Spontaneous cranial cerebrospinal fluid leak (CSF liquorrhea)
- Spontaneous Cranial Cerebrospinal Fluid Leak (CSF Liquorrhea): Overview
- Spontaneous Cranial Cerebrospinal Fluid Leak (CSF Liquorrhea) Causes:
- Spontaneous Cranial Liquorrhea: Clinical Signs and Symptoms
- Spontaneous Cranial Liquorrhea: Diagnosis
- Spontaneous Cranial Liquorrhea: Treatment
- Recovery and Prognosis After Spontaneous Cranial Liquorrhea Surgical Treatment
- References
Spontaneous Cranial Cerebrospinal Fluid Leak (CSF Liquorrhea): Overview
Spontaneous cranial cerebrospinal fluid (CSF) leak, also referred to as spontaneous CSF liquorrhea, describes the leakage of CSF — the clear, protective fluid surrounding the brain and spinal cord—from the subarachnoid space through an abnormal opening in the skull base and its overlying meningeal layers (dura mater and arachnoid mater) into an adjacent cavity (like the nasal passages, paranasal sinuses, middle ear, or orbit) or externally. Critically, this occurs without any preceding history of significant head trauma, neurosurgery, radiation therapy, or destructive skull base tumor clearly causing the defect [1]. The leakage arises from a structural failure at the skull base interface.
The character of the leak can vary significantly; it may be persistent or intermittent, manifesting as a slow drip, a steady flow, or occasionally a sudden gush, particularly precipitated by activities that increase intracranial pressure, such as bending forward, straining, coughing, or the Valsalva maneuver. In some instances, the leak is occult (hidden), with CSF draining posteriorly into the nasopharynx where it is swallowed largely unnoticed by the patient (presenting potentially with headache or salty taste), or being contained within adjacent tissues like the middle ear or mastoid air cells without obvious external drainage until infection or hearing loss occurs.
Spontaneous CSF leaks account for a minority of all cranial CSF leaks (around 3-5% in some series, though estimates vary), with traumatic leaks being far more common overall [2]. However, among leaks occurring without trauma or surgery, spontaneous ones represent a significant and increasingly recognized proportion. They are typically categorized based on the site where the CSF exits the cranial cavity:
- Nasal CSF Leak (Rhinorrhea): CSF drains into the nasal cavity or paranasal sinuses, exiting through the nose or draining posteriorly into the nasopharynx. This is the most common presentation of spontaneous cranial leaks.
- Otic CSF Leak (Otorrhea): CSF drains into the middle ear cavity or mastoid air cells. It may exit externally through a perforation in the tympanic membrane (eardrum) as clear ear discharge, or drain via the Eustachian tube into the nasopharynx (presenting as rhinorrhea or post-nasal drip, sometimes termed CSF otorrhea-rhinorrhea).
- Orbital CSF Leak: CSF leaks directly into the orbit; this is exceedingly rare spontaneously.
From an etiological perspective, spontaneous CSF leaks are often further classified based on the suspected underlying mechanism:
- Primary (Idiopathic) Spontaneous CSF Leak: Occurs without any clearly identifiable underlying pathology (like tumor, hydrocephalus, prior meningitis) causing the skull base defect, after thorough investigation. These cases are very strongly associated with underlying idiopathic intracranial hypertension (IIH), even if the measured CSF pressure is normal at the time of diagnosis (as the leak itself can act as a 'release valve', lowering the pressure). Features of IIH (obesity, female predominance, specific headache patterns, imaging signs) are common in this group [3].
- Secondary Spontaneous CSF Leak: Results from an underlying condition that structurally weakens the skull base or dura, or significantly elevates intracranial pressure, leading to fistula formation without acute trauma or surgery. Causes include congenital skull base defects/malformations, obstructive hydrocephalus, certain benign tumors near the skull base causing pressure erosion (though leaks due to direct tumor destruction are usually not termed 'spontaneous'), bone dysplasias, or conditions associated with chronically elevated intracranial pressure other than IIH.
Causes of Spontaneous Cranial Cerebrospinal Fluid Leak (CSF Leak)
Spontaneous CSF leaks occur due to a combination of factors: usually a pre-existing or acquired focal weakness/defect in the skull base bone and overlying dura mater, coupled with a pressure gradient that drives CSF through that defect. While termed "spontaneous" because they lack an acute traumatic or surgical trigger, many cases, particularly those classified as primary/idiopathic, are strongly linked to sustained or intermittent elevated intracranial pressure (ICP). Idiopathic intracranial hypertension (IIH), formerly known as pseudotumor cerebri, is the most recognized association, even if the measured CSF opening pressure is found to be normal at the time of diagnosis (as the leak itself can effectively lower the pressure) [4].
