Other causes of ischemic stroke (cerebral infarction)
- Cerebral Venous Sinus Thrombosis (CVST)
- Differential Diagnosis of CVST
- Systemic Hypotension as a Cause of Cerebral Ischemia
- Intracranial and Extracranial Artery Dissections (e.g., Internal Carotid or Vertebral Arteries)
- Cervical Artery Fibromuscular Dysplasia (FMD)
- Arteritis (Vasculitis) as a Cause of Stroke:
- Moyamoya Disease and Moyamoya Syndrome
- Oral Contraceptives and Stroke Risk
- Hematological Disorders and Hypercoagulable States
- Binswanger's Disease (Subcortical Arteriosclerotic Encephalopathy)
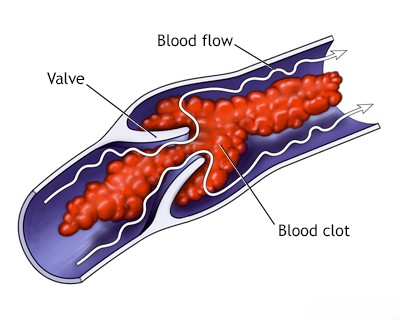
Cerebral Venous Sinus Thrombosis (CVST)
Thrombosis (clot formation) within the major dural venous sinuses (such as the transverse/lateral sinus or the superior sagittal sinus), or sometimes involving smaller cortical draining veins, can lead to serious neurological consequences [1, 2]. This condition, known as Cerebral Venous Sinus Thrombosis (CVST), can arise due to various underlying factors [1, 2, 3]:
- Infections: Both systemic infections (septicemia) and localized intracranial or head/neck infections (e.g., bacterial meningitis, subdural empyema) spreading from adjacent structures like the middle ear (otitis media), paranasal sinuses (sinusitis), face, or following open head trauma.
- Hypercoagulable States: Conditions that increase the blood's tendency to clot, such as inherited thrombophilias (e.g., Factor V Leiden, prothrombin gene mutation, protein C/S deficiency, antithrombin deficiency), acquired states like polycythemia (high red blood cell count), sickle cell anemia, dehydration, malignancy, certain autoimmune diseases (e.g., antiphospholipid syndrome, Behçet's disease), nephrotic syndrome, inflammatory bowel disease, pregnancy and the postpartum period, or the use of oral contraceptives or other hormonal therapies.
- Mechanical Factors: Head injury, neurosurgical procedures, or jugular vein catheterization (less common).
- Idiopathic: In some cases, no clear cause is identified.
When a major venous sinus or cortical vein becomes thrombosed, it obstructs venous outflow from the brain [1, 2]. This blockage can result in venous congestion and increased intracranial pressure, leading to symptoms like severe headaches (often diffuse and persistent), papilledema (optic disc swelling), nausea, and vomiting [1, 2]. Furthermore, impaired venous drainage can cause venous infarction (stroke due to lack of venous outflow, often hemorrhagic) and localized brain swelling (edema) [1, 2, 4]. These parenchymal changes can manifest as focal neurological deficits, including focal seizures, weakness (paresis), or paralysis, depending on the location and extent of venous territory affected [1, 2]. In severe cases involving extensive thrombosis and large venous infarcts with significant secondary edema, the resulting increase in intracranial pressure can be life-threatening, potentially leading to critical midline shift or brainstem herniation through the tentorial notch (incisura), which can rapidly cause coma and death [1].
