Increased intracranial pressure and hydrocephalus
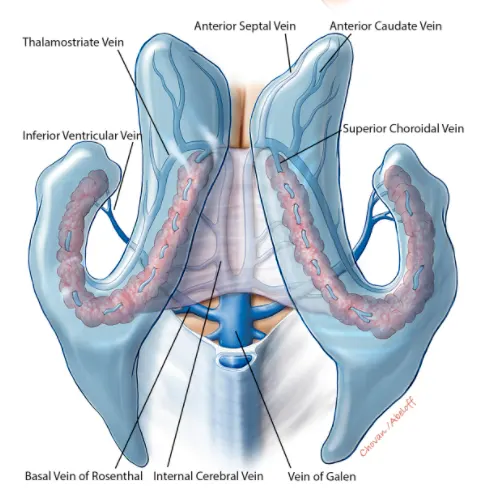
Cerebrospinal fluid (CSF)
Cerebrospinal fluid (CSF) is a clear, colorless biological fluid that circulates within the central nervous system (CNS), specifically within the brain's ventricles (internal cavities) and the subarachnoid space surrounding the brain and spinal cord.
In healthy adults, CSF is continuously produced, primarily by the choroid plexuses located within the ventricles, at a rate of approximately 0.3-0.4 mL/min, equating to about 20 mL/hour or roughly 500 mL per day (1). CSF production is relatively constant under normal conditions but can be influenced by factors like intracranial pressure (ICP) and certain medications (e.g., carbonic anhydrase inhibitors slightly reduce production). The total CSF volume in an adult is around 150 mL, meaning the entire volume is turned over approximately 3-4 times daily.
The key functions of cerebrospinal fluid (CSF) include (2):
- Mechanical Protection (Cushioning): CSF acts as a shock absorber, protecting the delicate brain and spinal cord from physical trauma and sudden movements.
- Buoyancy: By suspending the brain within the skull, CSF significantly reduces its effective weight (from ~1400g to ~50g), preventing the base of the brain from being compressed under its own weight.
- Homeostasis and Chemical Stability: CSF provides a stable chemical environment for neuronal function, regulating the distribution of ions and nutrients, and removing waste products.
- Waste Clearance (Glymphatic System): CSF plays a crucial role in clearing metabolic waste products (like amyloid-beta) from the brain interstitial fluid, particularly during sleep, via the recently described glymphatic system (3).
- Transport Medium: CSF transports nutrients, hormones, neurotransmitters, and signaling molecules within the CNS.
- Intracranial Pressure Regulation: CSF volume contributes to intracranial pressure and its regulation through production/absorption balance.
CSF is primarily produced by active secretion and ultrafiltration of blood plasma across the specialized epithelial cells of the choroid plexus located within the four brain ventricles (lateral, third, and fourth). A smaller amount may be produced by the ependymal lining of the ventricles and capillaries in the subarachnoid space.
The CSF system consists of interconnected internal (ventricular) and external (subarachnoid) spaces:
- Internal spaces (Ventricular System): Includes the two large C-shaped lateral ventricles within the cerebral hemispheres, the midline third ventricle between the thalami, and the fourth ventricle located between the brainstem (pons and medulla) and the cerebellum. The ventricles are lined by ependymal cells and contain choroid plexus.
- External spaces (Subarachnoid Space): Surrounds the entire brain and spinal cord, located between the arachnoid mater and the pia mater (the two inner meningeal layers). CSF fills this space, which contains cerebral and spinal blood vessels and cranial/spinal nerve roots. Widened areas of the subarachnoid space are called cisterns (e.g., cisterna magna, pontine cistern, interpeduncular cistern).
CSF Circulation Pathway: CSF produced mainly in the lateral ventricles flows through the paired interventricular foramina (of Monro) into the single third ventricle. From the third ventricle, it passes down through the narrow cerebral aqueduct (of Sylvius) into the fourth ventricle. CSF then exits the ventricular system into the subarachnoid space through three apertures in the roof/walls of the fourth ventricle: the median aperture (of Magendie) and the two lateral apertures (of Luschka). Once in the subarachnoid space, CSF circulates superiorly over the cerebral hemispheres and inferiorly around the spinal cord. This bulk flow is driven by the continuous production of CSF, arterial pulsations, respiratory movements, and possibly ciliary action of ependymal cells.
