Traumatic Brain Injury: Symptoms, Diagnosis, and Treatment
Types and Causes of Traumatic Brain Injury
Traumatic brain injury (TBI) encompasses a range of injuries resulting from external mechanical force, potentially leading to temporary or permanent impairment of cognitive, physical, and psychosocial functions. Common symptoms include headaches, confusion, dizziness, and memory loss. According to the CDC, mild TBI (often synonymous with concussion) affects over 1.5 million Americans annually (1). Severe TBIs can cause prolonged unconsciousness (coma), significant disability, or death if not promptly and appropriately managed. Initial diagnostic imaging often involves CT scans to detect acute bleeding or fractures, as outlined by neurotrauma guidelines (2).
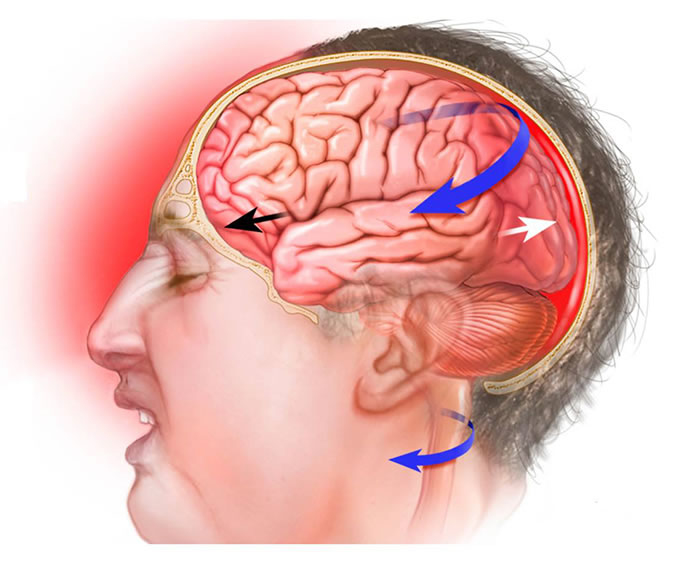
Ongoing research clarifies the complex cognitive and physiological consequences of TBI, including concussions. A concussion results from a blow to the head or body causing forces that make the brain move rapidly within the skull. Normally, the cerebrospinal fluid (CSF) acts as a cushion. However, strong impacts (common in falls, motor vehicle accidents, sports collisions, or assaults) can overwhelm this protection, causing the brain to strike the skull's inner surface. This can lead to focal injuries like contusions (bruising) and hemorrhages (bleeding), or diffuse injuries like axonal shearing. Subsequent inflammation is also a key component of the injury response (3).
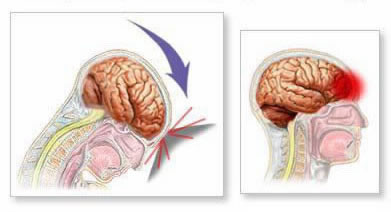
Sudden deceleration or acceleration causes the brain to impact the skull (coup injury) and then rebound against the opposite side (contrecoup injury). This mechanism can cause contusions at both impact sites and contributes to diffuse axonal injury (DAI) through shearing forces on nerve fibers. These injuries often trigger cerebral edema (swelling). Within the rigid confines of the skull, edema increases intracranial pressure (ICP), which can compress brain tissue and impede cerebral blood flow, potentially causing secondary ischemic damage.
TBIs are classified based on severity (mild, moderate, severe – often using the Glasgow Coma Scale), mechanism (closed vs. penetrating), and pathology. Key injury types include:
- Concussion: A mild TBI involving transient functional disturbance.
- Contusion: Bruising of brain tissue, often at coup/contrecoup sites.
- Hematomas (Blood Clots):
- Epidural Hematoma: Bleeding between the dura mater and skull, often arterial, can expand rapidly.
- Subdural Hematoma: Bleeding between the dura mater and arachnoid mater, often venous, can be acute or chronic.
- Intracerebral Hemorrhage: Bleeding within the brain tissue itself.
- Subarachnoid Hemorrhage: Bleeding into the space containing CSF.
- Diffuse Axonal Injury (DAI): Widespread shearing of nerve fibers due to rotational or acceleration/deceleration forces; often leads to severe, long-term deficits.
