by a specific blood vessel [6]. For example, the combination of hemiparesis (weakness on one side of the body) and aphasia (language impairment) typically points to involvement of the middle cerebral artery in the dominant hemisphere, whereas sudden loss of a visual field suggests involvement of the posterior cerebral artery [6]. Isolated hemiparesis might indicate a lacunar stroke affecting small penetrating branches of the middle cerebral or basilar arteries within the internal capsule or brainstem [6].
The severity of initial symptoms can vary widely and may fluctuate, sometimes gradually improving or worsening over time [6]. The progression pattern of neurological symptoms can help differentiate between thrombotic, embolic, or hemorrhagic causes [6]. However, a sudden onset of deep coma can result from various severe events, including basilar artery embolism, subarachnoid hemorrhage, or pontine hemorrhage secondary to hypertension [6]. Careful neurological examination and monitoring the progression of symptoms are crucial for determining the specific cause of coma [6]. Accurately documenting the early evolution of symptoms can be challenging, as patients are often unable to recall precise details without assistance from witnesses [6].
Lesion localization can sometimes be inferred from specific neurological findings, such as anosognosia (lack of awareness of one's deficit), which is often associated with strokes affecting the non-dominant hemisphere [6]. Obtaining crucial medical history from family members or bystanders is frequently necessary [6].
The diagnosis of stroke relies heavily on recognizing characteristic clinical signs and symptoms, encompassing both the patient's reported experiences (subjective) and the examiner's findings (objective) [6].
In hemorrhagic stroke, the clinical presentation is primarily determined by the location, size, and type (subarachnoid or intracerebral) of the hemorrhage [8]. In ischemic stroke, the presentation depends on the specific pathological process (e.g., thrombosis, embolism), the size and location of the resulting lesion, and the effectiveness of collateral circulation [6]. Adequate collateral blood flow can sometimes prevent infarction entirely or limit its size, thereby influencing the development and severity of symptoms [6, 12].
Sufficient collateral flow might prevent neurological symptoms and parenchymal damage even with complete occlusion of a major arterial trunk [12]. Conversely, occlusion of a large artery without adequate collateral support typically causes infarction throughout the affected vascular territory [6]. Infarcts can vary significantly in size, shape, and stage of development, largely influenced by the available collateral flow [6, 10]. The effectiveness of collateral circulation depends on individual vascular anatomy, the speed at which the occlusion develops, and systemic factors like blood pressure [12]. Factors such as hyperviscosity, polycythemia, and abnormalities in red blood cells can negatively impact blood flow, potentially exacerbating ischemia in territories with borderline perfusion [6].
Collateral circulation is often less robust for deep penetrating blood vessels, such as the lenticulostriate arteries arising from the middle cerebral artery and penetrating branches from the vertebrobasilar system and the circle of Willis [6, 12]. These vessels supply critical deep structures including white matter, gray matter nuclei (basal ganglia, thalamus), the brainstem, and the corona radiata [13]. Occlusion of these small penetrating vessels, often due to atherothrombosis, lipohyalinosis (associated with hypertension), or small emboli, results in small infarcts known as lacunar infarcts [6, 7].
The terms "progressive stroke" (or "stroke in evolution") and "completed stroke" are important clinical descriptors [6]. A progressive stroke is characterized by worsening or fluctuating neurological deficits observed over a period [6]. A completed stroke indicates that the neurological symptoms have stabilized and are no longer progressing [6].
Progressive stroke can result from several mechanisms, including the propagation of an arterial thrombus, the development of cerebral edema around the infarct, occlusion of collateral vessels, or systemic hypotension compromising perfusion [6]. Fluctuating deficits, however, often arise from the dynamic behavior of emboli (distribution, migration, lysis, dispersion) or variations in collateral blood flow through pathways like the circle of Willis and other anastomotic channels [6].
The patient's stroke symptoms and underlying risk factors can suggest specific types of vascular lesions [6]. Atherothrombotic stroke, for example, is often suspected in patients with known symptomatic or asymptomatic coronary artery disease or peripheral vascular disease [6]. Conversely, the presence of severe atherothrombotic disease in any major artery raises suspicion that atherothrombosis is the likely cause of an ischemic stroke [6].
