Small-vessel stroke (lacunar infarction)
Small-vessel stroke (lacunar infarction) Overview
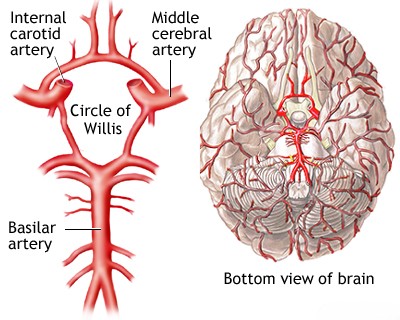
The term "lacunar infarction" (or lacunar stroke) refers to a type of ischemic stroke caused by the occlusion of a single, small penetrating artery deep within the brain [1, 2]. These occlusions are typically due to specific small vessel diseases, primarily lipohyalinosis (a degenerative process affecting small arteries and arterioles, strongly associated with chronic hypertension) or microatheroma (atherosclerosis within the small penetrating artery itself) [1, 2, 3]. These penetrating arteries arise from larger vessels like the arteries comprising the Circle of Willis, the main trunks of the Middle Cerebral Artery (MCA), and the vertebral and basilar arteries, supplying deep gray matter structures (like the thalamus, basal ganglia), the pons, and the internal capsule [1, 4].
Small-vessel stroke (lacunar infarction) Causes
The main trunks of the Middle Cerebral Artery (MCA), the arteries forming the Circle of Willis (including the A1 segments of the Anterior Cerebral Arteries, the Anterior and Posterior Communicating Arteries, and the P1 segments of the Posterior Cerebral Arteries), and the vertebral and basilar arteries all give rise to small penetrating branches [1, 4]. These arterioles typically have diameters ranging from 100 to 400 micrometers (µm) and perforate the deep gray matter structures (basal ganglia, thalamus), internal capsule, corona radiata, and brainstem (pons) [1, 2]. Occlusion of a single one of these small penetrating arteries leads to a lacunar infarct [1, 2]. This occlusion is usually caused by specific forms of small vessel disease: either lipohyalinosis (a degenerative process characterized by hyaline thickening of the vessel wall, strongly associated with chronic hypertension, typically affecting the vessel segment further from its origin) or microatheroma (atherosclerosis occurring at the origin or within the proximal part of the penetrating artery itself) [1, 2, 3]. Thrombosis occurring due to these underlying vessel pathologies results in small infarcts, generally defined as being less than 15 mm or 20 mm in diameter, often referred to as lacunes (from the Latin for "lake" or "gap") [1, 5]. Many are even smaller, measuring only 3-4 mm [1]. Chronic arterial hypertension is the single most important modifiable risk factor for developing these small vessel pathologies and subsequent lacunar infarcts [1, 2]. Lacunar infarcts account for approximately 15-25% of all ischemic strokes [1, 2].
Small-vessel stroke (lacunar infarction) Clinical Symptoms
The specific clinical patterns of neurological deficits caused by lacunar infarcts are referred to as lacunar syndromes [1, 2]. The onset of these symptoms can sometimes be preceded by related lacunar Transient Ischemic Attacks (TIAs) [1]. These TIAs may occur multiple times, often lasting only a few minutes each, before a persistent deficit develops [1].
The neurological deficit from a lacunar stroke may evolve over hours to days (sometimes called "stuttering lacunes") or appear suddenly and maximally at onset [1, 2]. Following the acute phase, the patient's condition typically stabilizes, and gradual improvement often occurs over weeks to months [1]. While many patients make a good recovery, some are left with persistent disabilities [1]. The degree of recovery can range from complete resolution to minimal or significant residual neurological deficits [1].
Numerous clinical syndromes attributed to lacunar infarcts have been described, though some are more clearly defined and commonly recognized than others [1, 2]. The most frequent and well-established lacunar syndromes include [1, 2]:
- Pure Motor Stroke (or Pure Motor Hemiparesis): Characterized by contralateral weakness affecting the face, arm, and leg relatively equally, without sensory loss, visual field deficits, aphasia, or neglect. It is typically caused by an infarction in the posterior limb of the internal capsule or the basis pontis.
- Pure Sensory Stroke: Presents as persistent or transient numbness, tingling, pain, burning, or other unpleasant sensations on one side of the body (face, arm, leg), without motor weakness or other deficits. Usually caused by an infarction in the ventral posterolateral (VPL) nucleus of the thalamus.
- Ataxic Hemiparesis: A combination of contralateral weakness (often more prominent in the leg) and ipsilateral limb ataxia (incoordination) that is out of proportion to the weakness. Commonly results from an infarction in the basis pontis, the posterior limb of the internal capsule, or the corona radiata.