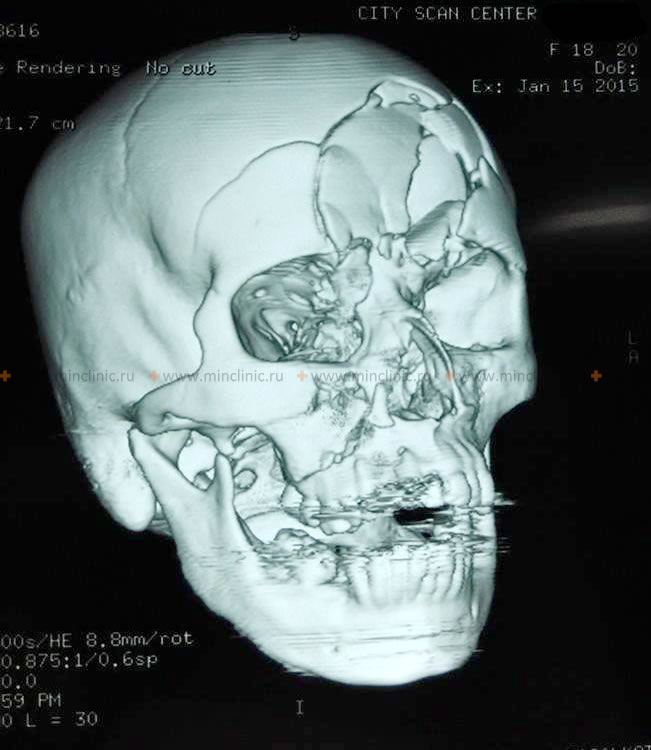
- Skull Fractures: Linear, depressed, comminuted, or basilar fractures. Open fractures communicate with the outside environment, increasing infection risk.
- Cranial Nerve Injuries: Damage to nerves exiting the brainstem due to direct trauma, stretching, or fracture involvement.
- Post-traumatic Seizures: Can occur early (within 7 days) or late (after 7 days).
Accurate diagnosis by physicians, often involving neurologists and neurosurgeons, is crucial for guiding appropriate medical management (e.g., controlling ICP, preventing seizures) or surgical intervention (e.g., hematoma evacuation, fracture repair). Specialized rehabilitation services, including physical, occupational, and cognitive therapy, are essential for recovery after TBI or spinal cord injury.
A concussion, representing the mild end of the TBI spectrum, involves temporary neurological dysfunction triggered by head impact or deceleration. Key signs and symptoms often include:
- Loss of consciousness (brief or none)
- Altered mental state (feeling dazed, confused, "foggy")
- Amnesia surrounding the event (anterograde - difficulty forming new memories; retrograde - loss of memory before the event)
- Nausea and/or vomiting
- Headache
- Dizziness or balance problems
- Visual disturbances (blurred vision, sensitivity to light)
- Nystagmus (involuntary eye movements, less common in simple concussion)
A brain contusion, being a structural injury (bruise), is more severe than a concussion and may accompany skull fractures. Fractures, particularly at the base of the skull (basilar fractures), can tear the dura mater, leading to leakage of cerebrospinal fluid (CSF) from the ear (CSF otorrhea) or nose (CSF rhinorrhea), identifiable by its clear appearance and sometimes a "halo" sign on gauze.
Brain injuries can directly damage cranial nerves via mechanical forces (stretching, tearing, compression) or indirectly via entrapment or injury from adjacent bone fragments.
Because of their anatomical course through bony foramina at the skull base, certain cranial nerves are more susceptible to TBI-related damage, including the olfactory (I), optic (II), oculomotor (III), trochlear (IV), trigeminal (V), abducens (VI), and facial (VII) nerves.
Diagnosing Neurologic Symptoms of Traumatic Brain Injury
The diagnostic process for TBI begins with a thorough clinical evaluation, including history of the injury mechanism and a detailed neurological examination (assessing mental status, cranial nerves, motor function, sensory function, reflexes, coordination, and gait). Skull X-rays may be used for initial screening, especially in resource-limited settings, but Computed Tomography (CT) is the primary imaging modality in the acute setting due to its speed and sensitivity for detecting skull fractures, acute hemorrhages (epidural, subdural, intracerebral, subarachnoid), and brain swelling (5). Brain MRI is more sensitive for detecting subtle contusions, diffuse axonal injury (DAI), and injuries to the brainstem and posterior fossa, and is often used later in the diagnostic process or for patients with persistent symptoms despite a normal CT scan (6). Patients with uncomplicated concussions (normal neurological exam, brief symptoms, normal CT if performed) usually do not require MRI acutely.