Differential Diagnosis of Symptoms Suggesting Cerebral Venous Sinus Thrombosis (CVST)
| Condition | Key Features / Distinguishing Points | Typical Investigations / Findings |
|---|---|---|
| Cerebral Venous Sinus Thrombosis (CVST) | Headache (often severe, progressive), seizures, focal neurological deficits (variable, can be bilateral/multifocal), signs of increased ICP (papilledema, CN VI palsy), altered mental status. Risk factors (infection, hypercoagulable state, pregnancy, OCPs) often present. | MR Venography (MRV) or CT Venography (CTV) is diagnostic: Confirms lack of flow / filling defect (thrombus) in dural sinus(es) or cortical veins. Contrast MRI Brain may show associated venous infarcts (often hemorrhagic) or intense dural enhancement near sinus. D-dimer may be elevated (but not specific). |
| Idiopathic Intracranial Hypertension (IIH) / Pseudotumor Cerebri | Headache (often daily, pulsatile), papilledema, visual disturbances, pulsatile tinnitus. Normal level of consciousness. Typically young, obese women. No focal deficits (except CN VI palsy). | Normal MRI brain structure. MRI/MRV may show secondary signs (empty sella, optic nerve sheath distension, venous sinus stenosis - *can overlap with CVST*). LP confirms elevated opening pressure (>25 cmH2O) with normal CSF composition. Crucially, MRV/CTV excludes thrombosis. |
| Meningitis / Encephalitis | Headache, fever, neck stiffness, altered mental status, photophobia. Focal deficits/seizures possible (esp. encephalitis). ICP can be elevated. | LP (if safe) shows inflammatory CSF (pleocytosis, protein/glucose changes) +/- pathogen identification. MRI may show meningeal enhancement or parenchymal changes (encephalitis). MRV/CTV usually normal unless complicated by secondary CVST. |
| Intracranial Mass Lesion (Tumor, Abscess, Hematoma) | Progressive headache, focal deficits corresponding to location, seizures. Signs of increased ICP. Specific history (fever for abscess, trauma for hematoma). | MRI/CT shows space-occupying lesion with mass effect. MRV/CTV usually normal unless tumor invades/compresses sinus. |
| Acute Hydrocephalus (Obstructive or Communicating) | Symptoms of raised ICP: Headache, nausea/vomiting, papilledema, lethargy. Underlying cause (e.g., tumor blockage, post-SAH/meningitis). | MRI/CT shows ventricular enlargement. MRV/CTV usually normal unless hydrocephalus is secondary to CVST. |
| Migraine (Severe or Complicated) | Severe headache, often unilateral, pulsating, with nausea/photophobia. Aura may include transient focal deficits. History of similar episodes. No papilledema usually. | Clinical diagnosis based on ICHD criteria. Normal neuroimaging (MRI/MRV/CTV) excludes CVST and other structural causes. |
| Subarachnoid Hemorrhage (SAH) | Sudden "thunderclap" headache, decreased LOC, neck stiffness. Less commonly presents like CVST unless venous infarct is hemorrhagic. | Non-contrast CT head shows subarachnoid blood. CTA/DSA identifies aneurysm. MRV/CTV usually normal unless secondary thrombosis. |
| Hypertensive Encephalopathy / PRES | Acute confusion, headache, seizures, visual changes in setting of severe hypertension. Papilledema common. | Markedly elevated BP. MRI shows characteristic posterior white matter edema (PRES). MRV/CTV normal. |
Note: CVST can sometimes be a complication of other conditions listed here (e.g., meningitis, tumor compression). Imaging, particularly MRV or CTV, is crucial for differentiation.
Systemic Hypotension as a Cause of Cerebral Ischemia
Systemic hypotension (a significant drop in overall blood pressure) can occasionally cause cerebral ischemia (insufficient blood flow to the brain), particularly affecting the most vulnerable "watershed" or border zone areas [1, 5]. Examples of conditions causing severe hypotension include Morgagni-Adams-Stokes attacks (episodes of syncope due to sudden cardiac arrhythmias causing drastically reduced cardiac output) [1]. However, brief episodes of hypotension typically do not cause permanent brain damage (infarction) [1]. Cerebral infarction (ischemic stroke) due to low blood pressure generally occurs only with severe and prolonged hypotension, such as that experienced during cardiac arrest or profound shock states [1, 5].
When infarction does occur due to systemic hypotension, it characteristically localizes to the border zones between the territories supplied by major intracranial arteries [1, 5]. These watershed areas include the distal territories where the supply from the anterior, middle, and posterior cerebral arteries meets [5]. This pattern results because these regions are farthest from the origin of the main feeding vessels and thus most susceptible to reductions in perfusion pressure [1, 5]. Clinically, this pattern of watershed infarction can lead to specific neurological deficits, such as weakness predominantly affecting the proximal parts of the limbs ("man-in-a-barrel" syndrome if bilateral upper limbs are affected) or cognitive and visual processing difficulties related to parietal lobe dysfunction [1].