Conditions like atrial fibrillation, valvular heart disease, recent myocardial infarction, or bacterial endocarditis point towards an embolic source for the stroke [6, 7]. Severe hypertension is strongly associated with lipohyalinosis (affecting small arteries), lacunar stroke, and also promotes atherothrombotic lesions, particularly at the carotid bifurcation, within the middle cerebral artery, and in the vertebrobasilar system [6, 7].
Hypertension is a major predisposing factor for deep intracerebral hemorrhage [8]. Consequently, effective antihypertensive therapy is a cornerstone of stroke prevention [4, 5]. Smoking and familial hyperlipidemia, although less common risk factors than hypertension, also significantly increase the risk of developing atherosclerosis and subsequent ischemic brain disease [4].
The clinical course of a stroke can be divided into several phases, including an acute phase, a recovery period (which can last from 10-15 days to several months, potentially extending up to 1-2 years), and a residual phase characterized by persistent neurological sequelae [6]. Differentiating clinically between hemorrhagic and ischemic stroke based solely on presentation can be challenging, with reported diagnostic error rates ranging from 10% to 27% without imaging [6].
Symptoms of Transient Ischemic Attack (TIA)
The clinical presentation and temporal pattern of a transient ischemic attack (TIA) provide valuable insights into the likely nature and location of the underlying arterial pathophysiology [6, 14]. A TIA is traditionally defined as a sudden, focal neurological deficit that resolves completely within 24 hours [14]. However, this definition is broad and can encompass syndromes that are not necessarily ischemic in origin, such as focal seizures (epilepsy) or migraine with aura [14, 15]. Furthermore, accumulating evidence suggests that neurological symptoms persisting beyond one hour may indicate underlying infarction, even if the symptoms eventually resolve completely [14].
Specific TIA symptoms can help pinpoint the involvement of particular arterial territories [6, 14]:
- Carotid artery territory.
- Middle cerebral artery territory.
- Vertebrobasilar system territory.
- Lacunar (small penetrating artery) territory.
The duration and frequency of stereotyped (identical) episodes can also suggest the underlying mechanism [6]. For instance, frequent (e.g., 5-10 times per day), very brief (≤15 minutes) episodes involving ipsilateral hand weakness combined with aphasia might suggest severe proximal arterial stenosis or occlusion with poor collateral flow, causing transient ischemia in the contralateral cortex [6]. Conversely, a single episode of isolated aphasia or hand weakness lasting several hours (up to 12 hours) is more likely indicative of embolic ischemia, potentially associated with a small infarction in the left frontal lobe [6].
Transient, brief episodes of pure pyramidal hemiparesis affecting the face, arm, and leg, without associated aphasia or neglect, suggest transient ischemia within the internal capsule [6]. This area is supplied by the lenticulostriate arteries, which branch off the middle cerebral artery [13]. An acute stroke involving these small penetrating arteries, resulting in lesions smaller than 1 cm (lacunes), can sometimes present initially as a lacunar TIA [6].
TIAs affecting the vertebrobasilar system are often caused by stenosis in the proximal basilar artery or bilateral distal vertebral arteries [6]. They typically manifest as brief episodes involving symptoms like dizziness (vertigo), double vision (diplopia), and slurred speech (dysarthria) [6]. Recurrent, short-lived episodes of this nature are more suggestive of transient hypoperfusion (low blood flow) rather than embolism [6].
In general, TIAs result from either focal hypoperfusion or embolism [6, 14]. Identifying the source of embolism is crucial for appropriate treatment in embolic TIAs [6]. The precise mechanisms underlying hypoperfusion-related TIAs are less well understood, but critical arterial stenosis or occlusion likely plays a key role by reducing blood flow to specific downstream brain regions [6].
While inadequacy of collateral blood flow certainly contributes to ischemia, transient ischemic episodes likely also involve complex interactions between factors like blood viscosity, vascular elasticity, and other mechanisms that are not yet fully understood [6]. These episodes are best classified as non-embolic TIAs [6].
Although TIA symptoms resolve completely by definition, they serve as a critical warning sign of significantly increased future stroke risk [14]. Therefore, the pathophysiology of stroke and TIA should be considered together. Effective management of TIA necessitates identifying and addressing the underlying cause [14].
Like stroke, TIA should be considered a syndrome that requires a specific etiological diagnosis for optimal management [14].