Lumbar puncture (LP) to analyze cerebrospinal fluid (CSF) has a limited role in acute TBI diagnosis. While it can detect subarachnoid hemorrhage (presence of blood) or signs of infection/inflammation (meningitis) as a later complication, it is strictly contraindicated if there is suspicion of elevated intracranial pressure (ICP), as indicated by clinical signs or imaging findings (e.g., mass effect, midline shift, effaced cisterns). Performing an LP in the presence of high ICP can precipitate life-threatening brain herniation (7).
Differential Diagnosis of Altered Mental Status/Neurologic Symptoms After Potential Head Trauma
| Condition | Key Features / Distinguishing Points | Typical Findings / Investigations |
|---|---|---|
| Traumatic Brain Injury (TBI) | Clear history of head trauma. Symptoms range from confusion, LOC, amnesia, headache (mild TBI) to focal deficits, coma (severe TBI). External signs of trauma may be present. | Neurological exam deficits (variable). CT/MRI may show hemorrhage, contusion, fracture, DAI, edema. GCS score reflects severity. |
| Stroke (Ischemic/Hemorrhagic) | Sudden onset of focal neurological deficits (weakness, sensory loss, aphasia). May or may not have headache or altered consciousness. History of trauma usually absent unless stroke caused fall. Vascular risk factors often present. | Focal neurological deficits. CT/MRI confirms stroke type (ischemia or hemorrhage) and location. CTA/MRA for vessel imaging. |
| Seizure | Abrupt onset, may involve motor activity (convulsions), altered awareness, sensory changes. Often followed by post-ictal confusion/drowsiness. Can be triggered by TBI (post-traumatic seizure). | Witness description crucial. Post-ictal state. EEG may show epileptiform activity. Brain imaging to rule out structural cause. |
| Syncope | Brief loss of consciousness due to transient global cerebral hypoperfusion. Often preceded by prodrome (lightheadedness, nausea). Rapid recovery upon becoming supine. Trauma may result *from* syncope. | History of prodrome, brief LOC, rapid recovery. Cardiac evaluation (ECG, Holter), orthostatic vital signs. Neurological exam usually normal post-event. |
| Intoxication (Alcohol/Drugs) | Altered mental status, confusion, slurred speech, ataxia. Odor of alcohol, drug paraphernalia. Trauma may co-exist. (Note: See also Alcoholic Neuropathy for chronic effects) | Clinical signs. Blood alcohol level, urine/serum toxicology screen. *Must still rule out concurrent TBI if trauma suspected.* |
| Metabolic Encephalopathy | Fluctuating level of consciousness, confusion, delirium. Often global neurological signs (asterixis). Caused by hypoglycemia, hypoxia, electrolyte imbalance, organ failure (liver/kidney). | Blood glucose, electrolytes, arterial blood gas, liver/renal function tests. Underlying cause identified by labs. |
| Migraine (Complicated/Aura) | Can present with transient neurological deficits (visual changes, sensory symptoms, weakness - hemiplegic migraine) preceding or during headache. Usually history of similar episodes. | Characteristic headache pattern. Neurological exam normal between attacks. Imaging usually normal (to rule out other causes). |
| Infection (Meningitis/Encephalitis) | Headache, fever, altered mental status, neck stiffness (meningismus). May have seizures or focal deficits. | Fever, nuchal rigidity. Lumbar puncture (if safe) shows CSF pleocytosis, altered glucose/protein. Brain imaging may show inflammation/abscess. |
| Subarachnoid Hemorrhage (SAH, non-traumatic) | Sudden, severe "thunderclap" headache. Often altered consciousness, nausea/vomiting, neck stiffness. Usually due to aneurysm rupture. | CT scan shows blood in subarachnoid space. LP (if CT negative but suspicion high) shows blood/xanthochromia. CTA/DSA identifies aneurysm. |
Clinical signs and symptoms evident immediately or shortly after TBI vary widely depending on the type and severity of injury. Common findings include:
- External signs of trauma: Scalp lacerations, bruising (including periorbital ecchymosis - "raccoon eyes," or mastoid ecchymosis - "Battle's sign," suggestive of basilar skull fracture), abrasions, swelling.
- Skull deformities or palpable fractures.
- CSF leakage from nose (rhinorrhea) or ears (otorrhea).
- Signs of meningeal irritation: Neck stiffness (nuchal rigidity).