Intracranial and Extracranial Artery Dissections (e.g., Internal Carotid or Vertebral Arteries)
Arterial dissection, a tear within the layers of an artery wall, affecting major neck (extracranial) or brain (intracranial) arteries can lead to subsequent impairment of arterial blood flow and potentially cause cerebral infarction (ischemic stroke) [1, 6]. Dissection is recognized as a significant cause of stroke, particularly in children and younger adults [6, 7]. The process typically involves a tear in the innermost layer (tunica intima), allowing blood to enter and track within the vessel wall, separating the intima from the middle layer (tunica media) or splitting layers within the media itself [6]. This creates a false lumen and intramural hematoma (blood clot within the wall), which can compress the true lumen causing stenosis (narrowing) or complete occlusion [6]. Alternatively, thrombus forming at the site of dissection can break off and travel downstream, causing embolic stroke [6]. Both transient ischemic attacks (TIAs) and completed ischemic strokes can result from either mechanism: vessel occlusion/stenosis (low flow) or arterio-arterial embolism [1, 6].
A significant number of arterial dissections affecting the brain and neck arteries occur following prior head or neck trauma, which can range from major injuries to relatively mild or even trivial events (e.g., chiropractic manipulation, severe coughing, sudden head movements) [6, 7]. However, spontaneous arterial wall dissection can also occur, sometimes in the context of underlying arteriopathies like fibromuscular dysplasia, certain connective tissue disorders (e.g., Ehlers-Danlos syndrome type IV, Marfan syndrome), homocystinuria, or potentially inflammatory arteritis, although many spontaneous dissections occur without an identifiable underlying condition [1, 6]. While internal carotid artery dissection is commonly observed, dissections can also affect the vertebral arteries (another frequent site, especially related to trauma), the basilar artery, or intracranial arteries like the middle cerebral artery (MCA) and anterior cerebral artery (ACA) [1, 6].
Internal carotid artery dissection occurring extracranially in the neck can compress or irritate the adjacent sympathetic nerve plexus, potentially resulting in Horner's syndrome (a combination of ipsilateral ptosis [drooping eyelid], miosis [constricted pupil], and sometimes anhidrosis [decreased sweating] or perceived enophthalmos [sunken eye]) [1, 6]. Neck pain, headache (often frontotemporal), or facial pain are also common presenting symptoms [6]. Audible arterial bruits might be detected in some patients, though perhaps not in over 50% as originally stated [1]. Tenderness may sometimes be elicited upon palpation over the carotid bulb area [1].
Often, these local symptoms (pain, Horner's syndrome) or transient neurological symptoms like transient monocular blindness (amaurosis fugax) or TIAs related to hypoperfusion or emboli may precede a major embolic stroke originating from the dissection site [1, 6]. This potential warning period allows for diagnosis and therapeutic intervention aimed at preventing a disabling stroke. However, the precise pathogenesis and natural history of arterial dissection are variable and not fully understood, which can complicate optimal management decisions in individual cases [6].
Treatment for carotid or vertebral artery dissection has evolved, with medical management now often considered first-line, especially for extracranial dissections without ongoing severe ischemic symptoms [6, 9]. Anticoagulation (typically starting with heparin followed by warfarin or a DOAC) or antiplatelet therapy (like aspirin, often combined with clopidogrel initially) are commonly used to prevent thromboembolic complications originating from the dissection site [6, 9]. The choice between anticoagulation and antiplatelet therapy is often debated and may depend on institutional protocols, specific patient factors, and whether the primary concern is embolism versus flow limitation [9]. Anticoagulation might be preferred initially, particularly if there are signs of ongoing TIA or embolic phenomena [6]. Surgical repair or endovascular stenting of a dissected artery is generally reserved for specific situations, such as refractory ischemic symptoms despite medical therapy, formation of a large or symptomatic pseudoaneurysm, or certain complex intracranial dissections [6, 9]. After an initial period (often 3-6 months), repeat imaging is usually performed, and long-term therapy (often transitioning to single antiplatelet agent) is decided based on vessel healing and residual abnormalities [6, 9].
In the acute phase of symptomatic vertebral, middle cerebral, or posterior cerebral artery dissection, patients are often treated initially with anticoagulation (e.g., heparin followed by warfarin or DOACs) or antiplatelet agents, similar to carotid dissections, depending on the clinical scenario and perceived mechanism of ischemia (embolic vs. hemodynamic) [6, 9].
