Saccular aneurysm and subarachnoid hemorrhage
- Saccular Aneurysms and Subarachnoid Hemorrhage (SAH) Overview
- Pathophysiology and Common Locations of Saccular Aneurysms
- Clinical Symptoms of Unruptured Intracranial Aneurysms
- Initial Manifestations of Ruptured Aneurysm and Acute Subarachnoid Hemorrhage
- Diagnosis of Ruptured Intracranial Aneurysm: Laboratory Evaluation and Imaging
- Medical Management of Ruptured Aneurysm and Subarachnoid Hemorrhage
- Delayed Neurologic Deficits After Intracranial Aneurysm Rupture
- Complications After Subarachnoid Hemorrhage
- Surgical and Endovascular Treatment of Intracranial Aneurysms
Saccular Aneurysms and Subarachnoid Hemorrhage (SAH) Overview
Types of brain aneurysms [1, 2]:
- Saccular ("berry") aneurysm – can be giant
- Fusiform aneurysm – can be giant
- Dissecting aneurysm
- Mycotic (infectious) aneurysm
Classification based on aneurysm size [1]:
- ≤6 mm – small aneurysm
- 7 to 12 mm – medium aneurysm
- 13 to 24 mm – large aneurysm
- ≥25 mm – giant aneurysm
The most common cause of spontaneous (non-traumatic) subarachnoid hemorrhage (SAH) is the rupture of an intracranial aneurysm [1, 2]. Rupture of mycotic aneurysms or those associated with other rare vessel wall pathologies is significantly less common [1]. Another important, though less frequent, cause of SAH compared to aneurysm rupture is the rupture of an arteriovenous malformation (AVM) [1].
While autopsy studies suggest aneurysms may be present in up to 5% of the population, the annual incidence of rupture causing SAH is estimated at approximately 4 per 100,000 people per year [1, 2 - Note: Incidence figures vary slightly between sources].
Rupture of an intracranial saccular aneurysm is a critical medical emergency [1, 2]. Approximately 25% of patients die within the first 24 hours, and roughly 50% die within the first 3 months following the hemorrhage [1, 2]. Among survivors, more than 50% experience significant long-term neurological deficits resulting from the initial bleed or subsequent complications, such as re-rupture, symptomatic cerebral vasospasm, or hydrocephalus [1, 2]. Furthermore, over 50% of survivors discharged after treatment remain functionally disabled [1]. Therefore, significant efforts focus on preventing the initial rupture (primary prevention) and, if rupture occurs, on preventing devastating secondary complications [1, 2].
Pathophysiology and Common Locations of Saccular Aneurysms
Saccular aneurysms typically form at the bifurcations (forks) of large cerebral arteries located at the base of the brain [1, 3]. Consequently, rupture usually releases blood directly into the basal cisterns (subarachnoid spaces at the base of the brain) [1].
In contrast, mycotic aneurysms are often located more distally, on branches overlying the cerebral cortex [1]. Therefore, mycotic aneurysm rupture tends to cause hemorrhage into the subarachnoid space over the brain surface rather than primarily into the basal cisterns [1].
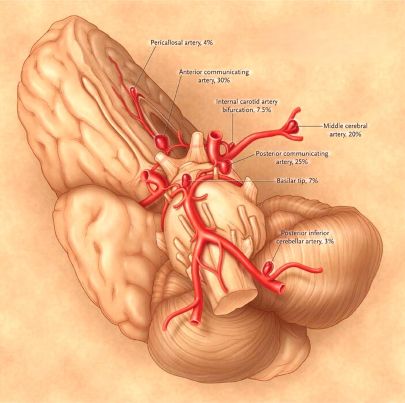
These location differences can influence the clinical presentation. Common locations for saccular aneurysms include [1, 3]:
- Junction of the anterior communicating artery (AComm) with the anterior cerebral artery (ACA)
- Junction of the posterior communicating artery (PComm) with the internal carotid artery (ICA)
- Bifurcation of the middle cerebral artery (MCA)
- The top (apex) of the basilar artery
- Junction of the basilar artery with the superior cerebellar artery (SCA) or anterior inferior cerebellar artery (AICA)
- Junction of the vertebral artery and the posterior inferior cerebellar artery (PICA)
Approximately 85% of saccular aneurysms occur in the anterior circulation (supplied by the internal carotid arteries) [1]. Multiple aneurysms are found in 12-31% of patients, and bilateral "mirror" aneurysms (identical locations on both sides) occur in 9-19% of those with multiple aneurysms [1].