Spontaneous CSF Rhinorrhea
For spontaneous nasal CSF leaks (rhinorrhea), the underlying dural and bony defect allowing CSF passage from the subarachnoid space (surrounding the frontal lobes or pituitary region) into the paranasal sinuses or nasal cavity is most commonly located in the anterior or central skull base. Common sites include [5, 6]:
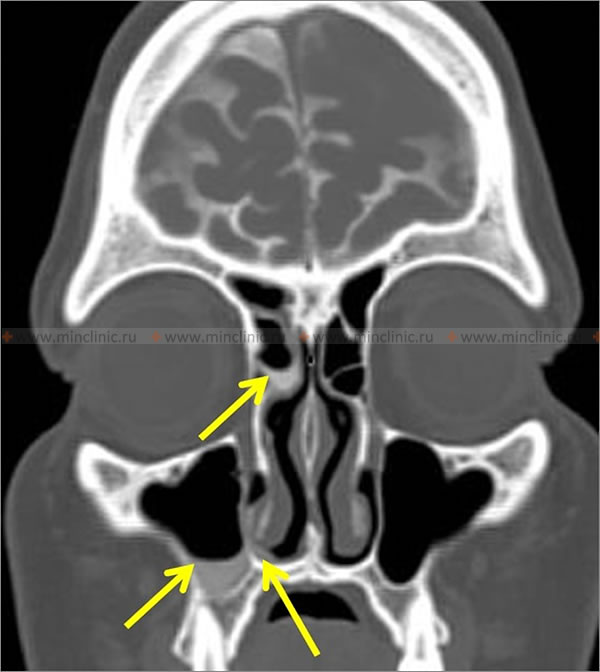
- Cribriform Plate / Ethmoid Roof (Fovea Ethmoidalis): Defects in the thin bone separating the anterior cranial fossa (overlying the olfactory bulbs/tracts) from the ethmoid sinuses or nasal cavity roof. This is a very common site, possibly due to inherent thinness or weaknesses around olfactory nerve rootlets.
- Sphenoid Sinus: Defects can occur through various parts of the sphenoid bone:
- Lateral Recess: A defect in a persistently pneumatized lateral recess of the sphenoid sinus (Sternberg's canal or lateral craniopharyngeal canal remnant) is a classic site, especially associated with underlying IIH.
- Planum Sphenoidale: The roof of the sphenoid sinus anteriorly.
- Sella Turcica Floor: Defects here can lead to CSF leak into the sphenoid sinus below the pituitary gland, often associated with empty sella syndrome and IIH.
- Clivus: Rarely, defects in the clivus (posterior aspect of sphenoid) can leak into the sphenoid sinus or nasopharynx.
- Frontal Sinus: Defects through the thin posterior wall of the frontal sinus, connecting the sinus lumen to the anterior cranial fossa.
- Petrous Temporal Bone (causing rhinorrhea): In rare instances, a defect in the medial temporal bone (e.g., petrous apex) allows CSF into the middle ear or Eustachian tube area, which then drains anteriorly into the nasopharynx, presenting clinically as rhinorrhea rather than otorrhea.
Spontaneous CSF Otorrhea
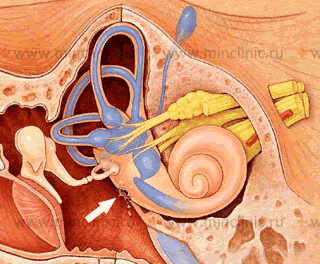
For spontaneous otic CSF leaks (otorrhea), where CSF exits via the ear canal (requiring a tympanic membrane perforation) or drains down the Eustachian tube (presenting as rhinorrhea or post-nasal drip), the defect involves communication between the subarachnoid space and the pneumatized spaces of the temporal bone (middle ear or mastoid air cells). The common locations for the underlying bony and dural defect include [7]:
- Tegmen Tympani or Tegmen Mastoideum: The thin bony roof separating the middle cranial fossa from the epitympanum (attic) and mastoid air cells, respectively. Defects here, often multiple small pits or erosions rather than a single large hole, are the most common cause of spontaneous CSF otorrhea/otorrhea-rhinorrhea in adults. These are frequently associated with herniation of brain tissue (encephalocele) or meninges (meningocele) through the defect(s). These defects are strongly linked to underlying IIH, thought to cause thinning and eventual breakdown of the tegmen over time.
- Posterior Cranial Fossa Plate: Defects in the bone overlying the sigmoid sinus or posterior fossa dura medial to the sinus can allow CSF leakage into the mastoid cavity.
- Petrous Apex / Other Temporal Bone Sites: Less commonly, defects in other areas like the petrous apex (potentially leaking into the sphenoid sinus or middle ear via Eustachian tube region) or near the internal auditory canal can be responsible.
- Inner Ear Malformations (especially in Children): Congenital defects involving the pathways between the inner ear and subarachnoid space (e.g., patent cochlear aqueduct, defects around oval/round windows, Mondini malformation) can lead to CSF leaking into the middle ear. These often present in childhood with recurrent meningitis and associated sensorineural hearing loss.
Predisposing factors and strongly associated conditions for spontaneous CSF leaks include:
- Idiopathic Intracranial Hypertension (IIH): This is considered the primary driver for many, if not most, primary spontaneous CSF leaks [4, 3]. Chronically elevated or intermittently high CSF pressure is thought to cause gradual erosion or remodeling of thin skull base bone at vulnerable sites, leading to dural tearing and leak formation. Many patients exhibit the typical IIH phenotype (obese females of childbearing age), report antecedent IIH-like headaches, or have objective signs of IIH on imaging (e.g., empty sella, dilated optic nerve sheaths, posterior globe flattening, transverse sinus stenosis) or upon CSF pressure measurement (if performed before leak significantly lowers pressure, or after repair).
- Obesity: A very strong risk factor, likely linked through its association with IIH.
- Obstructive Sleep Apnea (OSA): Increasingly recognized association with both IIH and spontaneous CSF leaks, possibly related to chronic intermittent hypoxia/hypercapnia affecting ICP regulation or venous pressure [8].
- Congenital Skull Base Defects/Weaknesses: Inherently thin areas (tegmen, cribriform plate), persistent embryological channels (e.g., Sternberg's canal), or minor developmental anomalies creating focal points susceptible to pressure erosion.