Cervical Artery Fibromuscular Dysplasia (FMD)
Fibromuscular dysplasia (FMD) is a non-atherosclerotic, non-inflammatory vascular disease that primarily affects the arterial wall, leading to abnormal cellular growth and development [1, 10]. It typically affects medium-sized arteries and is observed more commonly in young to middle-aged women [10]. When involving the cervical arteries, the carotid arteries (particularly the internal carotid artery distal to the bifurcation) and vertebral arteries are most frequently affected [10]. Radiologically, FMD often manifests with multiple segments of annular narrowing (stenosis) alternating with areas of dilation (aneurysms), creating a characteristic "string of beads" appearance on angiography [8, 10]. Arterial occlusion due to FMD itself is uncommon, but complications like dissection or aneurysm formation can lead to occlusion [10].
FMD affecting the cervical arteries may be asymptomatic or can present with non-specific symptoms like headache, pulsatile tinnitus, or neck pain [10]. Audible bruits may be present over the affected arteries [10]. More significantly, FMD can lead to transient ischemic attack (TIA) or stroke, primarily through mechanisms like spontaneous arterial dissection originating from the abnormal vessel wall, or less commonly, thromboembolism from thrombus forming within aneurysmal segments or at sites of dissection [1, 10]. Hypertension is frequently associated with FMD, often secondary to concurrent renal artery stenosis caused by FMD in those vessels [10].
The precise etiology and pathogenesis of FMD remain incompletely understood, although genetic and hormonal factors are thought to play a role [10]. When evaluating patients with TIA or stroke, caution should be exercised before attributing symptoms solely to the stenotic segments seen in FMD if the narrowing is not severe (e.g., residual lumen greater than 2 mm), as dissection or embolism are often the more likely culprits for acute events [1]. While surgical or endovascular treatment (like balloon angioplasty with or without stenting) for FMD-related stenosis or aneurysms is technically feasible, intervention is typically reserved for specific indications, such as symptomatic dissection, large or growing aneurysms, or hemodynamically significant stenosis causing refractory symptoms, due to potential procedural risks [10]. For many patients, particularly those who are asymptomatic or have experienced symptoms related to dissection that is managed medically, conservative management with antiplatelet therapy and blood pressure control is often the preferred approach [10]. Anticoagulation may be used temporarily in cases of acute dissection associated with FMD [6, 10].
Arteritis (Vasculitis) as a Cause of Stroke
Inflammation of blood vessel walls, known as arteritis or vasculitis, can occasionally lead to cerebral thrombosis and stroke, although it is generally an uncommon cause compared to atherosclerosis or embolism [1, 11]. In the modern era, infectious arteritis caused by bacteria or syphilis is now rare in developed countries due to effective antibiotic treatments, unlike in the pre-penicillin era where it was a more significant concern [1]. However, various non-infectious inflammatory conditions affecting arteries (primary central nervous system vasculitis or systemic vasculitis with CNS involvement) can potentially lead to thrombosis or stenosis of cerebral vessels, resulting in stroke [1, 11].
Necrotizing or Granulomatous Arteritis
Necrotizing arteritis (characterized by vessel wall inflammation and tissue death) or granulomatous arteritis (characterized by specific inflammatory cell clusters called granulomas) can affect cerebral blood vessels [1, 11]. This may occur as an isolated condition (primary CNS vasculitis) or as part of a systemic autoimmune disease like polyarteritis nodosa (PAN, typically affecting medium-sized arteries throughout the body) or granulomatosis with polyangiitis (formerly Wegener's granulomatosis, affecting respiratory tract, kidneys, and small-to-medium vessels) [1, 11, 12]. Cerebral involvement typically affects small distal branches (often less than 1 mm diameter) of intracerebral arteries [1]. Occlusion of these small vessels can lead to multiple small ischemic infarctions scattered throughout the brain, and potentially also affecting the optic nerves and spinal cord [1]. These forms of arteritis are generally rare and can cause a progressively debilitating neurological illness [1]. Treatment often involves immunosuppression, typically starting with high-dose corticosteroids (e.g., prednisone 40-60 mg/day or higher initially), and often requiring the addition of other immunosuppressant medications (like cyclophosphamide or rituximab) for long-term control [11, 12].