CSF Absorption: CSF is primarily resorbed from the subarachnoid space back into the venous bloodstream through specialized structures called arachnoid granulations (or villi). These are macroscopic protrusions of the arachnoid mater through the dura mater into the major dural venous sinuses (especially the superior sagittal sinus). Absorption occurs via a pressure-dependent bulk flow mechanism when CSF pressure exceeds venous sinus pressure. Some CSF absorption may also occur via lymphatic pathways associated with cranial and spinal nerves and the cribriform plate (1).
The blood-brain barrier (BBB) and the blood-CSF barrier (BCSFB) are crucial physiological barriers that tightly regulate the exchange of substances between the blood circulation and the CNS environment (brain interstitial fluid and CSF, respectively). The BBB is primarily formed by the specialized endothelial cells of brain capillaries, characterized by tight junctions, reduced pinocytosis, and specific transport systems, supported by astrocytes and pericytes. The BCSFB is mainly located at the choroid plexus epithelium, also featuring tight junctions and controlled transport mechanisms (4).
These barriers maintain the unique composition of CSF compared to blood plasma (e.g., lower protein, lower potassium, different glucose concentration) and protect the brain from potentially harmful substances, pathogens, and fluctuations in systemic physiology. They allow selective transport of essential nutrients (glucose, amino acids) into the CNS and facilitate removal of waste products. Disruption of these barriers occurs in various pathological states, including inflammation, infection, stroke, and trauma.
Increased intracranial pressure causes, hydrocephalus types
Increased intracranial pressure (ICP), also known as intracranial hypertension, refers to a sustained elevation of pressure within the rigid cranial vault. Hydrocephalus refers specifically to the pathological accumulation of excess CSF within the brain's ventricular system, usually leading to ventricular enlargement and often, but not always, resulting in increased ICP. Both are typically manifestations of underlying neurological pathology rather than independent diseases.
Increased ICP arises from an imbalance between the volumes of the intracranial contents (brain tissue, blood, CSF) within the fixed skull volume, according to the Monro-Kellie doctrine. An increase in the volume of one component (e.g., brain swelling/edema, intracranial mass, increased blood volume, increased CSF volume) must be compensated by a decrease in others, or ICP will rise.
Common causes of increased intracranial pressure and/or hydrocephalus include (5, 6):
- Increased Brain Volume:
- Cerebral Edema: Swelling of brain tissue due to trauma (TBI), stroke (ischemic or hemorrhagic), infection (encephalitis), hypoxia, metabolic disturbances (toxic/metabolic encephalopathy like hepatic failure), tumors.
- Increased Blood Volume:
- Obstruction of venous outflow (dural venous sinus thrombosis).
- Hypercapnia or hypoxia causing vasodilation (less common cause of sustained high ICP).
- Increased CSF Volume (Hydrocephalus): Due to:
- Impaired CSF Absorption (Communicating Hydrocephalus): CSF flows freely through the ventricles but is not adequately resorbed into the venous system. Causes include scarring/inflammation of arachnoid granulations after subarachnoid hemorrhage or meningitis, or elevated venous sinus pressure (e.g., from venous thrombosis). Normal Pressure Hydrocephalus (NPH) is a specific type of chronic communicating hydrocephalus in adults.
- Obstruction of CSF Flow Pathways (Non-communicating/Obstructive Hydrocephalus): Blockage within the ventricular system prevents CSF from reaching the subarachnoid space for absorption. Causes include congenital malformations (aqueductal stenosis, Chiari malformation), tumors within or compressing ventricles (e.g., colloid cyst, ependymoma, brainstem glioma), intraventricular hemorrhage, inflammatory adhesions/scarring (arachnoiditis).
- CSF Overproduction (Rare): Choroid plexus papilloma or carcinoma can rarely produce excessive CSF.
- Intracranial Mass Lesions: Expand within the fixed cranial volume, displacing normal structures and increasing pressure. Causes include primary brain tumors, metastatic tumors, abscesses, granulomas, large intracranial hematomas (epidural, subdural, intracerebral).
- Idiopathic Intracranial Hypertension (IIH): Increased ICP without hydrocephalus or identifiable structural cause, strongly associated with obesity in young women. Pathophysiology likely involves impaired CSF absorption or venous outflow issues.