- Alterations in consciousness: Ranging from brief confusion to deep coma (assessed by GCS).
- Pupillary abnormalities: Size, equality, reactivity to light (can indicate brainstem compression or cranial nerve injury).
- Focal neurological deficits: Weakness or paralysis (hemiparesis/hemiplegia), sensory loss, speech difficulties (aphasia/dysarthria), visual field defects.
A comprehensive neurological examination helps pinpoint the location and extent of brain injury, guiding further diagnostic steps and treatment decisions. Electroencephalography (EEG) may be used later to detect seizure activity or evaluate the degree of diffuse brain dysfunction.
Depending on the clinical picture, specific diagnostic tests are employed:
- Imaging Studies:
- Skull and cervical spine X-ray examination (Initial screening, fracture detection - limited role vs CT)
- Brain computed tomography (CT) (Standard for acute TBI: detects blood, fractures, edema, herniation)
- Brain magnetic resonance imaging (MRI) (Detects subtle contusions, DAI, posterior fossa/brainstem injury; often used subacutely)
- Cervical spine MRI (Evaluates associated spinal cord or ligamentous injury)
- Vascular Studies: (If vascular injury is suspected)
- Cerebrovascular Doppler ultrasonography (Non-invasive assessment of blood flow velocity)
- CT Angiography (CTA) or MR Angiography (MRA) (Visualize blood vessels for dissection, occlusion, aneurysm)
- Conventional Angiography (DSA) (Gold standard for detailed vascular imaging, allows intervention)
- Rheoencephalography (REG) (Less common, older technique for assessing cerebral circulation)
- Echoencephalography (EchoEG) (Limited use, primarily for detecting midline shift)
- Cerebrospinal Fluid Analysis:
- Lumbar puncture (LP) for cerebrospinal fluid (CSF) analysis (Limited role; contraindicated with high ICP)
Hospital admission is warranted for patients exhibiting persistent confusion, significant behavioral changes, decreased level of consciousness (low GCS), extreme dizziness, vomiting, severe headache, or focal neurologic signs (e.g., weakness, numbness, speech problems) after head injury. Urgent brain MRI or, more commonly, CT scan is mandatory in these cases.
Glasgow Coma Scale (GCS)
Assessment of TBI severity relies heavily on evaluating the patient's level of consciousness and neurological function. The Glasgow Coma Scale (GCS), developed by Professors Graham Teasdale and Bryan Jennett in 1974 (8), provides a standardized, objective method for recording and tracking the level of consciousness in response to defined stimuli.
The GCS assesses three components: Eye Opening (E), Verbal Response (V), and Motor Response (M):
| Index | Responses | Score |
|---|---|---|
| Motor Response (M) | Obeys commands | 6 |
| Localizes to pain (purposeful movement towards stimulus) | 5 | |
| Withdraws from pain (normal flexion) | 4 | |
| Abnormal flexion to pain (decorticate posturing) | 3 | |
| Abnormal extension to pain (decerebrate posturing) | 2 | |
| No motor response (flaccid) | 1 | |
| Verbal Response (V) | Oriented (to time, place, person) | 5 |
| Confused conversation | 4 | |
| Inappropriate words (random, exclamatory) | 3 | |
| Incomprehensible sounds (moaning, groaning) | 2 | |
| No verbal response | 1 | |
| Eye Opening (E) | Spontaneous | 4 |
| To verbal command | 3 | |
| To pain | 2 | |
| No eye opening | 1 | |
| Total GCS Score | (Sum of best M + V + E responses) | 3–15 |
Note on Interpretation: The total GCS score ranges from 3 (deep coma or death) to 15 (fully awake). Coma is generally defined as GCS ≤ 8. Lower scores correlate with higher mortality and poorer prognosis (9). For example, data suggests patients scoring 3-4 have a high probability of dying or remaining vegetative, while scores greater than 11 indicate a much better chance of moderate disability or good recovery (10). Intermediate scores correspond to proportional recovery chances.
Factors like intubation (preventing verbal response, denoted VT) or severe orbital/facial swelling (preventing eye opening, denoted EC) can make scoring components impossible. In such cases, modifiers are used and the score reflects only the assessable components, reported as individual scores (e.g., EC VT M4) or a partial sum with explanation.
Glasgow Coma Scale Calculator
This calculator helps determine the GCS score based on the best observed responses in each category.