A saccular aneurysm consists of a neck, connecting it to the parent artery, and a dome (or fundus) [1]. The dimensions of the neck and dome vary greatly, and these dimensions are critical factors in planning surgical or endovascular treatment to exclude the aneurysm from circulation [1]. Histologically, the internal elastic lamina of the parent artery wall is typically absent at the aneurysm neck [1, 3]. The media (middle layer) also thins, with smooth muscle cells often replaced by connective tissue [1, 3]. At the point of rupture (often the dome), the aneurysm wall can thin dramatically (e.g., less than 0.3 mm), and the actual tear may be very small (e.g., less than 0.5 mm) [1]. Predicting precisely which aneurysm will rupture in a specific patient is currently impossible [1]. However, size is recognized as a significant risk factor [1, 4]. Preventive treatment is often considered justified for aneurysms larger than 7 mm, although other factors (location, shape, patient factors) also play a crucial role in decision-making [1, 4].
Clinical Symptoms of Unruptured Intracranial Aneurysms
Neurological symptoms from an unruptured aneurysm, although less common than rupture, can indicate its location and may suggest progressive enlargement [1]. The onset of a third cranial nerve (oculomotor) palsy, particularly involving pupillary dilation (mydriasis), loss of light reflex, and localized eye/orbital pain, strongly suggests an expanding aneurysm, typically located at the junction of the posterior communicating artery (PComm) and the internal carotid artery (ICA) [1, 5]. Development of a third nerve palsy often implies the aneurysm is enlarging and may be around 7 mm or larger [1]. This clinical scenario often warrants urgent consideration for treatment [1].
A sixth cranial nerve (abducens) palsy, causing impaired outward eye movement (lateral rectus muscle weakness), can indicate an aneurysm within the cavernous sinus [1]. Visual field defects are often associated with enlarging aneurysms arising from the supraclinoid segment of the internal carotid artery [1]. Occipital or neck pain might signal an aneurysm of the posterior inferior cerebellar artery (PICA) or anterior inferior cerebellar artery (AICA) [1]. Pain in or behind the eye, or in the temporal region, can occur with an expanding middle cerebral artery (MCA) aneurysm [1].
Whether aneurysms can cause minor, intermittent bleeding ("warning leaks") into the subarachnoid space before a major rupture remains debated [1]. However, recognizing potential warning symptoms, such as an unusual, sudden, or severe headache preceding the major event, is clinically significant [1].
Any sudden, severe, unexplained headache ("thunderclap headache") should raise suspicion for SAH [1, 2]. Immediate neuroimaging, typically a non-contrast head CT scan, is necessary to detect blood in the subarachnoid space, especially the basal cisterns [1, 2]. If the initial CT scan is negative but suspicion for SAH remains high (especially if performed >6-12 hours after headache onset), a lumbar puncture (spinal tap) is often indicated to check for blood or xanthochromia (yellowish discoloration from blood breakdown products) in the cerebrospinal fluid (CSF) [1, 2].
Initial Manifestations of Ruptured Aneurysm and Acute Subarachnoid Hemorrhage
At the moment of rupture, the sudden influx of arterial blood into the subarachnoid space can cause intracranial pressure (ICP) to rise dramatically, potentially reaching levels close to mean arterial pressure (MAP) [1]. This drastically reduces cerebral perfusion pressure (CPP = MAP - ICP), impairing cerebral blood flow [1].
Approximately 45% of patients experience a sudden loss of consciousness at the onset, which may be transient [1]. This loss of consciousness may sometimes be preceded by a brief, excruciating headache [1]. Most patients who regain consciousness report an extremely severe headache [1].
In about 10% of cases, the initial hemorrhage is so severe that it causes prolonged unconsciousness lasting several days [1].
Approximately 45% of patients experience an abrupt, intensely severe headache without losing consciousness [1]. This headache often worsens with exertion [1]. Patients often describe this as "the worst headache of my life" [1, 2]. Terms like "thunderclap headache" or descriptions like feeling "hit in the head" are common [1]. Typically, patients describe the pain as involving the "whole head" or localized to the occipital/neck region [1].
Vomiting is a common symptom accompanying the headache at the onset of rupture [1]. The combination of sudden, severe headache and vomiting should always raise strong suspicion for acute SAH [1].