- Sinus Anatomy Variations: Extensive pneumatization (air cell development) of sinuses like the sphenoid or temporal bone (e.g., large mastoid air cell system) can create thinner bony partitions adjacent to the cranial base.
- Empty Sella Syndrome: An anatomical finding where the sella turcica appears empty or partially filled with CSF due to herniation of the subarachnoid space; often associated with IIH and potentially linked to sellar floor thinning/defects.
- Connective Tissue Disorders: Although rare, conditions causing dural weakness or ectasia (e.g., Marfan syndrome, Ehlers-Danlos syndrome) could theoretically predispose to spontaneous leaks.
The underlying pathophysiology often involves a "two-hit" process: a pre-existing structural vulnerability (thin bone, small defect) combined with a promoter (chronically elevated ICP) that leads to the eventual breakdown of the osteodural barrier and formation of a CSF fistula.
In adults, defects in the tegmen tympani/mastoideum are the most common cause of spontaneous CSF otorrhea, often associated with small meningoencephaloceles herniating through the bone defect(s), further supporting a role for increased ICP in their pathogenesis.
Spontaneous Orbital CSF Leak
Spontaneous CSF leak directly into the orbit, presenting as chemosis, proptosis, or periorbital swelling, is exceptionally rare. When it occurs, it is typically associated with defects in the orbital roof (floor of the anterior cranial fossa), often linked to chronic, erosive frontal sinusitis, large expanding mucoceles eroding through the orbital roof, or rarely, IIH causing defects in this location.
Overall, spontaneous cranial CSF leaks show a marked predilection for middle-aged (40s-50s), overweight or obese women, strongly reinforcing the link to the demographics and pathophysiology of idiopathic intracranial hypertension [4].
Clinical Signs and Symptoms of Spontaneous Cranial CSF Leaks
The clinical presentation of spontaneous CSF leaks varies depending on the site of the defect, the rate and volume of the leak, and the presence of associated intracranial pressure abnormalities (high or low).
- CSF Drainage (Liquorrhea): This is the hallmark symptom, though not always present or recognized.
- Rhinorrhea: Clear, watery, usually unilateral nasal discharge, often described as tasting salty or metallic. Typically increases when leaning forward, straining, or upon waking (pooling overnight). May be mistaken for allergies or rhinitis initially.
- Otorrhea: Clear, watery discharge from the ear canal. Requires a perforation or defect in the tympanic membrane to be visible externally. If the eardrum is intact, CSF may fill the middle ear (causing hearing loss, fullness) and drain via the Eustachian tube, presenting as rhinorrhea or post-nasal drip.
- Headache: Very common, but highly variable in character.
- Orthostatic Headache (Low Pressure Headache): Headache that develops or worsens significantly (often within minutes to an hour) upon assuming an upright posture (standing or sitting) and is relieved relatively quickly (often within 15-30 minutes) by lying flat. This pattern suggests intracranial hypotension caused by CSF volume depletion due to the ongoing leak [9]. Pain is often occipital or diffuse.
- Non-Orthostatic Headache (High or Normal Pressure Headache): Many patients with spontaneous leaks, especially those linked to underlying IIH, experience headaches that are not postural, or may even be worse when lying down (antiorthostatic). These headaches can be pulsatile, pressure-like, or resemble typical IIH or migraine headaches. Paradoxically, the onset of the leak may sometimes improve pre-existing high-pressure headaches, or the headache pattern may change over time.
- Associated Symptoms of Low ICP (Intracranial Hypotension): If the leak rate is significant enough to lower CSF volume/pressure, patients may experience nausea, vomiting, dizziness/lightheadedness, tinnitus (often low-frequency "roaring" or pulsatile), hearing changes (muffled hearing, hyperacusis), visual disturbances (blurred vision, diplopia from CN VI palsy), neck pain/stiffness (from meningeal irritation or sagging), fatigue, photophobia, phonophobia, and cognitive slowing ("brain fog") [9].
- Associated Symptoms/Signs of High ICP (Idiopathic Intracranial Hypertension): If underlying IIH is present and not fully decompressed by the leak, patients may exhibit headache (often worse in mornings, pulsatile, retrobulbar), pulsatile tinnitus ("whooshing" synchronous with pulse), transient visual obscurations (brief dimming/blackouts of vision, especially with position change), diplopia (CN VI palsy), and potentially papilledema on funduscopic examination.
- Neurological Signs (Less Common, often related to leak site or complications):
- Anosmia/Hyposmia: Loss or reduction of smell, often unilateral, strongly suggests a leak through the cribriform plate or anterior ethmoid roof region involving the olfactory apparatus.
- Visual Disturbances: Can result from high ICP (papilledema leading to field loss/atrophy), low ICP (sagging of optic nerves/chiasm), or direct involvement of the optic nerve/chiasm by the underlying skull base defect/pathology (e.g., encephalocele, inflammation near sphenoid).
- Hearing Loss (Otic Leaks): Conductive hearing loss is common with CSF filling the middle ear space (CSF tympanum). Sensorineural hearing loss may indicate involvement of the inner ear (e.g., labyrinthine fistula, associated malformation) or traction on the auditory nerve.
- Cranial Nerve Palsies: CN VI palsy (causing horizontal diplopia) is most common, usually related to raised ICP or sometimes low ICP (downward traction). Other cranial nerves can be affected by direct pressure from associated encephaloceles or skull base pathology.