Diagnosis of Ischemic Stroke, TIA, and Cerebral Ischemia
The diagnostic workup for suspected ischemic stroke, TIA, or cerebral ischemia typically includes [5, 6, 11]:
- A thorough neurological examination.
- Assessment of cervical spine biomechanics (evaluating muscle tone, range of motion, vertebral alignment at rest and during movement, potentially supplemented by functional radiographic studies).
- Studies of the cervical and cerebral vasculature, such as Doppler ultrasound and rheoencephalography (REG).
- MR angiography (MRA) of the cerebral vessels.
- CT scanning, including spiral CT angiography (CTA) of the cervical and cerebral vessels, often with intravenous contrast.
- Standard blood tests, including biochemical panels and a complete blood count (CBC).
- Electrocardiogram (ECG) to assess for cardiac abnormalities.
Monitoring dynamic changes in brain tissue following an ischemic stroke, particularly during thrombolytic therapy, is crucial [5]. This allows clinicians to assess the effectiveness of treatment in dissolving the thrombus and to promptly detect potential complications like parenchymal hemorrhage [5].
Differential Diagnosis of Cerebrovascular Diseases and Stroke Mimics
The diagnosis of vascular brain lesions fundamentally relies on recognizing characteristic stroke syndromes [6]. However, establishing a definitive stroke diagnosis can sometimes be challenging [15]. Three key criteria help in identifying a stroke [6]:
- The rate at which the clinical syndrome develops (temporal profile).
- The presence of focal neurological signs corresponding to a specific brain region.
- The patient's overall clinical status and associated medical conditions.
The temporal profile of a stroke is defined by any prodromal features (warning signs), the manner of onset (sudden vs. gradual), and the subsequent progression of neurological deficits, all considered alongside the patient's current clinical condition [6]. When the patient's history is limited or unclear, extended observation over days or even weeks may be necessary to fully elucidate the characteristics of the disease [6]. Insufficient data regarding the development and evolution of symptoms is a common source of diagnostic error [6].
Differential Diagnosis of Acute Focal Neurological Deficits ("Stroke Mimics") [6, 15]
| Condition | Key Features / Distinguishing Points | Typical Investigations / Findings |
|---|---|---|
| Ischemic Stroke / TIA | Sudden onset focal neurological deficit corresponding to vascular territory. Risk factors often present. TIA resolves completely (<24h, often <1h). | CT head excludes hemorrhage. MRI (DWI) confirms ischemia early. Vascular imaging identifies cause. |
| Intracerebral Hemorrhage (ICH) | Sudden onset focal deficit, often with headache, vomiting, decreased consciousness, severe hypertension. | Non-contrast CT head shows hemorrhage. |
| Seizure with Todd's Paralysis | Post-ictal focal weakness mimicking stroke. History of seizure. Transient (resolves <48h). | History. EEG may show abnormalities. Imaging usually normal unless underlying lesion. Transient nature. |
| Migraine with Aura (esp. Hemiplegic) | Transient neurological symptoms (often spreading gradually) followed by/accompanying headache. History of similar episodes. Full recovery. | Clinical diagnosis. Normal exam between attacks. Imaging usually normal. |
| Hypoglycemia | Can cause focal neurological deficits, confusion, seizures. History of diabetes relevant. | Low blood glucose. Symptoms improve with glucose. |
| Brain Tumor | Can present acutely with seizure/hemorrhage causing focal signs, but often progressive symptoms precede. | MRI with contrast shows mass lesion. |
| Subdural Hematoma | Can cause focal signs due to compression. Headache, altered mental status. History of trauma (may be minor). | CT/MRI shows subdural collection. |
| Metabolic Encephalopathy / Systemic Infection | Diffuse dysfunction (confusion, lethargy). Focal signs uncommon unless superimposed issue. Identifiable systemic cause (liver/kidney failure, sepsis, electrolyte imbalance). | Specific lab abnormalities. Imaging usually non-specific. Signs of infection. |
| Multiple Sclerosis Relapse | Acute/subacute onset focal deficits (sensory, motor, visual, cerebellar). History of prior episodes possible. | MRI shows characteristic demyelinating lesions. |
| Peripheral Vestibulopathy (e.g., Labyrinthitis) | Acute vertigo, nausea, vomiting. No other brainstem/cerebellar signs usually. Can mimic posterior circulation TIA/stroke. | Clinical exam (HINTS). Normal brain imaging. Audiometry if hearing loss. |
| Functional Neurological Disorder | Symptoms inconsistent with organic patterns. Positive clinical signs (e.g., Hoover's). | Diagnosis of exclusion. Normal imaging/labs. |
Several other neurological conditions can mimic the clinical presentation of vascular brain lesions ("stroke mimics") [15]. An incomplete or ambiguous patient history can particularly complicate the differential diagnosis, potentially leading to confusion with conditions like subdural hematoma, brain tumor, brain abscess, or even rapidly progressing dementia [6].