While the hallmark symptom is the sudden headache, focal neurological deficits can also occur at onset or develop shortly after [1].
A third cranial nerve palsy ipsilateral (on the same side) to the hemorrhage suggests rupture of a PComm artery aneurysm [1, 5].
A sixth cranial nerve palsy is less localizing but can occur, sometimes associated with increased ICP or rupture of a posterior circulation (subtentorial) aneurysm [1].
Rupture of an anterior communicating artery (AComm) or MCA bifurcation aneurysm can lead to blood collecting in the subdural space or forming a large clot within the subarachnoid space (e.g., Sylvian fissure for MCA), causing significant mass effect [1]. This can result in neurological deficits such as hemiparesis, aphasia (if the dominant hemisphere is affected), neglect or anosognosia (if the non-dominant hemisphere is affected), memory impairment, or abulia (lack of motivation) [1].
Rupture of an MCA bifurcation aneurysm specifically can cause bleeding into the Sylvian fissure, extending into the temporal lobe or superiorly into the frontal/parietal lobes [1]. Such hemorrhages can manifest with signs of a mass lesion and may be mistaken for primary intracerebral hemorrhage [1]. Associated cerebral edema often worsens the patient's condition and may necessitate urgent neurosurgical intervention (e.g., clot evacuation) [1].
Occasionally, patients develop acute unilateral cerebral hemispheric swelling immediately after rupture, associated with focal deficits and stupor [1]. The exact reasons for this early, severe swelling are not fully understood but might relate to transient circulatory arrest or severe early vasospasm affecting the main arterial trunk [1]. The precise cause of the initial neurological deficit can sometimes be difficult to determine, and symptoms may improve over time [1]. Careful documentation of the initial neurological deficit and its evolution over time is crucial for determining the cause, guiding management, and tracking its progression [1].
Clinical Grading Scales for Subarachnoid Hemorrhage [1]:
Grade |
Hunt and Hess Scale [6] |
World Federation of Neurosurgical Societies (WFNS) Scale [7] |
|---|---|---|
| 1 | Asymptomatic, or minimal headache and slight nuchal rigidity | Glasgow Coma Scale (GCS) score 15, no motor deficit |
| 2 | Moderate to severe headache, nuchal rigidity, no neurologic deficit other than cranial nerve palsy | GCS 13–14, no motor deficit |
| 3 | Drowsiness, confusion, or mild focal deficit | GCS 13–14, with motor deficit |
| 4 | Stupor, moderate to severe hemiparesis, possibly early decerebrate rigidity and vegetative disturbances | GCS 7–12, with or without motor deficit |
| 5 | Deep coma, decerebrate rigidity, moribund appearance | GCS 3–6, with or without motor deficit |
Note: Hunt and Hess descriptions slightly adapted for clarity. WFNS relies primarily on GCS and motor deficit presence.
The initial clinical severity of SAH, often graded using scales like the Hunt and Hess or the WFNS scale (based on the Glasgow Coma Scale - GCS), is a major prognostic factor [1]. Prognosis generally worsens with higher grades [1]. For example, patients presenting as Hunt and Hess grade 1 typically have a good prognosis if the aneurysm is treated promptly, whereas mortality rates for grades 4 and 5 can exceed 80%, even with treatment [1].
Diagnosis of Ruptured Intracranial Aneurysm: Laboratory Evaluation and Imaging
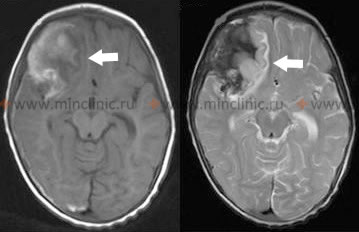
If imaging (typically non-contrast head CT) is performed within the first 24 hours after suspected aneurysm rupture, it will detect subarachnoid blood in over 95% of cases (sensitivity decreases over time) [1, 8]. The amount and distribution of blood seen on CT can help predict the location of the ruptured aneurysm and correlate with the initial neurological deficits [1, 8]. Furthermore, the pattern of blood on the initial CT scan (e.g., Fisher grade) can help predict the risk of developing delayed cerebral ischemia (DCI) due to vasospasm [1, 8].