Takayasu’s Arteritis (Aortic Arch Syndrome)
Takayasu's arteritis is a form of large-vessel giant cell arteritis that primarily affects the aorta and its major branches (like the subclavian, common carotid, and sometimes vertebral arteries) [1, 11, 12]. While it can cause stenosis or occlusion of these large vessels leading to symptoms like arm claudication, differential blood pressures, or loss of pulses (sometimes called "pulseless disease"), it only rarely causes direct thrombosis leading to stroke originating from the carotid or vertebral arteries themselves [1]. Takayasu's arteritis is an uncommon cause of aortic arch syndrome, particularly in populations of the Western Hemisphere, being more prevalent in young women of Asian descent [11, 12].
Temporal Arteritis / Giant Cell Arteritis (GCA)
Temporal arteritis, more broadly known as Giant Cell Arteritis (GCA), is a common systemic vasculitis affecting medium and large arteries, primarily diagnosed in older adults (typically over 50 years old) [1, 11, 12]. GCA frequently involves branches of the external carotid artery system, most classically the superficial temporal artery, leading to characteristic symptoms [1]. Pathologically, it involves subacute granulomatous inflammation of the artery wall, often containing lymphocytes, macrophages (monocytes), occasional neutrophils, and characteristic multinucleated giant cells [11]. Affected arterial segments can become narrowed, thrombosed, tender, and thickened [1]. The primary symptom is often a new-onset headache, frequently localized to the temporal areas [1, 11]. Other common symptoms include jaw claudication (pain with chewing), scalp tenderness, and systemic manifestations like anorexia, weight loss, malaise, low-grade fever, and polymyalgia rheumatica (PMR - stiffness and pain in the neck, shoulders, and hips) [1, 11]. Laboratory findings indicative of the inflammatory nature of GCA commonly include [1, 11]:
- Elevated erythrocyte sedimentation rate (ESR) and/or C-reactive protein (CRP)
- Moderate leukocytosis (elevated white blood cell count) may be present
- Anemia (normochromic, normocytic anemia of chronic inflammation)
A major and feared complication of GCA is visual loss due to ischemic optic neuropathy resulting from occlusion of ophthalmic artery branches (posterior ciliary arteries) [1, 11]. This can lead to sudden, irreversible blindness in one or both eyes in a significant proportion (over 25% if untreated) of patients [1, 11]. Ophthalmoplegia (double vision due to eye muscle weakness) from involvement of cranial nerves supplying eye muscles can also occur, though less commonly [1]. While the classic presentation involves extracranial arteries, postmortem studies and advanced imaging have occasionally revealed GCA involvement of the aorta (aortitis) and its major branches, including the carotid, subclavian, femoral, and coronary arteries [11]. Direct involvement causing severe intracerebral arteritis is rare, but GCA can potentially lead to stroke through mechanisms like dissection or thrombosis affecting the internal carotid or vertebral arteries [1]. Diagnosis is typically based on clinical suspicion (age, symptoms, elevated inflammatory markers), supported by temporal artery biopsy demonstrating characteristic histological findings (though biopsy can be negative due to skip lesions) [11]. Ultrasound of temporal arteries may also show characteristic signs (halo sign) [11].
Prompt treatment with high-dose corticosteroids is essential upon suspicion of GCA to alleviate symptoms and, crucially, to prevent irreversible blindness [11]. Prednisone is commonly prescribed, typically starting at high doses (e.g., 40-60 mg/day, sometimes higher initially) and then gradually tapered over many months (often 1-2 years) based on clinical response and normalization of inflammatory markers like ESR and CRP [11]. Medications like tocilizumab may also be used as steroid-sparing agents [11].
Moyamoya Disease and Moyamoya Syndrome
Moyamoya disease is a chronic, progressive occlusive arteriopathy (disease of arteries leading to blockage) primarily affecting large intracranial arteries [1, 13]. It is characterized by progressive stenosis (narrowing) or occlusion, typically involving the distal internal carotid arteries and the proximal segments of the middle cerebral artery (MCA) and anterior cerebral artery (ACA) [1, 13]. In response to this blockage, a network of fine, fragile collateral blood vessels develops, primarily involving perforating arteries like the lenticulostriate and thalamoperforating arteries at the base of the brain [1, 8, 13]. On cerebral angiography, this abnormal collateral network appears as a hazy 'puff of smoke,' which is the meaning of "moyamoya" in Japanese [8, 13]. Other collateral pathways, such as transdural anastomoses between superficial cortical branches (e.g., from the MCA) and external carotid artery branches supplying the scalp, may also develop [1]. While Moyamoya disease (idiopathic form) is more prevalent in individuals of East Asian descent, it can occur worldwide [13]. A similar angiographic pattern resulting from other known conditions (like sickle cell disease, Down syndrome, neurofibromatosis type 1, or cranial irradiation) is termed Moyamoya syndrome [1, 13]. Moyamoya should be considered in the differential diagnosis, particularly in children and young adults presenting with recurrent TIAs, ischemic strokes, or sometimes intracranial hemorrhage (more common presentation in adults than children) [1, 13].