- Complications:
- Meningitis: This is the most serious and potentially life-threatening complication. The skull base defect provides a direct pathway for bacteria (usually commensals from the sinonasal tract or middle ear like S. pneumoniae, H. influenzae) to enter the subarachnoid space, leading to bacterial meningitis. Spontaneous CSF leaks carry a significant lifetime risk of meningitis (estimated 10% per year if untreated in some studies, though variable) [10]. Recurrent episodes of bacterial meningitis (especially pneumococcal) should always prompt investigation for an underlying CSF leak, even if leakage is not obvious.
- Pneumocephalus: Air entering the cranial cavity through the defect, visible as intracranial air on imaging (CT scan is best). Can cause sudden severe headache or neurological symptoms if under tension.
- Local infections: Sinusitis or otitis media may develop secondary to CSF contamination or altered drainage.
Occult or low-volume leaks may present more subtly with only postural headaches, chronic "sinus" symptoms without infection, a persistent sensation of post-nasal drip, chronic cough, unexplained hearing loss, or recurrent meningitis without obvious external leakage.
![]() The significant risk of potentially fatal complications like bacterial meningitis underscores the critical need for prompt diagnosis and definitive treatment of all confirmed spontaneous cranial CSF leaks, regardless of symptom severity.
The significant risk of potentially fatal complications like bacterial meningitis underscores the critical need for prompt diagnosis and definitive treatment of all confirmed spontaneous cranial CSF leaks, regardless of symptom severity.
Spontaneous Cranial CSF Leak Diagnosis
Diagnosing a spontaneous CSF leak requires a two-step approach: 1) confirming that the draining fluid is indeed CSF, and 2) localizing the site of the skull base defect through which the CSF is leaking.
Differential Diagnosis of Clear Nasal Discharge (Rhinorrhea) or Ear Discharge (Otorrhea)
| Condition | Key Features / Distinguishing Points | Diagnostic Confirmation / Key Tests |
|---|---|---|
| CSF Leak (Rhinorrhea/Otorrhea) | Clear, watery, often unilateral discharge (may be bilateral). Increases with Valsalva/position change (leaning forward). Salty/metallic taste possible. +/- Headache (often positional), history suggestive of IIH (obesity, headaches), prior meningitis. Discharge typically non-viscous, does not stiffen handkerchief. | Beta-2 Transferrin assay on fluid sample = POSITIVE (Gold Standard). Glucose test unreliable. Imaging (HRCT Skull Base, MRI Brain, CT/MR Cisternography) needed to localize defect site. |
| Allergic Rhinitis | Clear, watery nasal discharge, usually bilateral. Associated nasal itching, sneezing, congestion. Often seasonal or related to specific allergen exposure (pollen, dust mites, pets). Often associated conjunctivitis. | Clinical history. Positive allergy testing (skin prick or serum IgE). Eosinophils may be present on nasal smear. Beta-2 Transferrin negative. |
| Vasomotor Rhinitis (Non-allergic rhinitis) | Clear, watery nasal discharge, significant congestion often prominent. Triggered by non-allergic factors (temperature/humidity changes, irritants like smoke/perfume, strong odors, eating - 'gustatory rhinitis'). Itching/sneezing usually absent or minimal. | Clinical diagnosis of exclusion. Negative allergy testing. Beta-2 Transferrin negative. Response to topical anticholinergics (ipratropium) can support diagnosis. |
| Viral Rhinitis (Common Cold / URI) | Nasal discharge initially clear/watery, often becomes thicker/mucoid/colored (yellow/green) after a few days. Associated sore throat, cough, malaise, +/- low-grade fever. Typically self-limited course (7-10 days). | Clinical diagnosis based on typical symptoms and course. Beta-2 Transferrin negative. |
| Serous Otitis Media (Otitis Media with Effusion - OME) | Fluid buildup (serous or mucoid) behind an intact eardrum. Causes hearing loss, ear fullness/pressure, "popping". No external ear drainage unless tympanostomy tubes are present or the eardrum perforates secondarily (rarely clear fluid). | Otoscopy shows effusion (air-fluid level, bubbles, retracted/dull TM). Tympanometry confirms middle ear fluid (Type B curve). Fluid itself is Beta-2 Transferrin negative (unless there is a coincident CSF leak into the middle ear). |
| Watery Eye Discharge (Epiphora / Excessive Tearing) | Excess tears flowing onto face or draining into nose via nasolacrimal duct (can mimic unilateral rhinorrhea). Caused by tear overproduction (ocular surface irritation, dry eye reflex) or blocked nasolacrimal drainage system. | Ophthalmologic exam identifies ocular source. Dye disappearance test, lacrimal duct probing/irrigation can confirm blockage. Fluid collected from eye/face is tears, Beta-2 Transferrin negative. |
| Perilymphatic Fistula (PLF) | Leak of inner ear fluid (perilymph) into middle ear, usually at oval or round window, often post-traumatic, post-stapes surgery, or due to barotrauma. Causes fluctuating/progressive sensorineural hearing loss, tinnitus, episodic vertigo/imbalance often triggered by pressure changes (Valsalva, straining, loud sounds - Tullio phenomenon). External otorrhea is rare unless TM perforated. | History suggestive. Audiometry shows SNHL. Vestibular testing may be abnormal. Fistula test (nystagmus with tragal pressure) may be positive. Fluid analysis (if collectible) for Beta-2 Transferrin could theoretically be positive (perilymph contains it), but rarely feasible. Often requires exploratory tympanotomy for definitive diagnosis/repair. |
Diagnosing a spontaneous CSF leak involves confirming the nature of the fluid and then precisely localizing the site of the skull base defect.