Conversely, decreased intracranial pressure (intracranial hypotension) typically results from loss of CSF volume, most commonly due to CSF leaks (spontaneous, traumatic, or post-procedural like post-lumbar puncture), but can also be associated with severe dehydration, certain systemic illnesses, or over-shunting in treated hydrocephalus.
Increased intracranial pressure and hydrocephalus diagnosis
Diagnosing the presence and underlying cause of increased intracranial pressure (ICP) or hydrocephalus involves a combination of clinical assessment, ophthalmological evaluation, neuroimaging, and sometimes direct pressure measurement.
- Clinical Assessment (Neurological Examination): Evaluating symptoms suggestive of raised ICP (headache - often worse in morning or with Valsalva, nausea, vomiting, transient visual obscurations, diplopia), altered level of consciousness (lethargy, confusion, coma), and neurological signs (cranial nerve palsies - especially CN VI, motor deficits, ataxia, Cushing's triad [hypertension, bradycardia, irregular respiration] - a late and ominous sign). Specific symptoms may suggest hydrocephalus (cognitive decline, gait disturbance, incontinence - triad of NPH).
- Fundoscopic Examination (Ophthalmoscopy): Direct visualization of the optic nerve head is crucial to detect papilledema, the swelling of the optic disc specifically caused by transmission of raised ICP along the optic nerve sheath. This is a key objective sign of intracranial hypertension. Absence of papilledema does not rule out raised ICP, especially if acute.
- Neuroimaging: Essential for identifying structural causes and assessing ventricular size.
- Brain MRI: Preferred modality for detailed anatomical evaluation. Can show ventricular enlargement (hydrocephalus), identify obstructing lesions (tumors, cysts, aqueductal stenosis), show complications of raised ICP (herniation), detect causes of communicating hydrocephalus (e.g., signs of prior hemorrhage/infection), reveal signs suggestive of IIH (empty sella, optic nerve sheath distension, posterior globe flattening, venous sinus stenosis on MRV), or show underlying pathology causing cerebral edema (stroke, tumor, inflammation) (7). Specific sequences (e.g., CISS/FIESTA) can evaluate CSF flow dynamics.
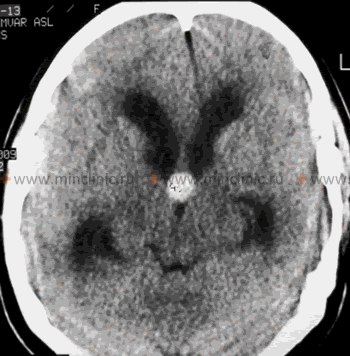
- Brain CT: Faster and more readily available, excellent in emergencies to detect acute hemorrhage, hydrocephalus (ventricular size, periventricular lucency indicating transependymal edema), large masses, or skull fractures. Less sensitive than MRI for subtle structural lesions, posterior fossa pathology, or specific signs of IIH.
- Lumbar Puncture (LP): Allows direct measurement of CSF opening pressure (normal in adults typically <20 cmH2O, or <25 cmH2O in obese individuals for IIH diagnosis) and collection of CSF for analysis (to rule out infection, inflammation, hemorrhage, malignancy). Extreme caution or contraindication applies if neuroimaging shows a mass lesion causing significant midline shift or posterior fossa mass effect, due to the risk of precipitating brain herniation (5). LP can be both diagnostic and therapeutic in IIH and sometimes communicating hydrocephalus (temporary relief with large volume removal).
- Direct ICP Monitoring: Invasive methods involving placement of a monitoring device (e.g., intraventricular catheter [EVD], intraparenchymal probe, epidural/subdural sensor) provide continuous ICP readings. Primarily used in critical care settings for managing severe TBI, large strokes, post-surgical monitoring, or fulminant hydrocephalus/ICP crisis (8).
- Older/Less Common Tests: Skull radiography (can show chronic signs like "copper beaten skull" or sella erosion, but insensitive). Cerebral blood flow studies (e.g., Transcranial Doppler, REG) may show indirect hemodynamic changes related to high ICP but are not primary diagnostic tools. Echoencephalography is largely obsolete for ICP assessment.