The underlying etiology of idiopathic Moyamoya disease remains unknown, although genetic factors are implicated [13]. Pathological studies typically show stenosis or occlusion of the affected arteries associated with intimal thickening due to fibrocellular deposition, without significant inflammation or atherosclerosis [1]. Managing patients with Moyamoya presents challenges [13]. The use of anticoagulants needs careful consideration due to the increased risk of hemorrhage, particularly subarachnoid hemorrhage, potentially arising from rupture of the fragile collateral vessels (including transdural anastomoses) [1]. Surgical revascularization procedures, such as extracranial-intracranial (EC-IC) bypass surgery (e.g., superficial temporal artery to middle cerebral artery [STA-MCA] bypass), are often considered, especially in symptomatic patients, to improve blood flow to the ischemic brain and potentially reduce reliance on the fragile moyamoya collaterals [1, 13]. However, the long-term efficacy and optimal surgical technique remain subjects of ongoing research [13]. Furthermore, the surgical procedure itself (e.g., craniotomy for bypass) carries risks, including potential disruption of existing functional transdural anastomoses, which could paradoxically worsen neurological deficits in some cases, or potentially lead to thrombosis of the proximal native vessel after bypass creation [1].
Oral Contraceptives and Stroke Risk
Epidemiological studies have shown that the use of combined oral contraceptives (containing estrogen and progestin) is associated with a small but statistically significant increased risk of ischemic stroke, particularly in young women [1, 14]. Reported relative risks vary, but older studies suggested incidence rates roughly like 13 per 100,000 women-years among users versus 3 per 100,000 among non-users, with higher risks associated with older formulations containing higher estrogen doses [1]. In many cases of stroke occurring in oral contraceptive users, cerebral angiography reveals patent (open) arteries, or if an occlusion is initially detected, subsequent angiography may show recanalization (reopening) of the vessel [1]. This pattern suggests that embolism (perhaps from an undetected cardiac or paradoxical source, or possibly related to transient hypercoagulability) might be a significant underlying mechanism in many of these strokes, rather than primary arterial thrombosis or atherosclerosis [1]. However, the precise source of potential emboli is often unclear, and autopsy studies in such cases typically show no significant underlying arterial or cardiovascular abnormalities directly attributable to the contraceptive itself [1].
It's also important to note that other established risk factors for stroke in young women, such as migraine (especially migraine with aura) and cigarette smoking, appear to interact with oral contraceptive use, synergistically increasing the stroke risk much more significantly than any factor alone [1, 14]. The underlying mechanism might involve effects on blood coagulation (hypercoagulable states), endothelial function, or vasoreactivity, potentially leading to cerebral artery thrombosis or facilitating embolism [14].
Hematological Disorders and Hypercoagulable States
Several hematological disorders and conditions associated with hypercoagulability (an increased tendency for blood clotting) can significantly increase the risk of ischemic stroke (cerebral infarction) [1, 15]. The mechanism often involves abnormal thrombus formation within either the arterial or venous system, which can then lead to direct arterial occlusion or subsequent embolism [1, 15]. Conditions known to be associated with increased stroke risk include [1, 15]:
- Polycythemia Vera: A myeloproliferative disorder characterized by excessive production of red blood cells, increasing blood viscosity and thrombosis risk.
- Essential Thrombocythemia (Idiopathic Thrombocytosis): Characterized by excessive platelet production, increasing the risk of both thrombosis and bleeding.
- Thrombotic Thrombocytopenic Purpura (TTP): A rare disorder causing widespread microvascular thrombi, leading to low platelets, microangiopathic hemolytic anemia, and organ damage, including stroke.