This diagnostic challenge also applies to conditions like chronic vertebrobasilar insufficiency (VBI) [6]. VBI is characterized by intermittently or chronically impaired blood flow through the vertebral and basilar arteries, which supply the posterior portions of the brain, including the cerebellum and brainstem [6]. Symptoms commonly include gait instability, dizziness or vertigo, fluctuations in intracranial pressure, nausea, and vomiting [6].
Treatment of Ischemic Stroke, Transient Ischemic Attack (TIA), and Cerebral Ischemia
Following an accurate diagnosis (confirming ischemic stroke, transient ischemic attack [TIA], or chronic cerebral ischemia), the patient will be offered appropriate treatment options, which may include conservative management or surgical intervention, depending on the specific condition and its severity [5].
Research clearly indicates that intravenous thrombolytic therapy (clot-busting medication) for ischemic stroke yields the best outcomes when administered within the first 4.5 hours of symptom onset [5, 17]. Timely thrombolysis within this critical time window significantly reduces mortality rates, lessens the severity of long-term neurological deficits (such as paresis and paralysis measured at 90 days), and lowers the risk of major complications, including symptomatic intracerebral hemorrhage [5, 17].
Carotid artery stenting is a minimally invasive endovascular procedure designed to restore normal blood flow to the brain by treating narrowing (stenosis) in a carotid artery. A small, expandable metal mesh tube, known as a stent, is deployed within the stenotic segment to keep the artery open [18].
Treatment strategies for cerebral blood flow impairment are tailored to the specific type and cause of the impairment and may encompass a combination of approaches, including [5, 6]:
- Pharmacological therapy (using vascular agents, nootropic drugs to support brain function, and other supportive medications).
- Therapeutic massage.
- Physiotherapy (physical therapy and rehabilitation programs).
- Medical gymnastics (structured therapeutic exercise programs).
- Acupuncture and related reflex therapies.
- Surgical treatment (such as endarterectomy, stenting, or other interventions when indicated).
References
- Feigin VL, Roth GA, Naghavi M, et al. Global Burden of Stroke and Risk Factors in 188 Countries, during 1990-2013. N Engl J Med. 2016 Jul 14;375(2):198. (Or similar global burden of disease study for epidemiology).
- Ropper AH, Samuels MA, Klein JP, Prasad S. Adams and Victor's Principles of Neurology. 11th ed. McGraw Hill; 2019. Chapter 34: Cerebrovascular Diseases (Introduction).
- Libby P, Buring JE, Badimon L, Hansson GK, Deanfield J, Bittencourt MS, et al. Atherosclerosis. Nat Rev Dis Primers. 2019 Aug 29;5(1):56.
- Sacco RL, Roth GA, Reddy KS, et al. The Heart of 25 × 25: Achieving the Goal of Reducing Global and Regional Premature Deaths From Cardiovascular Diseases and Stroke: A Presidential Advisory From the American Heart Association/American Stroke Association. Circulation. 2016 Jun 14;133(23):e674-90. (Discusses prevention potential).
- Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2019 Dec;50(12):e344-e418.
- Ropper AH, Samuels MA, Klein JP, Prasad S. Adams and Victor's Principles of Neurology. 11th ed. McGraw Hill; 2019. Chapter 34: Cerebrovascular Diseases.
- Grotta JC, Albers GW, Broderick JP, et al. Stroke: Pathophysiology, Diagnosis, and Management. 7th ed. Elsevier; 2021. Chapter on Mechanisms of Ischemic Stroke.
- Grotta JC, Albers GW, Broderick JP, et al. Stroke: Pathophysiology, Diagnosis, and Management. 7th ed. Elsevier; 2021. Chapter on Intracerebral Hemorrhage.