- Dysarthria-Clumsy Hand Syndrome: Characterized by facial weakness, severe dysarthria (slurred speech), dysphagia (difficulty swallowing), and clumsiness/slowness of the contralateral hand. Typically caused by an infarction in the basis pontis or the genu (knee) of the internal capsule.
- Sensorimotor Stroke: Combines contralateral motor weakness and sensory loss (hemianesthesia). Often results from infarcts involving both the thalamus and the adjacent posterior limb of the internal capsule.
Multiple, often bilateral, lacunar infarcts accumulating over time, particularly in the context of poorly controlled chronic hypertension, can lead to progressive neurological decline [1, 6]. This may manifest as vascular cognitive impairment or vascular dementia, gait disturbance (marche à petits pas), parkinsonism, emotional lability (pseudobulbar affect), apathy, abulia, and urinary incontinence [1, 6]. While effective antihypertensive treatment has likely reduced the incidence of the most severe classic "pseudobulbar palsy" presentations, cognitive and motor deficits due to small vessel disease remain common [1].
Other neurological syndromes can result from occlusion of penetrating arteries or small branches in the brainstem, though not all fit the strict definition of classic lacunar syndromes [1]. Examples potentially related to small vessel occlusions include:
- Specific brainstem syndromes resulting from small infarcts, such as isolated cranial nerve palsies or specific combinations like ataxia with ipsilateral leg weakness (from pontine infarcts).
- Certain syndromes linked to occlusion of penetrating branches from the Posterior Cerebral Artery (PCA), including various thalamic syndromes beyond pure sensory loss.
- Syndromes from occlusion of penetrating branches of the basilar artery, potentially causing isolated motor or sensory deficits, or specific crossed deficits (e.g., hemiparesis with contralateral abducens nerve palsy).
- Lateral Medullary Syndrome (Wallenberg Syndrome): While caused by occlusion of a branch supplying the lateral medulla (usually from PICA or the vertebral artery), this typically involves a larger territory than a single lacune and presents with a characteristic constellation of symptoms (vertigo, nystagmus, ipsilateral ataxia, Horner's syndrome, loss of pain/temperature sensation on the ipsilateral face and contralateral body, dysphagia, hoarseness). It's generally not classified as a lacunar syndrome.
- Medial Medullary Syndrome: Caused by occlusion of penetrating branches from the vertebral or anterior spinal artery, leading to contralateral hemiparesis (often sparing the face initially) and loss of proprioception/vibration, along with ipsilateral tongue weakness. Can sometimes be caused by small vessel occlusion.
Small-vessel stroke (lacunar infarction) Diagnosis
Computed Tomography (CT) scanning of the brain has limited sensitivity for detecting lacunar infarcts, especially in the acute phase or when they are very small [1, 5]. Chronic lacunes may appear as small, well-defined hypoattenuating (dark) lesions [5]. Magnetic Resonance Imaging (MRI) is significantly more sensitive than CT for identifying both acute and chronic lacunar infarcts in supratentorial and infratentorial locations [1, 5]. Specific sequences like Diffusion-Weighted Imaging (DWI) are crucial for detecting acute lacunes, while FLAIR and T2-weighted images are excellent for visualizing chronic lacunes and assessing the overall burden of small vessel disease [5].
MRI clearly demonstrates the characteristic locations of lacunar infarcts within the deep gray matter structures (e.g., basal ganglia, thalamus), internal capsule, corona radiata, and brainstem (pons), sparing the cerebral cortex [1, 5]. Critically, MRI can also detect small infarcts that involve the cerebral cortex or are located superficially in the subcortical white matter immediately adjacent to the cortex [1]. Such infarcts, even if small, are typically caused by embolism (from the heart, aorta, or large arteries) rather than the intrinsic occlusion of a single small penetrating artery (due to lipohyalinosis or microatheroma) that defines a lacunar infarct [1, 2]. Therefore, the presence of cortical involvement on imaging effectively excludes a diagnosis of lacunar infarction and points towards an embolic etiology [1].