Differential Diagnosis of Sudden Severe Headache ("Thunderclap Headache") [1, 9]
| Condition | Key Features / Distinguishing Points | Typical Investigations / Findings |
|---|---|---|
| Subarachnoid Hemorrhage (SAH) - Aneurysmal | Sudden, severe "worst headache of life". Neck stiffness, altered consciousness, nausea/vomiting common. +/- Focal deficits, CN III palsy. | CT head shows SAH (blood in cisterns/sulci). LP if CT neg but suspicion high (blood/xanthochromia). CTA/DSA identifies aneurysm. |
| Reversible Cerebral Vasoconstriction Syndrome (RCVS) | Recurrent thunderclap headaches over days/weeks. May have focal deficits during headache. Often triggered (postpartum, vasoactive drugs). No SAH typically. | CTA/MRA/DSA shows multifocal segmental vasoconstriction (may be normal initially). MRI brain usually normal or may show small infarcts/posterior edema (PRES). CSF normal. Vasoconstriction resolves over weeks/months. |
| Cerebral Venous Thrombosis (CVT) | Headache often progressive but can be sudden/severe. Seizures, focal deficits, papilledema common. Risk factors (prothrombotic state, infection, dehydration). | MRV or CTV confirms sinus/vein thrombosis. MRI brain may show venous infarcts (often hemorrhagic). D-dimer may be elevated. LP may show high pressure. |
| Cervical Artery Dissection (Carotid/Vertebral) | Sudden headache and/or neck pain. May have focal neurological signs (stroke/TIA), Horner's syndrome (carotid), tinnitus. +/- History of minor trauma/manipulation. Can rarely cause SAH. | CTA or MRA of head/neck confirms dissection (intimal flap, pseudoaneurysm, stenosis/occlusion). MRI brain checks for infarct. |
| Intracerebral Hemorrhage (ICH) | Sudden focal deficit, often with headache, vomiting, altered LOC, severe HTN. Headache may not be "thunderclap" severity unless associated SAH/IVH. | Non-contrast CT head shows parenchymal blood. Location helps determine cause (deep vs. lobar). |
| Pituitary Apoplexy | Sudden severe headache, visual loss, ophthalmoplegia (CN palsies), altered consciousness. Often history of known pituitary adenoma. May have signs of adrenal insufficiency. | MRI shows hemorrhage/infarction within pituitary adenoma, often with suprasellar extension/chiasm compression. Endocrine assessment crucial. |
| Meningitis (esp. Bacterial) | Severe headache, fever, neck stiffness, altered mental status. Onset may be rapid but usually not instantaneous thunderclap. | LP shows CSF pleocytosis/bacterial pattern. Imaging may show meningeal enhancement. |
| Primary Thunderclap Headache | Sudden severe headache reaching peak intensity within 1 minute. Diagnosis of exclusion after ruling out secondary causes (SAH, RCVS, CVT, etc.). Often recurrent. | Normal neurological exam. Normal CT, LP, MRI/MRA/MRV. |
| Ischemic Stroke | Headache can occur but usually not primary/most severe symptom. Focal deficits dominate. Exception: posterior circulation stroke sometimes presents with severe headache/neck pain. | CT excludes hemorrhage. MRI (DWI) confirms ischemia. |
| Spontaneous Intracranial Hypotension | Orthostatic headache (worse upright). Can sometimes present acutely/severely. | MRI may show diffuse pachymeningeal enhancement, sagging brain. LP shows low opening pressure. |
The initial diagnostic step for suspected ruptured intracranial aneurysm is typically a non-contrast head CT scan [1, 8]. Once SAH is confirmed (or if suspicion is very high despite negative CT/LP), vascular imaging is performed to identify the source [1, 8]. CT Angiography (CTA) is often performed immediately after the non-contrast CT [8]. MR Angiography (MRA) is another non-invasive option [8]. These can detect aneurysms and differentiate them from other vascular lesions like AVMs [8]. As mentioned earlier, if the initial non-contrast CT is negative but clinical suspicion for SAH remains high, a lumbar puncture is performed to analyze the CSF for blood or xanthochromia [1, 2]. CT/MRI also assesses for complications like hydrocephalus or intracerebral hematoma [1, 8].
Digital Subtraction Angiography (DSA), also known as conventional or catheter-based cerebral angiography, is considered the gold standard for visualizing aneurysms and planning treatment [1, 8]. DSA provides detailed anatomical information about the aneurysm (size, neck, orientation) and the surrounding vessels, and can also dynamically assess for vasospasm [1, 8]. It is often performed early in the diagnostic process or just prior to intervention [1].