- Grotta JC, Albers GW, Broderick JP, et al. Stroke: Pathophysiology, Diagnosis, and Management. 7th ed. Elsevier; 2021. Chapter on Pathophysiology of Ischemic Stroke.
- Kumar V, Abbas AK, Aster JC. Robbins & Cotran Pathologic Basis of Disease. 10th ed. Elsevier; 2020. Chapter 28: The Central Nervous System (Section on Vascular Diseases).
- Osborn AG, Hedlund GL, Salzman KL. Osborn's Brain: Imaging, Pathology, and Anatomy. 2nd ed. Elsevier; 2017. Section on Stroke and Vascular Disease.
- Ropper AH, Samuels MA, Klein JP, Prasad S. Adams and Victor's Principles of Neurology. 11th ed. McGraw Hill; 2019. Chapter 34: Cerebrovascular Diseases, sections on Collateral Circulation.
- Blumenfeld H. Neuroanatomy through Clinical Cases. 2nd ed. Sinauer Associates; 2010. Relevant chapters on brain structures and vascular supply.
- Easton JD, Saver JL, Albers GW, et al; American Heart Association; American Stroke Association Stroke Council; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; Interdisciplinary Council on Peripheral Vascular Disease. Definition and evaluation of transient ischemic attack: a scientific statement for healthcare professionals from the American Heart Association/American Stroke Association Stroke Council; Council on Cardiovascular Surgery and Anesthesia; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; and the Interdisciplinary Council on Peripheral Vascular Disease. The American Academy of Neurology affirms the value of this statement as an educational tool for neurologists. Stroke. 2009 Jun;40(6):2276-93.
- Caplan LR. Stroke Mimics. Semin Neurol. 2016 Apr;36(2):203-12.
- Goyal M, Menon BK, van Zwam WH, et al; HERMES collaborators. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016 Apr 23;387(10029):1723-31.
- Hacke W, Kaste M, Bluhmki E, et al; ECASS Investigators. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008 Sep 25;359(13):1317-29. (Example of thrombolysis trial).
- Brott TG, Halperin JL, Abbara S, et al; American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines; American Stroke Association; American Association of Neuroscience Nurses; American Association of Neurological Surgeons; American College of Radiology; American Society of Neuroradiology; Congress of Neurological Surgeons; Society of Atherosclerosis Imaging and Prevention; Society for Cardiovascular Angiography and Interventions; Society of Interventional Radiology; Society of NeuroInterventional Surgery; Society for Vascular Medicine; Society for Vascular Surgery. 2011 ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/SAIP/SCAI/SIR/SNIS/SVM/SVS guideline on the management of patients with extracranial carotid and vertebral artery disease. Stroke. 2011 Aug;42(8):e464-540. (Or more recent updates).
See also
- Ischemic stroke, cerebral ischemia
- Vertebrobasilar insufficiency (VBI) with vertigo symptom
- Somatoform autonomic dysfunction
- Dizziness, stuffiness in ear and tinnitus
- Ischemic brain disease:
- Atherosclerotic thrombosis
- Atherothrombotic occlusion of internal carotid artery
- Asymptomatic carotid bifurcation stenosis with noise
- Atherothrombotic occlusion of vertebrobasilar and posterior cerebral arteries
- Atherothrombotic occlusion of posterior cerebral artery
- Atherothrombotic occlusion of vertebral and posterior inferior cerebellar arteries (PICA)
- Atherothrombotic occlusion of basilar artery
- Small-vessel stroke (lacunar infarction)
- Other causes of ischemic stroke (cerebral infarction)
- Cerebral embolism
- Spontaneous intracranial (subarachnoid) and intracerebral hemorrhage:
- Arteriovenous malformations of the brain
- Hypertensive intracerebral hemorrhage
- Cerebral arteries inflammatory diseases (cerebral arteritis)
- Giant intracranial aneurysms
- Other causes of intracerebral hemorrhage
- Lobar intracerebral hemorrhage
- Saccular aneurysm and subarachnoid hemorrhage
- Mycotic intracranial aneurysms
- Repeated cerebral artery aneurysm rupture
- Communicating hydrocephalus after intracerebral hemorrhage with ruptured aneurysm
- Cerebral vasospasm
- Cerebrovascular diseases - ischemic stroke, transient ischemic attack (TIA):
- Transient ischemic attack (TIA)
- Sigmoid sinus suppurative thrombophlebitis with thrombosis