Differential Diagnosis of Lacunar Stroke Syndromes [1, 7]
| Condition | Key Features / Distinguishing Points | Typical Investigations / Findings |
|---|---|---|
| Lacunar Infarction | Classic lacunar syndrome (Pure Motor, Pure Sensory, Ataxic Hemiparesis, Dysarthria-Clumsy Hand, Sensorimotor). Deficits usually involve face/arm/leg. No cortical signs (aphasia, neglect, visual field cut). Often hypertensive. | MRI shows small (<1.5-2.0 cm) infarct in typical deep location (basal ganglia, thalamus, pons, internal capsule, deep white matter), sparing cortex. CT often negative acutely. |
| Small Cortical/Subcortical Embolic Stroke | May present with similar focal deficits initially, but often associated with cortical signs (e.g., mild aphasia, neglect, visual field disturbance) even if subtle. Larger territory usually involved on imaging. Source of embolism often identifiable (cardiac, large artery). | MRI (DWI) shows acute infarct involving cortex or superficial subcortical white matter, potentially larger than lacune definition. Workup reveals embolic source. |
| Small Intracerebral Hemorrhage (ICH) | Sudden onset focal deficit, may have headache. Can occur in deep locations similar to lacunes (esp. hypertensive ICH). | Non-contrast CT head shows hemorrhage. MRI confirms location/size. |
| Brain Tumor | Can occasionally present acutely (e.g., due to hemorrhage/seizure), but often more progressive symptoms. Headache common. Deficits may not fit classic lacunar syndrome. | MRI with contrast shows mass lesion. |
| Brain Abscess | Subacute onset usually. Focal deficits, headache, fever, seizures. History of infection source. | MRI shows ring-enhancing lesion, restricted diffusion. Inflammatory markers elevated. |
| Multiple Sclerosis (MS) Plaque | Acute/subacute onset focal deficit (sensory, motor, ataxia). Can occur in locations mimicking lacunes (pons, internal capsule). Often younger patients, may have prior episodes or other neurological symptoms. | MRI shows characteristic demyelinating lesion(s) (T2/FLAIR hyperintense, +/- enhancement), often periventricular, juxtacortical, infratentorial, or spinal cord involvement. |
| Migraine Aura | Transient focal neurological symptoms (sensory, motor). Typically spreads gradually, followed by headache. Full recovery. History of migraine. | Clinical diagnosis. Normal imaging usually. |
| Hypoglycemia | Can mimic stroke with focal deficits. | Low blood glucose. Resolves with glucose. |
| Seizure with Todd's Paralysis | Transient post-ictal focal weakness. | History. EEG. Transient. |
Strokes larger than the typical size definition for lacunes (generally >1.5 cm or 2 cm in diameter), even if they present clinically with symptoms resembling a classic lacunar syndrome (like pure motor hemiparesis), should not be classified as lacunar infarcts [1]. Their larger size implies involvement beyond the territory of a single small penetrating artery and often suggests a different underlying cause, most commonly artery-to-artery or cardioembolism [1]. While Computed Tomography (CT) might sometimes fail to show subtle cortical involvement in these larger deep infarcts, Magnetic Resonance Imaging (MRI), particularly with DWI sequences, is much more reliable for assessing infarct size, precise location, and crucially, for excluding any cortical involvement [5]. A definitive diagnosis of lacunar infarction requires an infarct size consistent with the definition (typically <1.5 or <2.0 cm), a location typical for penetrating artery supply (e.g., basal ganglia, thalamus, pons, internal capsule, deep white matter), *and* the absence of cortical involvement on imaging (best confirmed by MRI) [1, 5]. Larger deep infarcts within the territory of major vessels like the Middle Cerebral Artery (MCA) are highly suggestive of an embolic source [1].
Electroencephalography (EEG) findings can sometimes provide supportive evidence [1]. Because lacunar infarcts involve deep brain structures and spare the cerebral cortex, the EEG is typically normal [1]. In contrast, strokes that involve the cerebral cortex often cause abnormalities on EEG, such as focal slowing or epileptiform discharges, reflecting the disruption of cortical electrical activity [1]. Therefore, obtaining a normal EEG shortly after the onset of stroke symptoms can support the clinical suspicion of a deep, subcortical infarct location, consistent with (but not definitively diagnostic of) a lacunar stroke [1].
Small-vessel stroke (lacunar infarction) Treatment
The cornerstone of managing small vessel disease responsible for lacunar infarcts is secondary prevention, primarily through meticulous long-term control of arterial hypertension [1, 2, 8]. However, acutely lowering blood pressure aggressively during an ongoing lacunar stroke is generally avoided as it may potentially worsen neurological deficits by reducing perfusion to ischemic tissue [8]. Blood pressure management strategies in the acute phase often aim for permissive hypertension initially, with gradual reduction initiated once the patient's neurological status has stabilized [8].