TBI severity is typically classified based on the initial GCS score, duration of loss of consciousness (LOC), and duration of post-traumatic amnesia (PTA) (11):
| Severity | Structural Imaging (MRI, CT) | Loss of Consciousness (LOC) | Alteration of Consciousness (AOC)/Confusion | Post-traumatic Amnesia (PTA) | Glasgow Coma Scale (GCS) |
|---|---|---|---|---|---|
| Mild (Concussion) | Normal | 0–30 min | Moment to <24 hours | 0 to 1 day | 13–15 |
| Moderate | Normal or Abnormal | >30 min but <24 hours | >24 hours | >1 day but <7 days | 9–12 |
| Severe | Normal or Abnormal | >24 hrs | >24 hours | >7 days | 3–8 |
Pathophysiological changes and critical care in traumatic brain injury
The initial impact (primary injury) triggers a cascade of secondary injury mechanisms that evolve over hours to days, significantly influencing patient outcomes. Critical care focuses on mitigating these secondary insults (12):
- Cerebral Blood Flow (CBF) and Autoregulation: The brain normally maintains stable CBF despite fluctuations in systemic blood pressure (mean arterial pressure [MAP] approx. 60-160 mmHg) via autoregulation. Severe TBI disrupts this mechanism (often shifting the curve rightward), making CBF dangerously dependent on MAP. Hypotension can cause ischemia, while hypertension can worsen edema and hyperemia. Maintaining adequate Cerebral Perfusion Pressure (CPP = MAP - ICP), typically targeted between 60-70 mmHg in adults, is crucial, but requires careful balancing against ICP management (2).
- Intracranial Pressure (ICP) Dynamics: Increased ICP (normal generally considered < 15-20 mmHg) results from hematomas, edema, or increased cerebral blood volume (CBV). High ICP compresses brain tissue, reduces CPP, and can lead to herniation syndromes (brain tissue displacement). Management aims to keep ICP less than 20-22 mmHg using tiered therapies (head elevation, sedation/analgesia, osmotic agents, CSF drainage via EVD, controlled hyperventilation, potentially barbiturate coma, decompressive craniectomy) (2, 13).
- Cerebral Metabolism and Oxygenation: TBI increases metabolic demand while potentially impairing oxygen delivery due to disrupted CBF or systemic hypoxia. Monitoring brain tissue oxygenation (PbtO2) via specialized probes (target >15-20 mmHg) can help guide therapies to prevent metabolic crisis and secondary ischemic injury (14).
- Secondary Injury Cascade: Beyond hemodynamics, secondary injury involves complex processes including:
- Excitotoxicity: Excessive release of excitatory neurotransmitters (e.g., glutamate) leading to calcium influx, mitochondrial dysfunction, and neuronal death.
- Oxidative Stress: Generation of reactive oxygen species (free radicals) damaging lipids, proteins, and DNA.
- Inflammation: Activation of microglia and astrocytes, release of pro-inflammatory cytokines, and blood-brain barrier disruption contributing to edema and further neuronal damage.
- Apoptosis: Programmed cell death is triggered in injured and surrounding neurons.
- Systemic Effects: TBI impacts other body systems. Sympathetic surge ("catecholamine storm") can cause hypertension, tachycardia, and cardiac stress. Neurogenic pulmonary edema can occur. Coagulopathy (TBI-induced coagulopathy) is common and associated with worse outcomes. Maintaining systemic homeostasis (normoxia, normocapnia [avoiding excessive hyper/hypocapnia], normothermia/fever control, euglycemia [avoiding hyper- and hypoglycemia], euvolemia) is vital to prevent secondary brain insults.
- Hyperventilation Caution: While controlled hyperventilation (targeting PaCO2 30-35 mmHg) can temporarily lower ICP via vasoconstriction, prolonged or excessive prophylactic hyperventilation (PaCO2 less than 30 mmHg) risks cerebral ischemia by reducing CBF and should generally be avoided, especially in the first 24-48 hours. Its use should be cautious, temporary, and ideally guided by advanced monitoring (e.g., PbtO2, jugular venous oximetry, CBF monitoring) (2).
- Fluid and Glucose Management: Osmotic agents like mannitol or hypertonic saline reduce brain water content to lower ICP but require careful monitoring of serum osmolality, electrolytes (especially sodium), and fluid balance to avoid dehydration, hypotension, or renal injury. Hyperglycemia is associated with worse outcomes and requires strict glucose control (e.g., targeting 140-180 mg/dL), while hypoglycemia must also be vigilantly avoided (2, 16).