If the bleeding pattern on CT/MRI suggests an AVM or mycotic aneurysm (e.g., hemorrhage primarily over the convexity rather than in the basal cisterns), angiography is crucial for confirmation and diagnosis [1].
Urgent angiography is also indicated if a large intracerebral hematoma from the rupture is causing significant mass effect and neurological deterioration, potentially requiring emergency surgical evacuation (often combined with aneurysm treatment) [1]. Accurate definition of the aneurysm's anatomy via angiography is mandatory before surgical intervention [1].
ECG changes are common after SAH and are often secondary to the neurological event (neurogenic cardiac stunning or injury) [1]. These can include T-wave changes (inversion or elevation), QT interval prolongation, and ST segment depression or elevation, sometimes mimicking acute coronary syndrome [1]. Careful cardiac evaluation may be needed [1].
Hyponatremia (low blood sodium) is a common complication, potentially due to the Syndrome of Inappropriate Antidiuretic Hormone secretion (SIADH) or Cerebral Salt Wasting (CSW) [1]. Differentiating these conditions is important as their fluid management strategies differ [1]. Therefore, regular monitoring of serum electrolytes and fluid balance is essential in SAH patients [1].
Medical Management of Ruptured Aneurysm and Subarachnoid Hemorrhage
In patients who are stuporous or comatose after SAH, intracranial pressure (ICP) may be elevated [1]. Management aims to maintain adequate cerebral perfusion pressure (CPP) while avoiding excessively high blood pressure (which could increase re-rupture risk before the aneurysm is secured) [1, 10]. Arterial blood gas monitoring is used to guide respiratory support and ensure adequate oxygenation and ventilation (avoiding hypercapnia, which can increase ICP) [1]. Significant hypercapnia requires mechanical ventilation [1]. If a subdural or intracerebral hematoma causes worsening neurological status due to mass effect, surgical evacuation may be necessary [1]. During surgery, the neurosurgeon may also clip the aneurysm if feasible [1].
To minimize the risk of re-bleeding before the aneurysm is secured, patients are typically managed with bed rest in a quiet, low-stimulation environment (e.g., intensive care unit) [1]. Blood pressure control is crucial [1, 10]. Stool softeners and laxatives are prescribed to prevent straining during bowel movements, which can increase ICP and re-rupture risk [1]. However, excessive restriction can cause agitation. Limited activities like reading or quiet visits may be permitted depending on the patient's condition [1].
Mild sedatives and analgesics are used to manage severe headache and neck pain [1]. Antiplatelet agents like aspirin are generally avoided due to bleeding risk [1]. Acetaminophen (paracetamol) is preferred for pain/fever [1]. Opioids may be used cautiously [1]. Excessive sedation should be avoided as it can mask changes in neurological status [1].
True epileptic seizures at the time of initial rupture are relatively uncommon, although tremors, twitching, or posturing related to high ICP can occur [1]. Because seizures can increase ICP and potentially the risk of re-rupture, prophylactic anticonvulsant medication (e.g., phenytoin, levetiracetam) is often administered, at least in the early period after SAH [1, 10].
Steroids may help reduce headache and neck pain caused by the chemical meningitis from blood in the subarachnoid space [1]. However, there is no clear evidence supporting their routine use for managing cerebral edema after SAH, and they are generally not recommended for this purpose [1, 10].
Delayed Neurologic Deficits After Intracranial Aneurysm Rupture
Delayed neurological deterioration after the initial stabilization period can arise from three main causes [1, 10]:
- Re-rupture of the aneurysm (highest risk before treatment)
- Communicating hydrocephalus (impaired CSF absorption)
- Delayed Cerebral Ischemia (DCI) due to cerebral vasospasm
Differentiating between these complications requires careful clinical monitoring (neurological exams) and knowledge of the patient's initial neurological status [1]. Repeat imaging (CT, MRI) and sometimes angiography or transcranial Doppler (TCD) ultrasound are important tools for diagnosing these delayed complications [1, 8, 10]. Imaging helps assess ventricular size (for hydrocephalus), detect signs of re-rupture or new infarction, or evaluate blood flow changes related to vasospasm [1, 8].