- Confirmation of CSF Leak:
- Clinical Assessment: History of clear, watery, often unilateral rhinorrhea or otorrhea, particularly positional (worse leaning forward) or increasing with Valsalva, is highly suggestive. Patient description of a salty or metallic taste (rhinorrhea) is also indicative. Observation of clear fluid dripping from nose/ear can be helpful. The "halo sign" or "double ring sign" (fluid dropped on filter paper separates into a central blood spot surrounded by a clearer CSF ring if blood-tinged) is historically mentioned but has low sensitivity/specificity. Testing fluid glucose content with bedside glucometer strips is unreliable and not recommended (nasal secretions/tears contain glucose).
- Biochemical Analysis (Laboratory Test): Beta-2 Transferrin Assay is the gold standard test for confirming CSF [11]. Beta-2 transferrin is an isoform of transferrin found almost exclusively in CSF, perilymph, and vitreous humor. Detecting it in nasal or ear discharge using sensitive methods like immunofixation or nephelometry definitively confirms the presence of CSF. Requires collecting a sufficient sample (at least 0.5-1 mL) of the suspected fluid, which can be challenging with intermittent leaks (patients may need to collect fluid when it occurs).
- Beta-Trace Protein (Prostaglandin D Synthase) Assay: Another highly specific CSF marker, used as an alternative or adjunct to beta-2 transferrin in some centers [12].
- Localization of the Leak Site: Once a CSF leak is biochemically confirmed, identifying the exact anatomical location of the dural and bony defect is crucial for planning targeted treatment, especially surgical repair. Imaging techniques are key:
- High-Resolution Computed Tomography (HRCT) of the Skull Base and/or Temporal Bones: Thin-slice (≤1mm) axial and coronal CT scans without contrast provide excellent visualization of bony anatomy. Essential for identifying bony defects (gaps, thinning, erosion) in the skull base (cribriform plate, ethmoid roof/fovea ethmoidalis, planum sphenoidale, sella floor, tegmen tympani/mastoideum, petrous apex). May also show associated findings like sinus opacification or meningo/encephalocele herniating through defect. While HRCT shows the potential site of leak (bone defect), it doesn't directly visualize the CSF leak itself unless combined with cisternography.
- CT Cisternography: Involves performing a lumbar puncture to inject non-ionic iodinated contrast material into the intrathecal CSF space, followed by high-resolution CT scanning of the skull base, often with specific patient positioning (e.g., prone, head-down) to encourage leakage and pooling of contrast. Direct visualization of contrast pooling outside the intracranial space adjacent to a bony defect confirms the active leak site. Considered highly specific and useful for active leaks, though sensitivity varies (reported ~50-90%) and depends on leak activity during scan [13]. Invasive due to LP.
- Magnetic Resonance Imaging (MRI): Standard brain MRI is important for evaluating associated findings like signs of IIH (empty sella, dilated optic nerve sheaths, posterior globe flattening, transverse sinus stenosis), hydrocephalus, encephaloceles/meningoceles (brain/meninges herniating through defect), and ruling out underlying tumors. Specific MRI techniques are used for leak localization:
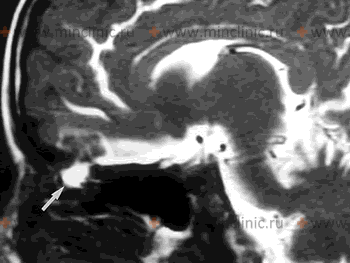
- High-Resolution T2-Weighted Sequences (MR Cisternography): Sequences like CISS (Constructive Interference in Steady State) or FIESTA (Fast Imaging Employing Steady-state Acquisition) provide high spatial resolution and bright signal from CSF. Can directly visualize CSF signal intensity extending through a skull base defect or pooling abnormally in adjacent sinuses/nasal cavity/mastoid [14]. Non-invasive alternative to CT cisternography, sensitivity reported around 80-90% in some studies.
- Contrast-Enhanced MRI (after Intrathecal Gadolinium): Similar principle to CT cisternography but uses MRI after intrathecal injection of gadolinium contrast (diluted). Intrathecal gadolinium is an off-label use in many regions and carries potential risks (neurotoxicity, arachnoiditis); typically reserved for complex or difficult cases where other methods have failed to localize the leak [15].
- Standard MRI sequences (T1, T2, FLAIR) can sometimes show indirect signs like fluid collections in sinuses/mastoid or brain/meningeal herniation into a defect.
- Radionuclide (Isotope) Cisternography: Involves injecting a radioactive tracer (e.g., Indium-111 DTPA) into the CSF via LP. Absorbent cotton pledgets are placed strategically in different areas of the nasal cavity (e.g., near sphenoethmoidal recess, middle meatus, olfactory cleft). Pledgets are removed after several hours and scanned for radioactivity. Detecting radioactivity significantly higher than background/serum levels on specific pledgets confirms a leak and can help broadly localize it (anterior vs. posterior skull base), but precise anatomical localization is poor. Less commonly used now due to lower spatial resolution compared to CT/MR cisternography.