Treatment of Traumatic Brain Injury Symptoms
TBI management requires a multidisciplinary approach addressing the specific injury type, severity, and patient factors. Treatment aims to stabilize the patient, prevent secondary injury, manage symptoms, and facilitate recovery. Options include:
- Immediate / Emergency Management:
- Airway, Breathing, Circulation (ABC) stabilization.
- Cervical spine immobilization until injury is excluded.
- Rapid neurological assessment (GCS, pupils).
- Urgent neuroimaging (usually non-contrast head CT scan).
- Medical Management (Critical Care for Moderate/Severe TBI):
- Intracranial Pressure (ICP) monitoring and management (target generally < 20-22 mmHg) (2).
- Cerebral Perfusion Pressure (CPP) optimization (target generally 60-70 mmHg) (2).
- Mechanical ventilation to ensure adequate oxygenation (PaO2 > 60 mmHg or SpO2 > 90%) and controlled CO2 levels (normocapnia preferred, PaCO2 35-45 mmHg).
- Sedation and analgesia to reduce metabolic demand, improve ventilator synchrony, and control agitation.
- Osmotic therapy (mannitol, hypertonic saline) for elevated ICP.
- Temperature control (aggressive treatment of fever > 38°C; targeted temperature management/hypothermia may be considered in specific refractory ICP cases but not routine) (17).
- Seizure prophylaxis (anti-epileptic drugs like levetiracetam or phenytoin often used for the first 7 days post-injury in severe TBI to prevent early seizures) (2).
- Nutritional support (early enteral feeding preferred).
- Deep vein thrombosis (DVT) prophylaxis (pharmacologic and mechanical methods).
- Conservative Management (Mild TBI/Concussion, or post-acute phase):
- Rest (physical and cognitive) initially, followed by gradual, symptom-limited return to activity.
- Symptom management (Medication for headache, nausea, sleep disturbance; avoiding medications that impair cognition or lower seizure threshold).
- Physiotherapy (for balance, vestibular dysfunction, neck pain).
- Occupational therapy (for activities of daily living, return to work/school planning).
- Speech therapy (for cognitive-communication or swallowing problems).
- Cognitive rehabilitation / Neuropsychological support (for persistent memory, attention, executive function deficits).
- Reflexotherapy (acupuncture) (may be considered as adjunctive therapy for pain/symptoms, evidence varies).
- Interventional Procedures:
- Trigger point injections or peripheral nerve blocks (for associated myofascial pain or specific headaches).
- ICP monitor placement (various types: intraventricular, intraparenchymal, subarachnoid, epidural).
- External Ventricular Drain (EVD) placement (allows both CSF drainage for ICP control and ICP monitoring).
- Lumbar puncture (rarely therapeutic in TBI unless communicating hydrocephalus; primarily diagnostic if infection suspected and ICP permits).
- Surgical Interventions (Indicated for specific pathologies based on clinical and imaging findings):
Indications and Types of Surgical Intervention for TBI:
- Craniotomy: Surgical opening of the skull. Used primarily for:
- Evacuation of significant space-occupying hematomas (e.g., epidural hematoma > 30 cm³, acute subdural hematoma > 10 mm thick or causing > 5 mm midline shift, large symptomatic intracerebral hematoma) (18, 19).
- Repair of penetrating injuries or extensive dural tears.
- Clipping/repair of traumatic vascular injuries (aneurysms, fistulas).
- Decompressive Craniectomy: Removal of a large portion of the skull (bone flap not immediately replaced) to allow the swollen brain to expand outwards, thereby reducing life-threatening intracranial pressure refractory to maximal medical management (20).
- Cranioplasty: Later surgical procedure to replace the bone flap or reconstruct the skull defect (using autograft, titanium mesh, or synthetic materials) after brain swelling has resolved following decompressive craniectomy.
- Burr Hole Evacuation: Drilling small holes in the skull, often used for draining chronic subdural hematomas or placing ICP monitors/EVDs.
- Ventriculostomy / External Ventricular Drain (EVD) Placement: Insertion of a catheter into the brain's ventricles to drain CSF, monitor ICP, and sometimes administer medication. Critical for managing obstructive hydrocephalus or refractory intracranial hypertension.
- Skull Fracture Repair: Elevation of significantly depressed skull fractures (especially if > skull thickness, causing neurological deficit, overlying eloquent cortex/dural sinus, cosmetic deformity, or associated with dural tear) or debridement/repair of open/compound fractures to prevent infection and brain injury from bone fragments (4).