- Sickle Cell Anemia: An inherited disorder causing abnormal red blood cell shape, leading to vaso-occlusion, hemolysis, inflammation, and increased stroke risk, particularly in children.
- Inherited Thrombophilias: Genetic deficiencies of natural anticoagulants like Protein C, Protein S, or Antithrombin III, or mutations like Factor V Leiden or Prothrombin gene mutation, predisposing to venous and sometimes arterial thrombosis.
- Antiphospholipid Syndrome: An autoimmune disorder associated with antibodies causing hypercoagulability and risk of both arterial and venous thrombosis.
- Other conditions like malignancy or severe dehydration can also induce a hypercoagulable state.
Binswanger's Disease (Subcortical Arteriosclerotic Encephalopathy)
Binswanger's disease, also known as Subcortical Arteriosclerotic Encephalopathy (SAE), is a form of small vessel vascular dementia characterized by widespread, chronic ischemic damage predominantly affecting the deep subcortical white matter of the brain [1, 16]. A key feature is relative sparing of the short association U-fibers located just beneath the cortex [1]. Brain imaging, particularly MRI (more sensitive than CT), typically reveals diffuse areas of low signal intensity on T1-weighted images and high signal intensity on T2-weighted/FLAIR images (leukoaraiosis) in the periventricular and deep white matter, often accompanied by multiple lacunar infarcts in the deep gray matter structures (basal ganglia, thalamus) and white matter [8, 16]. The underlying pathology is believed to be related to chronic damage to small penetrating arteries and arterioles supplying the deep brain structures, most commonly associated with long-standing, often poorly controlled, hypertension [1, 16]. Histologically, these small vessels often show lipohyalinosis (a degenerative change involving lipid deposition and fibrinoid necrosis in the vessel wall) and arteriosclerosis (thickening and hardening) [1]. These vascular changes lead to chronic hypoperfusion and recurrent small infarcts in the deep white and gray matter, affecting watershed areas between penetrating arteries arising from the Circle of Willis and those penetrating down from the cortex [1]. While the exact pathophysiology linking the vascular changes to the specific pattern of white matter damage remains debated, Binswanger's disease is recognized as a significant cause of progressive cognitive decline (often affecting executive function prominently), gait disturbance, mood changes (like apathy or abulia - lack of initiative), and overall disability, particularly in elderly hypertensive patients [1, 16].
References
- Ropper AH, Samuels MA, Klein JP, Prasad S. Adams and Victor's Principles of Neurology. 11th ed. McGraw Hill; 2019. Chapter 34: Cerebrovascular Diseases (Sections on specific stroke etiologies like Venous Thrombosis, Hypotension, Dissection, Vasculitis, Moyamoya, Hematologic Disorders, etc.).
- Saposnik G, Barinagarrementeria F, Brown RD Jr, et al; American Heart Association Stroke Council and the Council on Epidemiology and Prevention. Diagnosis and management of cerebral venous thrombosis: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011 Apr;42(4):1158-92.
- Ferro JM, Bousser MG, Canhão P, et al; ISCVT Investigators. European Stroke Organization guideline for the diagnosis and treatment of cerebral venous thrombosis - endorsed by the European Academy of Neurology. Eur J Neurol. 2017 Oct;24(10):1203-1213.
- Osborn AG, Hedlund GL, Salzman KL. Osborn's Brain: Imaging, Pathology, and Anatomy. 2nd ed. Elsevier; 2017. Section on Venous Thrombosis and Infarction.
- Grotta JC, Albers GW, Broderick JP, et al. Stroke: Pathophysiology, Diagnosis, and Management. 7th ed. Elsevier; 2021. Chapter on Hemodynamic Stroke.
- Caplan LR. Caplan's Stroke: A Clinical Approach. 5th ed. Cambridge University Press; 2016. Chapter on Cervical Artery Dissection.
- Debette S, Leys D. Cervical-artery dissections: predisposing factors, diagnosis, and outcome. Lancet Neurol. 2009 Jul;8(7):668-78.
- Osborn AG, Hedlund GL, Salzman KL. Osborn's Brain: Imaging, Pathology, and Anatomy. 2nd ed. Elsevier; 2017. Section on Vascular Diseases (Dissection, FMD, Moyamoya, Vasculitis).
- Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2019 Dec;50(12):e344-e418. (Includes sections on specific etiologies like dissection).