Differential Diagnosis of Symptoms Suggesting Increased Intracranial Pressure (ICP)
| Condition | Key Clinical & Exam Features | Primary Diagnostic Findings |
|---|---|---|
| Intracranial Mass Lesion (Tumor, Abscess, Hematoma) | Often progressive headache, focal neurological deficits corresponding to location, seizures common. +/- Papilledema, nausea/vomiting. History relevant (infection/fever for abscess, trauma/anticoagulation for hematoma). | MRI/CT shows space-occupying lesion with mass effect, +/- surrounding edema or obstructive hydrocephalus. Specific imaging features depend on lesion type (enhancement, DWI, location). |
| Hydrocephalus (Obstructive or Communicating, excluding NPH) | Symptoms of raised ICP: Headache, nausea/vomiting, papilledema, CN VI palsy, lethargy/altered consciousness. Gait/cognitive changes possible. Onset can be acute (e.g., aqueduct stenosis, tumor blockage) or subacute/chronic (post-SAH/meningitis). | MRI/CT shows ventricular enlargement often with signs of CSF obstruction (e.g., dilated temporal horns, aqueductal narrowing) or impaired absorption. LP opening pressure typically elevated. |
| Idiopathic Intracranial Hypertension (IIH) | Headache (often daily, pulsatile), papilledema (usually bilateral), visual disturbances (transient obscurations, field loss), pulsatile tinnitus, +/- CN VI palsy. Normal level of consciousness. Typically young, obese women. Neurological exam otherwise normal (except papilledema/CN VI palsy). | Normal MRI/CT brain structure (no mass/hydrocephalus). MRI/MRV may show secondary signs (empty sella, optic nerve sheath distension, posterior globe flattening, venous sinus stenosis). LP confirms elevated opening pressure (>25 cmH2O) with normal CSF composition. |
| Meningitis / Encephalitis | Headache, fever, neck stiffness (meningismus), altered mental status, photophobia. ICP can be elevated due to inflammation, cerebral edema, or secondary hydrocephalus/venous thrombosis. | LP (if safe) is diagnostic: CSF shows characteristic inflammatory changes (pleocytosis, protein/glucose changes) +/- positive pathogen identification (Gram stain/culture/PCR). MRI may show meningeal enhancement or parenchymal changes (encephalitis). |
| Dural Venous Sinus Thrombosis (DVST) | Often severe headache (can mimic IIH or migraine). Seizures, focal deficits (due to venous infarcts/hemorrhage), papilledema/raised ICP signs common. Risk factors often present (prothrombotic state, pregnancy, infection, dehydration). | MR Venography (MRV) or CT Venography (CTV) confirms lack of flow / thrombus ("filling defect") in dural sinuses or cortical veins. Brain MRI/CT may show venous infarcts (often hemorrhagic) or edema. |
| Subarachnoid Hemorrhage (SAH) | Sudden, severe "thunderclap" headache. Neck stiffness, altered consciousness, photophobia. ICP often acutely and severely elevated. May develop hydrocephalus. | Non-contrast CT head shows blood in subarachnoid space (sulci, cisterns, fissures). LP (if CT negative but suspicion high) shows blood/xanthochromia. CTA/DSA identifies ruptured aneurysm (most common cause). |
| Hypertensive Encephalopathy / PRES | Acute onset neurological symptoms (headache, confusion, visual changes, seizures) in setting of severely elevated blood pressure. Papilledema common. | Markedly elevated BP on exam. MRI Brain shows characteristic posterior white matter vasogenic edema (T2/FLAIR hyperintensity) - PRES pattern. Symptoms/imaging typically reverse with controlled BP lowering. |
| Severe Traumatic Brain Injury (TBI) | History of significant head trauma. Altered consciousness (low GCS score). ICP often elevated due to primary injury (hematoma, contusion) and secondary cerebral edema. | CT/MRI shows traumatic lesions (hematoma, contusion, edema, shear injury). ICP monitoring (if used) confirms elevation. |
| Migraine with Aura / Other Severe Primary Headache | Can cause severe headache, nausea, vomiting, visual/sensory disturbances (aura). However, no objective signs of raised ICP (no papilledema). Neurological exam normal between attacks. Consciousness usually preserved (unless complicated migraine). | Clinical diagnosis based on ICHD criteria. Normal neuroimaging (if performed to rule out secondary causes). Normal LP opening pressure and CSF composition. |
This table highlights key differentiating features; overlap exists, and multiple conditions can co-occur.