- Lobectomy or Resection of Contused Tissue: Rarely performed; removal of non-functional, severely contused brain tissue (e.g., temporal or frontal pole) may be considered as a last resort for intractable ICP or mass effect in specific locations.
- Shunt Placement (e.g., Ventriculoperitoneal Shunt): Permanent internal drainage system implanted to treat chronic post-traumatic hydrocephalus by diverting excess CSF to another body cavity (usually the peritoneum).
- Neurovascular Repair: Surgical or endovascular repair of damaged blood vessels (e.g., traumatic dissection, aneurysm, fistula). Aims to control hemorrhage or prevent ischemia. May involve clipping, coiling, stenting, or grafting.
- Intracranial Pressure (ICP) Monitor Placement: Insertion of a device (e.g., EVD, parenchymal probe) into the cranial cavity to directly measure ICP. Essential for guiding management in severe TBI (typically GCS ≤ 8 with abnormal CT, or normal CT with risk factors like age > 40, posturing, or hypotension) (2). *Note: This is diagnostic/monitoring, not treatment itself.*
Lumbar puncture (LP) may be considered for CSF analysis after specific events like brain surgery or intracranial hemorrhage, only if imaging and clinical assessment definitively rule out significantly elevated intracranial pressure or mass effect. If deemed safe, 10-20 ml of CSF can be collected to check for infection, inflammation, or persistent bleeding.
![]() Attention! Any concerning symptoms after a head injury, especially persistent or worsening headaches, altered consciousness, seizures, focal neurological deficits, vomiting, or unusual behavior, require immediate medical evaluation by qualified professionals, ideally including a neurologist or neurosurgeon at a facility equipped to manage TBI. Attempting self-diagnosis or relying on non-specialist opinions is dangerous and can delay life-saving treatment. Advanced imaging and clinical expertise are necessary for accurate diagnosis and management. Always consult a specialist experienced in neurotrauma.
Attention! Any concerning symptoms after a head injury, especially persistent or worsening headaches, altered consciousness, seizures, focal neurological deficits, vomiting, or unusual behavior, require immediate medical evaluation by qualified professionals, ideally including a neurologist or neurosurgeon at a facility equipped to manage TBI. Attempting self-diagnosis or relying on non-specialist opinions is dangerous and can delay life-saving treatment. Advanced imaging and clinical expertise are necessary for accurate diagnosis and management. Always consult a specialist experienced in neurotrauma.
References
- Taylor CA, Bell JM, Breiding MJ, Xu L. Traumatic Brain Injury–Related Emergency Department Visits, Hospitalizations, and Deaths — United States, 2007 and 2013. MMWR Surveill Summ 2017;66(No. SS-9):1–16. DOI: http://dx.doi.org/10.15585/mmwr.ss6609a1
- Carney N, Totten AM, O'Reilly C, et al. Guidelines for the Management of Severe Traumatic Brain Injury, Fourth Edition. Neurosurgery. 2017 Jan 1;80(1):6-15. doi: 10.1227/NEU.0000000000001432
- Werner C, Engelhard K. Pathophysiology of traumatic brain injury. Br J Anaesth. 2007 Jul;99(1):4-9. doi: 10.1093/bja/aem131
- Bullock MR, Chesnut R, Ghajar J, et al; Surgical Management of Traumatic Brain Injury Author Group. Surgical management of depressed cranial fractures. Neurosurgery. 2006 Mar;58(3 Suppl):S2-54-60; discussion Si-iv. doi: 10.1227/01.neu.0000210361.20173.3c
- Jagoda AS, Bazarian JJ, Bruns JJ Jr, et al; American College of Emergency Physicians Clinical Policies Subcommittee on Mild Traumatic Brain Injury. Clinical policy: neuroimaging and decisionmaking in adult mild traumatic brain injury in the acute setting. Ann Emerg Med. 2008 Dec;52(6):714-48. doi: 10.1016/j.annemergmed.2008.08.021
- Amyot F, Arciniegas DB, Brazaitis MP, et al. A Review of the Effectiveness of Neuroimaging Modalities for the Detection of Traumatic Brain Injury. J Neurotrauma. 2015 Nov 1;32(21):1693-721. doi: 10.1089/neu.2014.3601
- Greenberg MS. Handbook of Neurosurgery. 9th ed. Thieme; 2020.