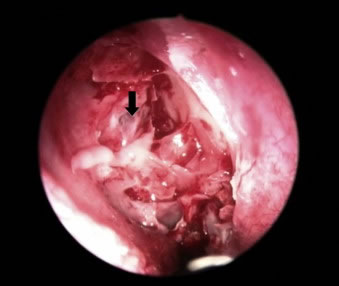
- Intrathecal Fluorescein: Involves injecting sterile, preservative-free fluorescein dye (diluted) into the CSF via LP, usually 30-60 minutes before surgery. During subsequent nasal endoscopy (or ear surgery) performed under blue light illumination, the surgeon looks for the characteristic bright yellowish-green fluorescence of CSF leaking from the defect site. Highly sensitive for intraoperative localization but carries small risks (seizures, neurological deficits, allergic reaction) and requires careful dosing and expertise. Primarily used during surgery, not typically as a pre-operative diagnostic localization tool alone [16].
- Nasal Endoscopy: Performed by an otolaryngologist. Can sometimes directly visualize CSF pooling or dripping from specific regions (sphenoethmoidal recess, olfactory cleft) especially if the leak is active. May also identify associated polyps, inflammation, or encephaloceles. Visualization can be enhanced by asking patient to lean forward or perform Valsalva.
Brain magnetic resonance imaging (MRI) and specialized imaging techniques like CT or MR cisternography are key diagnostic tools used to identify and precisely localize the site of skull base defects responsible for spontaneous cranial CSF leaks.
The optimal diagnostic strategy often involves combining high-resolution imaging modalities. HRCT excels at defining the bony defect, while high-resolution T2 MRI sequences (MR Cisternography) are excellent for visualizing CSF pathways and potential leaks non-invasively. CT Cisternography remains valuable for confirming active leaks when other methods are inconclusive. The choice depends on clinical suspicion, leak activity, local expertise, and availability.
It is crucial to remember that a history of recurrent bacterial meningitis (especially pneumococcal) in an adult should always trigger a thorough investigation for an underlying occult CSF leak, even if active leakage is not currently observed or reported by the patient.
Spontaneous Cranial Liquorrhea Treatment
Treatment is strongly recommended for virtually all confirmed spontaneous cranial CSF leaks due to the significant and ongoing risk of bacterial meningitis, which can be life-threatening or cause severe neurological sequelae. The approach involves addressing both the anatomical defect and, often, the underlying pressure abnormality (especially IIH).
- Conservative Management: Unlike traumatic CSF leaks where conservative measures (bed rest, head elevation, avoiding straining) have a reasonable success rate, spontaneous leaks are much less likely to resolve without intervention, particularly those associated with underlying IIH [1]. A brief trial might be considered in select cases with minimal leakage or low suspicion of high ICP, but persistent leaks require definitive repair. Prophylactic antibiotics are generally not recommended during observation as they do not prevent meningitis and may select for resistant organisms; antibiotics are reserved for treating established meningitis.
- Management of Intracranial Pressure (if Elevated/IIH suspected): This is a crucial component, especially for primary spontaneous leaks, as failure to address underlying high ICP significantly increases the risk of surgical repair failure or recurrence at the same or different site [3, 4]. Management strategies include:
- Weight loss: Strongly encouraged for obese patients, as it can effectively lower ICP in IIH. Bariatric surgery may be considered in refractory cases.
- Medications to lower ICP: Acetazolamide (carbonic anhydrase inhibitor reducing CSF production) is the mainstay medical therapy for IIH. Topiramate may also be used (weaker evidence, multiple mechanisms including CA inhibition).
- CSF Shunting: Placement of a permanent CSF diversionary shunt (typically ventriculoperitoneal [VP] or lumboperitoneal [LP] shunt) may be necessary to manage refractory IIH, either before, during, or after skull base repair, particularly if ICP remains elevated despite medical therapy and weight loss, or if leak repair fails due to pressure [17]. Shunting alone without defect repair is usually insufficient for spontaneous leaks.
- Surgical Repair of the Defect: The definitive treatment for sealing the leak. The goal is meticulous identification of the dural and bony defect(s) and creation of a durable, watertight seal. The surgical approach depends primarily on the location and size of the defect:
- Endoscopic Endonasal Approach: Now considered the preferred method for most spontaneous leaks originating from the anterior skull base (cribriform plate, ethmoid roof/fovea, planum sphenoidale, sella, clivus, medial sphenoid sinus) [18, 2]. Performed entirely through the nostrils using endoscopes and specialized long instruments. Allows excellent visualization of the skull base, precise defect identification, and minimally invasive repair. Involves removing overlying mucosa, defining bone edges, and placing multiple layers of repair materials (e.g., underlay graft like fascia lata or Alloderm®, overlay graft, sometimes bone/cartilage support, often covered with a vascularized pedicled mucosal flap like the nasoseptal flap for robust closure). Success rates for endoscopic repair are high (typically >90% for primary repair) with significantly less morbidity (e.g., avoids brain retraction, lower risk of anosmia) compared to traditional transcranial approaches.
- Transcranial Approach (Open Craniotomy): Required for leaks inaccessible endoscopically (e.g., far lateral frontal sinus defects, some extensive tegmen defects, complex defects involving critical vascular structures) or sometimes for failed endoscopic repairs. Approaches vary by location (e.g., bifrontal craniotomy for anterior fossa, pterional for middle fossa, subtemporal for tegmen). Allows wide exposure for direct dural repair, often using pericranial flaps, fascia lata grafts, or synthetic dural substitutes, sometimes combined with bone grafting. Carries higher morbidity associated with craniotomy, including risk of anosmia (especially bifrontal), frontal lobe retraction injury, seizures, cosmetic issues, and longer recovery time.