Papilledema is specifically non-inflammatory edema (swelling) of the optic nerve head (optic disc) resulting from increased intracranial pressure transmitted through the subarachnoid space surrounding the optic nerve within its dural sheath. Fundoscopic examination by an experienced clinician reveals characteristic findings: elevation of the optic disc surface, blurring or obliteration of the optic disc margins (especially nasally first), hyperemia (redness) of the disc, loss of the physiological optic cup, dilated and tortuous retinal veins, loss of spontaneous venous pulsations (an early sign, but its presence doesn't rule out raised ICP), and in more severe or chronic cases, peripapillary hemorrhages (flame-shaped) or cotton wool spots (retinal nerve fiber layer infarcts). Chronic papilledema can lead to secondary optic atrophy (pale disc) and permanent vision loss.
MRI findings suggestive of Intracranial Hypertension (often seen in IIH but can occur with other causes of chronic raised ICP) [9]:
Increased intracranial pressure and hydrocephalus treatment
Treatment strategies for increased intracranial pressure (ICP) and hydrocephalus are dictated by the underlying cause, the severity and acuity of the pressure elevation, and the presence of neurological symptoms or deficits. The primary goal is always to treat the root cause whenever possible.
General / Emergency Management of Acutely Raised ICP: (Often employed in critical care settings while investigating/treating the cause) [10]
- Positioning: Elevate the head of the bed (30-45 degrees) with the head in a neutral midline position to optimize cerebral venous drainage.
- Hyperosmolar Therapy: Administration of osmotic agents to draw fluid out of the brain tissue into the bloodstream.
- Mannitol: Intravenous osmotic diuretic (requires intact BBB).
- Hypertonic Saline (e.g., 3% or 23.4% NaCl): Creates an osmotic gradient, used increasingly. Requires careful monitoring of serum sodium and osmolality.
- Ventilation Control: Maintain normocapnia (PaCO2 35-40 mmHg). Controlled hyperventilation (targeting PaCO2 30-35 mmHg) can rapidly lower ICP by causing cerebral vasoconstriction but should be used only as a temporary bridge to more definitive therapy (e.g., surgery for mass lesion) or for refractory ICP, due to risk of cerebral ischemia if prolonged or excessive. Guided by advanced monitoring (e.g., PbtO2, SjvO2) if possible.
- Sedation and Analgesia: To reduce cerebral metabolic rate (CMRO2), control agitation, pain, and facilitate mechanical ventilation, thereby lowering ICP.
- CSF Drainage: Placement of an External Ventricular Drain (EVD) via neurosurgery allows direct ICP monitoring and therapeutic drainage of CSF to control pressure. This is often the most effective method for rapidly lowering ICP caused by hydrocephalus or diffuse edema. Therapeutic lumbar puncture (removing CSF) can lower pressure but is contraindicated if there's risk of herniation from a mass lesion.
- Temperature Control: Aggressive treatment of fever (antipyretics, cooling devices) as fever increases cerebral metabolism and ICP. Therapeutic hypothermia is generally not recommended routinely but may be considered in refractory ICP.
- Blood Pressure Management: Maintain adequate cerebral perfusion pressure (CPP = Mean Arterial Pressure - ICP), typically targeting CPP > 60-70 mmHg in adults, while avoiding excessive hypertension that could worsen edema.
- Seizure Control: Treat seizures promptly with antiepileptic drugs, as seizures markedly increase cerebral metabolism and ICP. Prophylaxis may be considered in high-risk situations (e.g., severe TBI).
Definitive Management (Addressing the Cause):
- Surgical removal or treatment (e.g., radiation, chemotherapy) of mass lesions (tumors, hematomas, abscesses).
- Treatment of infections (e.g., antibiotics/antivirals for meningitis/encephalitis).
- Management of traumatic brain injury (TBI) according to specific protocols (e.g., hematoma evacuation, decompressive craniectomy for refractory ICP).
- Addressing cerebrovascular issues like hemorrhage (e.g., aneurysm clipping/coiling) or venous sinus thrombosis (anticoagulation).
- Specific treatment for IIH (weight loss, acetazolamide, potentially shunting or venous sinus stenting if stenosis present).
- Management of metabolic/toxic encephalopathies (correcting imbalance, removing toxin).