- Olin JW, Gornik HL, Kadian-Dodov D, et al. Fibromuscular Dysplasia: State of the Science and Critical Unanswered Questions: A Scientific Statement From the American Heart Association. Circulation. 2014 Feb 18;129(9):1048-78.
- Salvarani C, Cantini F, Hunder GG. Polymyalgia rheumatica and giant-cell arteritis. Lancet. 2008 Jul 19;372(9634):234-45. (Or comprehensive review on GCA/Vasculitis).
- Fauci AS, Langford CA. Harrison's Principles of Internal Medicine. 20th ed. McGraw Hill; 2018. Chapters on Vasculitis.
- Scott RM, Smith ER. Moyamoya disease and moyamoya syndrome. N Engl J Med. 2009 Mar 19;360(12):1226-37.
- Bushnell CD, McCullough LD, Awad IA, et al; American Heart Association Stroke Council; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; Council on Epidemiology and Prevention; Council for High Blood Pressure Research. Guidelines for the prevention of stroke in women: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014 May;45(5):1545-88.
- Grotta JC, Albers GW, Broderick JP, et al. Stroke: Pathophysiology, Diagnosis, and Management. 7th ed. Elsevier; 2021. Chapter on Uncommon Causes of Stroke (Hematologic).
- Pantoni L. Cerebral small vessel disease: from pathogenesis and clinical characteristics to therapeutic challenges. Lancet Neurol. 2010 Jul;9(7):689-701. (Or specific review on Binswanger's/SAE).
- Kattah JC, Talkad AV, Wang DZ, Hsieh YH, Newman-Toker DE. HINTS to diagnose stroke in the acute vestibular syndrome: three-step bedside oculomotor examination more sensitive than early MRI diffusion-weighted imaging. Stroke. 2009 Nov;40(11):3504-10.
- Ropper AH, Samuels MA, Klein JP, Prasad S. Adams and Victor's Principles of Neurology. 11th ed. McGraw Hill; 2019. Chapter 15: Coma and Related Disorders of Consciousness (Section on Herniation).
- Albers GW, Marks MP, Kemp S, et al; DEFUSE 3 Investigators. Thrombectomy for Stroke at 6 to 16 Hours with Selection by Perfusion Imaging. N Engl J Med. 2018 Feb 22;378(8):708-718.
- Kernan WN, Ovbiagele B, Black HR, et al; American Heart Association Stroke Council, Council on Cardiovascular and Stroke Nursing, Council on Clinical Cardiology, and Council on Peripheral Vascular Disease. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014 Jul;45(7):2160-236.
See also
- Ischemic stroke, cerebral ischemia
- Vertebrobasilar insufficiency (VBI) with vertigo symptom
- Somatoform autonomic dysfunction
- Dizziness, stuffiness in ear and tinnitus
- Ischemic brain disease:
- Atherosclerotic thrombosis
- Atherothrombotic occlusion of internal carotid artery
- Asymptomatic carotid bifurcation stenosis with noise
- Atherothrombotic occlusion of vertebrobasilar and posterior cerebral arteries
- Atherothrombotic occlusion of posterior cerebral artery
- Atherothrombotic occlusion of vertebral and posterior inferior cerebellar arteries (PICA)
- Atherothrombotic occlusion of basilar artery
- Small-vessel stroke (lacunar infarction)
- Other causes of ischemic stroke (cerebral infarction)
- Cerebral embolism
- Spontaneous intracranial (subarachnoid) and intracerebral hemorrhage:
- Arteriovenous malformations of the brain
- Hypertensive intracerebral hemorrhage
- Cerebral arteries inflammatory diseases (cerebral arteritis)
- Giant intracranial aneurysms
- Other causes of intracerebral hemorrhage
- Lobar intracerebral hemorrhage
- Saccular aneurysm and subarachnoid hemorrhage
- Mycotic intracranial aneurysms
- Repeated cerebral artery aneurysm rupture
- Communicating hydrocephalus after intracerebral hemorrhage with ruptured aneurysm
- Cerebral vasospasm
- Cerebrovascular diseases - ischemic stroke, transient ischemic attack (TIA):
- Transient ischemic attack (TIA)
- Sigmoid sinus suppurative thrombophlebitis with thrombosis


.jpg)