- Transmastoid Approach: Used specifically for otic CSF leaks originating from defects in the tegmen mastoideum or posterior fossa dura accessible via the mastoid cavity. Involves performing a mastoidectomy to expose the defect and repairing it from below using materials like bone wax, bone pâté, fascia, muscle plugs, cartilage grafts, or synthetic bone cements. Often suitable for small to moderate sized tegmen defects.
- Middle Cranial Fossa Approach: A specific transcranial approach providing wide exposure of the middle fossa floor (tegmen tympani and mastoideum). Often preferred for multiple or extensive temporal bone defects, large associated encephaloceles, or defects involving the petrous apex, allowing repair from above under direct vision [7].
- Lumbar Drain Placement: Temporary drainage of CSF via a lumbar catheter placed before or during surgery can reduce CSF pressure on the repair site, potentially improving healing. Its routine use is debated; often employed adjunctively for 2-5 days postoperatively, particularly after transcranial repairs, complex endoscopic repairs, or if high ICP is a concern [20]. Requires careful monitoring to avoid overdrainage.
The optimal strategy often involves addressing both the leak site (surgical repair) and the underlying pressure issue (medical/surgical ICP management if IIH is present).
Recovery and Prognosis After Spontaneous Cranial CSF Leak Surgical Treatment
Surgical repair of spontaneous CSF leaks, particularly utilizing modern multilayered endoscopic transnasal techniques for anterior/central skull base defects or appropriate transmastoid/middle fossa approaches for temporal bone defects, generally has high primary success rates, often reported between 90-98 percent in experienced centers [18, 7]. Recovery time is typically shorter, and morbidity lower, after endoscopic procedures compared to open transcranial surgery.
However, failure of the initial repair or recurrence of the leak can occur, necessitating further intervention. Factors contributing to unsuccessful outcomes or recurrence include [21, 22]:
- Inaccurate Preoperative Localization: Failure to precisely identify all defect site(s) before surgery.
- Multiple Defects: The presence of multiple, sometimes subtle, defects, particularly common in spontaneous leaks associated with IIH affecting the tegmen or cribriform region. Missing one defect during repair leads to persistent leakage.
- Large Defects: Defects exceeding 1.5-2.0 cm in diameter may be more challenging to seal durably, often requiring vascularized flap reconstruction (e.g., nasoseptal flap).
- Specific Locations: Defects in technically challenging areas like the far lateral sphenoid recess or complex frontal sinus defects may have higher failure rates.
- Graft Failure: Displacement, inadequate placement, necrosis, or poor integration/healing of the repair materials.
- Persistent Elevated Intracranial Pressure (ICP): Failure to diagnose and effectively manage underlying IIH (with weight loss, medication, or shunting) is considered a major cause of both early repair failure and late recurrence, as sustained high pressure stresses the repair site or can cause new leaks to develop at other inherently weak points in the skull base [3, 4].
- Postoperative Factors: Early strenuous activity, coughing/sneezing, meningitis, or local wound infection can compromise the repair.
- Patient Factors: Poor wound healing related to comorbidities like diabetes, smoking, prior radiation therapy.
Recurrence rates for spontaneous CSF leaks are generally considered higher than for traumatic leaks, likely reflecting the frequent underlying pathophysiology involving IIH and potential for multiple or progressive defects. Reported recurrence rates after primary endonasal repair range roughly from 2-15 percent in various series, depending on technique, defect size/location, management of ICP, and length of follow-up [2]. Rates after primary transcranial repair may be similar or slightly higher historically. Recurrent leaks typically require careful re-evaluation to confirm the site (which may be the original site or a new location) and revision surgery, often utilizing a different or reinforced surgical approach, combined with aggressive investigation and management of any underlying intracranial hypertension.
Long-term follow-up with clinical assessment for symptoms of leak or raised ICP, and sometimes periodic imaging, is necessary to monitor for recurrence. Successful and durable repair significantly reduces the lifetime risk of meningitis. Prognosis regarding associated neurological symptoms depends on whether permanent damage occurred prior to treatment (e.g., irreversible vision loss from chronic papilledema, permanent hearing loss from otic leaks or associated meningitis/labyrinthitis).
![]() Attention! Spontaneous CSF leaks require specialized diagnosis and treatment due to the high risk of meningitis. Persistent clear nasal discharge, especially unilateral or positional, unexplained recurrent meningitis, or positional headaches should prompt medical evaluation. Management involves confirming the leak, localizing the defect, surgical repair, and often addressing underlying intracranial pressure issues.
Attention! Spontaneous CSF leaks require specialized diagnosis and treatment due to the high risk of meningitis. Persistent clear nasal discharge, especially unilateral or positional, unexplained recurrent meningitis, or positional headaches should prompt medical evaluation. Management involves confirming the leak, localizing the defect, surgical repair, and often addressing underlying intracranial pressure issues.