Regarding conservative therapies potentially linked to vertebrobasilar insufficiency (VBI) or cervical spine issues: It's important to clarify that standard management of confirmed increased ICP or hydrocephalus does not typically involve therapies like manual therapy, cervical massage, or acupuncture as primary treatments for lowering ICP or resolving hydrocephalus itself. These approaches might be considered for managing associated or co-existing cervicogenic headaches or neck pain, which can sometimes mimic or overlap with symptoms of raised ICP. However, applying them requires caution and accurate diagnosis to ensure they are appropriate for the patient's condition and do not exacerbate an underlying instability or pathology. Management of suspected VBI focuses on vascular risk factor control and potentially antiplatelet agents, not typically manual therapies [11].
- Conservative modalities for associated musculoskeletal symptoms:
- Manual therapy and specific cervical spine massage techniques (applied cautiously by trained professionals for associated neck pain/stiffness).
- Physical therapy modalities (e.g., heat, cold, TENS) aimed at reducing muscle spasm and pain.
- Acupuncture may be used by some for headache or neck pain symptom relief.
- Therapeutic exercises (physiotherapy) focusing on neck/postural rehabilitation for cervicogenic issues.
- Temporary use of a cervical collar for support during acute neck pain phases.
- Pharmacological therapy targeting associated musculoskeletal pain or vascular health if VBI is confirmed (e.g., anti-inflammatories, muscle relaxants, antiplatelets).
Surgical Treatment for Hydrocephalus: Primarily aims to restore normal CSF circulation or permanently divert excess CSF [12]. Options include:
- Treating the underlying obstructive cause: Surgical removal of a tumor (e.g., colloid cyst, ependymoma), cyst fenestration, or lysis of adhesions blocking CSF pathways.
- CSF Shunting: Implanting a shunt system is the most common treatment for persistent hydrocephalus. A ventriculoperitoneal (VP) shunt is most frequently used, consisting of a ventricular catheter placed into a lateral ventricle, a pressure-regulating valve, and a distal catheter tunneled under the skin to drain CSF into the peritoneal cavity. Other distal sites (atrium - VA shunt, pleura - VPl shunt) are less common. Shunts effectively control hydrocephalus but are prone to complications like infection, obstruction, overdrainage, requiring lifelong monitoring and potential revisions.
- Endoscopic Third Ventriculostomy (ETV): A minimally invasive neuroendoscopic procedure where a small opening is created in the floor of the third ventricle, allowing CSF to bypass an obstruction (typically at the level of the cerebral aqueduct) and flow directly into the basal subarachnoid cisterns for absorption. ETV avoids shunt hardware and its associated long-term complications. It is the preferred treatment for obstructive hydrocephalus due to aqueductal stenosis and is increasingly used for other causes. Success depends on the cause of hydrocephalus and patient age (less effective in infants <6-12 months). Sometimes combined with choroid plexus cauterization (CPC) to reduce CSF production, especially in infants [13].
![]() Attention! Symptoms suggesting increased intracranial pressure or hydrocephalus (severe headache, vomiting, vision changes, altered consciousness, gait issues) require urgent medical evaluation. Diagnosis and treatment depend critically on identifying the underlying cause and should be managed by neurological and neurosurgical specialists.
Attention! Symptoms suggesting increased intracranial pressure or hydrocephalus (severe headache, vomiting, vision changes, altered consciousness, gait issues) require urgent medical evaluation. Diagnosis and treatment depend critically on identifying the underlying cause and should be managed by neurological and neurosurgical specialists.
References
- Sakka L, Coll G, Chazal J. Anatomy and physiology of cerebrospinal fluid. Eur Ann Otorhinolaryngol Head Neck Dis. 2011 Dec;128(6):309-16. doi: 10.1016/j.anorl.2011.03.002
- Johanson CE, et al. Multiplicity of cerebrospinal fluid functions: New challenges in health and disease. Cerebrospinal Fluid Res. 2008;5:10. doi: 10.1186/1743-8454-5-10
- Iliff JJ, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci Transl Med. 2012;4(147):147ra111. doi: 10.1126/scitranslmed.3003748
- Saunders NR, et al. The rights and wrongs of the blood-brain barrier concept: a current view of cerebrospinal fluid systems. J Anat. 2014;225(1):31-42. doi: 10.1111/joa.12166
- Chapter 5: Confusion, Delirium, and Acute Encephalopathy. In: Ropper AH, Samuels MA, Klein JP, Prasad S. Adams and Victor's Principles of Neurology. 11th ed. McGraw Hill; 2019.