- Teasdale G, Jennett B. Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974 Jul 13;2(7872):81-4. doi: 10.1016/s0140-6736(74)91639-0
- Jennett B, Teasdale G, Braakman R, et al. Predicting outcome in individual patients after severe head injury. Lancet. 1976 May 15;1(7968):1031-4. doi: 10.1016/s0140-6736(76)92860-7
- Teasdale GM, Maas AI, Lecky F, et al; Traumatic Coma Data Bank. The Glasgow Coma Scale at 40 years: standing the test of time. Lancet Neurol. 2014 Aug;13(8):844-54. doi: 10.1016/S1474-4422(14)70120-6
- Mild Traumatic Brain Injury Committee of the Head Injury Interdisciplinary Special Interest Group of the American Congress of Rehabilitation Medicine. Definition of mild traumatic brain injury. J Head Trauma Rehabil. 1993;8(3):86–7.
- Ng SY, Lee AYW. Traumatic Brain Injuries: Pathophysiology and Potential Therapeutic Targets. Front Cell Neurosci. 2019 Nov 29;13:528. doi: 10.3389/fncel.2019.00528
- Hawryluk GWJ, et al. Guidelines for the Management of Severe Traumatic Brain Injury: 2020 Update of the Decompressive Craniectomy Recommendations. Neurosurgery. 2020 Sep 1;87(3):427-434. doi: 10.1093/neuros/nyaa278
- Okorie C, et al. Brain Tissue Oxygenation Monitoring in Neurocritical Care. J Clin Med. 2021 Oct 28;10(21):5079. doi: 10.3390/jcm10215079
- Simon DW, McGeachy MJ, Bayır H, Clark RSB, Loane DJ, Kochanek PM. The dark side of the force: a review of severe brain injury-induced secondary neuroinflammation. Transl Res. 2017 May;183:107-131. doi: 10.1016/j.trsl.2016.12.002
- Hermanides J, et al. Glucose control in intensive care. Lancet. 2018 Jul 28;392(10144):346-347. doi: 10.1016/S0140-6736(18)31596-6
- Andrews PJD, et al; Eurotherm3235 Trial Collaborators. Hypothermia for Intracranial Hypertension after Traumatic Brain Injury. N Engl J Med. 2015 Dec 17;373(25):2403-12. doi: 10.1056/NEJMoa1507581
- Bullock MR, et al; Surgical Management of Traumatic Brain Injury Author Group. Surgical management of acute subdural hematomas. Neurosurgery. 2006 Mar;58(3 Suppl):S2-15-24; discussion Si-iv. doi: 10.1227/01.neu.0000210365.61200.95
- Bullock MR, et al; Surgical Management of Traumatic Brain Injury Author Group. Surgical management of traumatic parenchymal lesions. Neurosurgery. 2006 Mar;58(3 Suppl):S2-25-46; discussion Si-iv. doi: 10.1227/01.neu.0000210363.91299.65
- Hutchinson PJ, et al; RESCUEicp Trial Collaborators. Trial of Decompressive Craniectomy for Traumatic Intracranial Hypertension. N Engl J Med. 2016 Sep 22;375(12):1119-30. doi: 10.1056/NEJMoa1605215
- Fishman RA. Cerebrospinal Fluid in Diseases of the Nervous System. 2nd ed. Saunders; 1992.
See also
- Anatomy of the nervous system
- Central nervous system infection:
- Brain abscess (lobar, cerebellar)
- Eosinophilic granuloma, Langerhans cell histiocytosis (LCH), Hennebert's symptom
- Epidural brain abscess
- Sinusitis-associated intracranial complications
- Otogenic intracranial complications
- Sinusitis-associated ophthalmic complications
- Bacterial otogenic meningitis
- Subdural brain abscess
- Sigmoid sinus suppurative thrombophlebitis
- Cerebral 3rd Ventricle Colloid Cyst
- Cerebral and spinal adhesive arachnoiditis
- Corticobasal Ganglionic Degeneration (Limited Brain Atrophy)
- Encephalopathy
- Headache, migraine
- Traumatic brain injury (concussion, contusion, brain hemorrhage, axonal shearing lesions)
- Increased intracranial pressure and hydrocephalus
- Parkinson's disease
- Pituitary microadenoma, macroadenoma and nonfunctioning adenomas (NFPAs), hyperprolactinemia syndrome
- Spontaneous cranial cerebrospinal fluid leak (CSF liquorrhea)