The optimal acute treatment strategy regarding anticoagulants and antiplatelet agents specifically for lacunar strokes and related TIAs, particularly those with fluctuating symptoms, remains debated and is an area of ongoing research [1, 8]. Standard practice typically involves initiating antiplatelet therapy (like aspirin) [8]. Caution is sometimes advised regarding early anticoagulation, partly due to the underlying pathology in many lacunes: lipohyalinosis is associated with vessel wall fragility and an increased risk of intracerebral hemorrhage, including microbleeds [1, 3]. Indeed, pathological studies sometimes reveal evidence of prior microbleeds (hemosiderin-laden macrophages) within or near areas of lacunar infarction [1].
While the routine use of anticoagulants like heparin in acute lacunar stroke is generally not standard practice due to the microbleed risk and lack of definitive evidence of benefit over antiplatelets, some clinicians might consider it in specific situations [1]. For example, in patients with progressive or fluctuating deficits thought potentially related to thrombosis of a microatheroma at the origin of a penetrating artery (arising from larger vessels like the basilar or MCA trunk), heparin has sometimes been used empirically, although strong supporting evidence is limited [1].
Long-term anticoagulation is generally not indicated for secondary prevention after a lacunar stroke unless there is a concurrent indication, such as atrial fibrillation [8]. The primary focus for long-term secondary prevention is aggressive risk factor modification, most importantly, achieving and maintaining optimal blood pressure control to prevent the progression of hypertensive small vessel disease and reduce the risk of recurrent lacunar events [1, 8].
References
- Ropper AH, Samuels MA, Klein JP, Prasad S. Adams and Victor's Principles of Neurology. 11th ed. McGraw Hill; 2019. Chapter 34: Cerebrovascular Diseases (Section on Lacunar Infarction).
- Caplan LR. Caplan's Stroke: A Clinical Approach. 5th ed. Cambridge University Press; 2016. Chapter on Lacunar and Small Vessel Disease Strokes.
- Fisher CM. Lacunar strokes and infarcts: a review. Neurology. 1982 Aug;32(8):871-6. (Classic paper by Fisher).
- Blumenfeld H. Neuroanatomy through Clinical Cases. 2nd ed. Sinauer Associates; 2010. Relevant chapters on deep brain structures and vascular supply (e.g., Ch 4, Ch 16, Ch 17, Ch 18).
- Osborn AG, Hedlund GL, Salzman KL. Osborn's Brain: Imaging, Pathology, and Anatomy. 2nd ed. Elsevier; 2017. Section on Lacunar Infarcts and Small Vessel Disease.
- Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010 Jul;9(7):689-701.
- Caplan LR. Stroke Mimics. Semin Neurol. 2016 Apr;36(2):203-12.
- Kernan WN, Ovbiagele B, Black HR, et al; American Heart Association Stroke Council, Council on Cardiovascular and Stroke Nursing, Council on Clinical Cardiology, and Council on Peripheral Vascular Disease. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014 Jul;45(7):2160-236. (Or more recent updates addressing secondary prevention, including BP targets).
See also
- Ischemic stroke, cerebral ischemia
- Vertebrobasilar insufficiency (VBI) with vertigo symptom
- Somatoform autonomic dysfunction
- Dizziness, stuffiness in ear and tinnitus
- Ischemic brain disease:
- Atherosclerotic thrombosis
- Atherothrombotic occlusion of internal carotid artery
- Asymptomatic carotid bifurcation stenosis with noise
- Atherothrombotic occlusion of vertebrobasilar and posterior cerebral arteries
- Atherothrombotic occlusion of posterior cerebral artery
- Atherothrombotic occlusion of vertebral and posterior inferior cerebellar arteries (PICA)
- Atherothrombotic occlusion of basilar artery
- Small-vessel stroke (lacunar infarction)
- Other causes of ischemic stroke (cerebral infarction)
- Cerebral embolism
- Spontaneous intracranial (subarachnoid) and intracerebral hemorrhage:
- Arteriovenous malformations of the brain
- Hypertensive intracerebral hemorrhage
- Cerebral arteries inflammatory diseases (cerebral arteritis)
- Giant intracranial aneurysms
- Other causes of intracerebral hemorrhage
- Lobar intracerebral hemorrhage
- Saccular aneurysm and subarachnoid hemorrhage
- Mycotic intracranial aneurysms
- Repeated cerebral artery aneurysm rupture
- Communicating hydrocephalus after intracerebral hemorrhage with ruptured aneurysm
- Cerebral vasospasm
- Cerebrovascular diseases - ischemic stroke, transient ischemic attack (TIA):
- Transient ischemic attack (TIA)
- Sigmoid sinus suppurative thrombophlebitis with thrombosis

 in the basal ganglia, and the lower images show those infarcts (arrows) in the deep white matter.jpg)