Complications After Subarachnoid Hemorrhage
Patients recovering from SAH due to ruptured aneurysm are susceptible to various systemic complications [1, 10]:
- Venous thromboembolism: Deep vein thrombosis (DVT) and pulmonary embolism (PE)
- Gastrointestinal complications: Stress-induced ulcers
- Cardiopulmonary complications: Neurogenic pulmonary edema, myocardial stunning/injury, arrhythmias (as mentioned earlier)
- Infections: Pneumonia, urinary tract infections
SAH triggers a surge in sympathetic nervous system activity [1]. This can lead to cardiac complications, including myocardial stunning or injury (myofibrillar degeneration) and arrhythmias [1]. Beta-blockers may be considered in some patients to manage cardiac effects, but require caution, especially in the presence of heart block or hypotension [1].
Hyponatremia, as mentioned earlier, is another frequent complication [1]. It can result from SIADH or Cerebral Salt Wasting (CSW) [1]. Management depends on the underlying cause and involves careful fluid and electrolyte management, often aiming to maintain adequate intravascular volume (euvolemia) or providing salt/fluid replacement for CSW [1, 10].
Surgical and Endovascular Treatment of Intracranial Aneurysms
The primary goal of treatment is to definitively exclude the aneurysm from the circulation to prevent initial or recurrent rupture [1, 10].
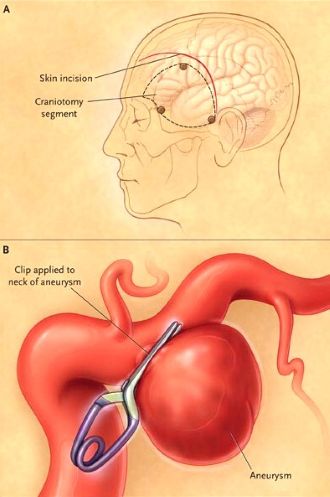
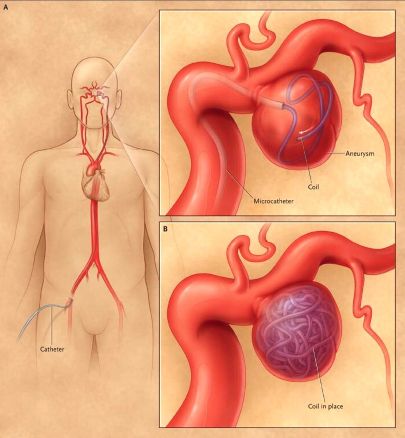
Microsurgical clipping, performed by a neurosurgeon via craniotomy, involves placing a small metal clip across the neck of the aneurysm [1, 11]. This is typically performed using an operating microscope for magnification [11]. This definitive treatment prevents future rupture of the treated aneurysm.
Historically, surgery for ruptured aneurysms was often delayed (e.g., 10-14 days) to allow the patient to stabilize and brain swelling to subside [1]. This delay aimed to minimize surgical complications related to brain swelling and potentially reduce the risk of operating during peak vasospasm [1]. However, current practice increasingly favors early intervention (within 24-72 hours) if the patient's condition permits [1, 10]. Early treatment definitively prevents re-rupture, which carries a high mortality rate [1, 10]. Early surgery may also allow for removal of subarachnoid blood clots from the basal cisterns, potentially reducing the risk or severity of vasospasm, although the effectiveness of clot removal for vasospasm prevention is debated [1].
Endovascular coiling is a minimally invasive alternative performed via catheter angiography [1, 10]. Detachable platinum coils are carefully packed into the aneurysm sac, promoting thrombosis within the aneurysm and excluding it from circulation [10]. Other endovascular techniques include stent-assisted coiling and flow diversion stents, particularly for complex or wide-necked aneurysms [10].
Complex aneurysms, such as some involving the origin of critical arteries like the PICA, may require more complex surgical strategies, sometimes involving clipping combined with a bypass procedure (e.g., occipital artery to PICA anastomosis) to maintain blood flow to vital brain regions and prevent infarction (like lateral medullary syndrome) [1, 11].
The choice between surgical clipping and endovascular treatment depends on various factors, including aneurysm location, size, and morphology, the patient's clinical condition, and institutional expertise [1, 10]. While clipping the aneurysm itself may be technically straightforward in some cases, managing associated large hematomas or operating on a swollen brain carries significant risks [1, 11]. Therefore, the optimal treatment strategy and its timing (early vs. delayed) are individualized for each patient [1, 10].