References
- Schlosser RJ, Woodworth BA. Spontaneous CSF leaks: a paradigm for definitive repair and management of intracranial pressure. Otolaryngol Head Neck Surg. 2006 Nov;135(5):821-7. doi: 10.1016/j.otohns.2006.08.006
- Psaltis AJ, et al. Management of spontaneous cerebrospinal fluid leaks. Otolaryngol Clin North Am. 2012;45(5):1027-40. doi: 10.1016/j.otc.2012.06.006
- Woodworth BA, et al. Spontaneous CSF leaks: a paradigm for definitive repair and management of intracranial pressure. Otolaryngol Head Neck Surg. 2008;138(6):715-20. doi: 10.1016/j.otohns.2008.02.010
- Perez MA, et al. Spontaneous cerebrospinal fluid leaks and idiopathic intracranial hypertension: a systematic review. Int Forum Allergy Rhinol. 2021;11(11):1549-1557. doi: 10.1002/alr.22826
- Lobo BC, et al. Surgical management of spontaneous cerebrospinal fluid rhinorrhea. Otolaryngol Head Neck Surg. 2004;130(4):457-62. doi: 10.1016/j.otohns.2003.09.028
- Schlosser RJ, et al. Spontaneous spinal cerebrospinal fluid leaks and intracranial hypotension. J Neurol Neurosurg Psychiatry. 2006;77(3):358-63. doi: 10.1136/jnnp.2005.069868
- Kutz JW Jr, et al. Spontaneous cerebrospinal fluid otorrhea. Otolaryngol Clin North Am. 2011;44(2):333-44, vii-viii. doi: 10.1016/j.otc.2011.01.008
- Sugerman HJ. Increased intra-abdominal pressure and idiopathic intracranial hypertension. Obes Surg. 2007;17(7):871-4. doi: 10.1007/s11695-007-9160-8
- Schievink WI. Spontaneous spinal cerebrospinal fluid leaks and intracranial hypotension. JAMA. 2006 May 17;295(19):2286-96. doi: 10.1001/jama.295.19.2286
- Prokopakis EP, et al. Spontaneous cerebrospinal fluid rhinorrhea: pathophysiology and management. Curr Opin Otolaryngol Head Neck Surg. 2005;13(1):17-23. doi: 10.1097/01.moo.0000151475.79250.45
- Warnecke A, et al. Diagnostic relevance of beta-2 transferrin for the detection of cerebrospinal fluid fistulas. Arch Otolaryngol Head Neck Surg. 2004;130(10):1178-84. doi: 10.1001/archotol.130.10.1178
- Bachmann-Harildstad G. Diagnostic values of beta-2 transferrin and beta-trace protein for detection of cerebrospinal fluid fistula. Rhinology. 2008;46(2):82-5.
- Stone JA, et al. Evaluation of suspected cerebrospinal fluid rhinorrhea: a new algorithm incorporating high-resolution CT cisternography. AJNR Am J Neuroradiol. 2003;24(5):998-1001.
- Zapala DA, et al. Detection of CSF leaks with MR cisternography. Otolaryngol Head Neck Surg. 2008;138(5):565-9. doi: 10.1016/j.otohns.2008.01.017
- Alijani B, et al. Intrathecal Gadolinium-Enhanced MR Cisternography for Detection of Suspected CSF Rhinorrhea. AJNR Am J Neuroradiol. 2016;37(8):1553-8. doi: 10.3174/ajnr.A4749
- Seth R, et al. Outcomes of endoscopic skull base surgery for cerebrospinal fluid leaks. Otolaryngol Head Neck Surg. 2010;143(3):388-93. doi: 10.1016/j.otohns.2010.05.019
- Friedman DI, et al. Idiopathic intracranial hypertension. Lancet Neurol. 2021;20(5):388-401. doi: 10.1016/S1474-4422(21)00021-X
- Harvey RJ, et al. Endoscopic skull base reconstruction of large dural defects: a systematic review. J Neurosurg. 2010;112(5):1176-85. doi: 10.3171/2009.7.JNS09570
- Hadad G, et al. A novel reconstructive technique after endoscopic expanded endonasal approaches: vascular pedicle nasoseptal flap. Laryngoscope. 2006;116(10):1882-6. doi: 10.1097/01.mlg.0000239591.04319.e2
- Abu-Seida MA, et al. Role of lumbar drain in reducing the recurrence rate of cerebrospinal fluid rhinorrhea following endoscopic endonasal repair. Eur Arch Otorhinolaryngol. 2019;276(3):741-746. doi: 10.1007/s00405-019-05308-x
- Zwagerman NT, et al. Predictors of Cerebrospinal Fluid Leak and Meningitis After Endoscopic Skull Base Surgery. Otolaryngol Head Neck Surg. 2016;154(4):760-5. doi: 10.1177/0194599815626213
- Illing E, et al. Predictors of failure in endoscopic skull base reconstruction. Laryngoscope. 2017;127(1):58-65. doi: 10.1002/lary.26096
See also
- Anatomy of the nervous system
- Central nervous system infection:
- Brain abscess (lobar, cerebellar)
- Eosinophilic granuloma, Langerhans cell histiocytosis (LCH), Hennebert's symptom
- Epidural brain abscess
- Sinusitis-associated intracranial complications
- Otogenic intracranial complications
- Sinusitis-associated ophthalmic complications
- Bacterial otogenic meningitis
- Subdural brain abscess
- Sigmoid sinus suppurative thrombophlebitis
- Cerebral 3rd Ventricle Colloid Cyst
- Cerebral and spinal adhesive arachnoiditis
- Corticobasal Ganglionic Degeneration (Limited Brain Atrophy)
- Encephalopathy
- Headache, migraine
- Traumatic brain injury (concussion, contusion, brain hemorrhage, axonal shearing lesions)
- Increased intracranial pressure and hydrocephalus
- Parkinson's disease
- Pituitary microadenoma, macroadenoma and nonfunctioning adenomas (NFPAs), hyperprolactinemia syndrome
- Spontaneous cranial cerebrospinal fluid leak (CSF liquorrhea)

 rhinorrhea.jpg)