- Greenberg MS. Handbook of Neurosurgery. 9th ed. Thieme; 2020.
- Bradley WG Jr. Magnetic resonance imaging of the brain in the evaluation of hydrocephalus. Neurosurg Clin N Am. 2001;12(4):671-84.
- Brain Trauma Foundation; American Association of Neurological Surgeons; Congress of Neurological Surgeons; Joint Section on Neurotrauma and Critical Care, AANS/CNS, Bratton SL, Chestnut RM, Ghajar J, et al. Guidelines for the management of severe traumatic brain injury. VIII. Intracranial pressure thresholds. J Neurotrauma. 2007;24 Suppl 1:S55-8.
- Hoffman J, et al. MRI of idiopathic intracranial hypertension. AJR Am J Roentgenol. 2013;200(1):W1-W9. doi: 10.2214/AJR.12.8921
- Cook AM, et al. Guidelines for the Management of Severe Traumatic Brain Injury, 4th Ed. Neurocrit Care. 2019;31(Suppl 1):S1-S45.
- Sacco S, et al; European Stroke Organisation (ESO) Executive Committee; ESO Writing Committee. European Stroke Organisation (ESO) guidelines on the diagnosis and treatment of vertebrate dissection. Eur Stroke J. 2021;6(2):LXXXIX-CXLVII. doi: 10.1177/23969873211013823
- Rekate HL. The definition and classification of hydrocephalus: a personal recommendation to standardize terminology. Cerebrospinal Fluid Res. 2011;8:1. doi: 10.1186/1743-8454-8-1
- Warf BC. Comparison of endoscopic third ventriculostomy alone and combined with choroid plexus cauterization in infants younger than 1 year of age: a prospective study in 550 African children. J Neurosurg Pediatr. 2008;2(4):290-6. doi: 10.3171/PED.2008.2.10.290
- Plum F, Posner JB. The Diagnosis of Stupor and Coma. 3rd ed. FA Davis; 1980.
- McKee AC, et al. The spectrum of disease in chronic traumatic encephalopathy. Brain. 2013 Jan;136(Pt 1):43-64. doi: 10.1093/brain/aws307
- Pavlakis SG, et al. Mitochondrial myopathy, encephalopathy, lactic acidosis, and strokelike episodes: a distinctive clinical syndrome. Ann Neurol. 1984;16(4):481-8. doi: 10.1002/ana.410160409
- Vaughan CJ, Delanty N. Hypertensive emergencies. Lancet. 2000;356(9227):411-7. doi: 10.1016/S0140-6736(00)02539-3
- Hinchey J, et al. A reversible posterior leukoencephalopathy syndrome. N Engl J Med. 1996;334(8):494-500. doi: 10.1056/NEJM199602223340803
- Fugate JE, Rabinstein AA. Posterior reversible encephalopathy syndrome: clinical and radiological manifestations, pathophysiology, and outstanding questions. Lancet Neurol. 2015 Sep;14(9):914-25. doi: 10.1016/S1474-4422(15)00111-8
See also
- Anatomy of the nervous system
- Central nervous system infection:
- Brain abscess (lobar, cerebellar)
- Eosinophilic granuloma, Langerhans cell histiocytosis (LCH), Hennebert's symptom
- Epidural brain abscess
- Sinusitis-associated intracranial complications
- Otogenic intracranial complications
- Sinusitis-associated ophthalmic complications
- Bacterial otogenic meningitis
- Subdural brain abscess
- Sigmoid sinus suppurative thrombophlebitis
- Cerebral 3rd Ventricle Colloid Cyst
- Cerebral and spinal adhesive arachnoiditis
- Corticobasal Ganglionic Degeneration (Limited Brain Atrophy)
- Encephalopathy
- Headache, migraine
- Traumatic brain injury (concussion, contusion, brain hemorrhage, axonal shearing lesions)
- Increased intracranial pressure and hydrocephalus
- Parkinson's disease
- Pituitary microadenoma, macroadenoma and nonfunctioning adenomas (NFPAs), hyperprolactinemia syndrome
- Spontaneous cranial cerebrospinal fluid leak (CSF liquorrhea)