References
- Ropper AH, Samuels MA, Klein JP, Prasad S. Adams and Victor's Principles of Neurology. 11th ed. McGraw Hill; 2019. Chapter 34: Cerebrovascular Diseases (Section on Intracranial Aneurysms and Subarachnoid Hemorrhage).
- Connolly ES Jr, Rabinstein AA, Carhuapoma JR, et al; American Heart Association Stroke Council; Council on Cardiovascular Radiology and Intervention; Council on Cardiovascular Nursing; Council on Cardiovascular Surgery and Anesthesia; Council on Clinical Cardiology. Guidelines for the management of aneurysmal subarachnoid hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2012 Jun;43(6):1711-37.
- Greenberg MS. Handbook of Neurosurgery. 9th ed. Thieme; 2019. Chapter 37: Intracranial Aneurysms.
- Thompson BG, Brown RD Jr, Amin-Hanjani S, et al; American Heart Association Stroke Council, Council on Cardiovascular and Stroke Nursing, Council on Clinical Cardiology, Council on Functional Genomics and Translational Biology, and Council on Hypertension. Guidelines for the Management of Patients With Unruptured Intracranial Aneurysms: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2015 Aug;46(8):2368-400.
- Ropper AH, Samuels MA, Klein JP, Prasad S. Adams and Victor's Principles of Neurology. 11th ed. McGraw Hill; 2019. Chapter 13: Disorders of Ocular Movement and Pupillary Function.
- Hunt WE, Hess RM. Surgical risk as related to time of intervention in the repair of intracranial aneurysms. J Neurosurg. 1968 Jan;28(1):14-20.
- Report of World Federation of Neurological Surgeons Committee on a Universal Subarachnoid Hemorrhage Grading Scale. J Neurosurg. 1988 Jun;68(6):985-6.
- Osborn AG, Hedlund GL, Salzman KL. Osborn's Brain: Imaging, Pathology, and Anatomy. 2nd ed. Elsevier; 2017. Section on Aneurysms and Subarachnoid Hemorrhage.
- Edlow JA, Caplan LR. Avoiding pitfalls in the diagnosis of subarachnoid hemorrhage. N Engl J Med. 2000 Jan 6;342(1):29-36.
- Diringer MN, Bleck TP, Claude Hemphill J 3rd, et al; Neurocritical Care Society. Critical care management of patients following aneurysmal subarachnoid hemorrhage: recommendations from the Neurocritical Care Society's Multidisciplinary Consensus Conference. Neurocrit Care. 2011 Sep;15(2):211-40.
- Winn HR. Youmans and Winn Neurological Surgery. 7th ed. Elsevier; 2017. Volume 4, Section XII: Vascular Neurosurgery (Chapters on Aneurysms, SAH management, Surgical and Endovascular Treatment).
See also
- Ischemic stroke, cerebral ischemia
- Vertebrobasilar insufficiency (VBI) with vertigo symptom
- Somatoform autonomic dysfunction
- Dizziness, stuffiness in ear and tinnitus
- Ischemic brain disease:
- Atherosclerotic thrombosis
- Atherothrombotic occlusion of internal carotid artery
- Asymptomatic carotid bifurcation stenosis with noise
- Atherothrombotic occlusion of vertebrobasilar and posterior cerebral arteries
- Atherothrombotic occlusion of posterior cerebral artery
- Atherothrombotic occlusion of vertebral and posterior inferior cerebellar arteries (PICA)
- Atherothrombotic occlusion of basilar artery
- Small-vessel stroke (lacunar infarction)
- Other causes of ischemic stroke (cerebral infarction)
- Cerebral embolism
- Spontaneous intracranial (subarachnoid) and intracerebral hemorrhage:
- Arteriovenous malformations of the brain
- Hypertensive intracerebral hemorrhage
- Cerebral arteries inflammatory diseases (cerebral arteritis)
- Giant intracranial aneurysms
- Other causes of intracerebral hemorrhage
- Lobar intracerebral hemorrhage
- Saccular aneurysm and subarachnoid hemorrhage
- Mycotic intracranial aneurysms
- Repeated cerebral artery aneurysm rupture
- Communicating hydrocephalus after intracerebral hemorrhage with ruptured aneurysm
- Cerebral vasospasm
- Cerebrovascular diseases - ischemic stroke, transient ischemic attack (TIA):
- Transient ischemic attack (TIA)
- Sigmoid sinus suppurative thrombophlebitis with thrombosis