Cerebral embolism
Causes of Cerebral Embolism
Cerebral embolism, the lodging of an embolus (a traveling particle or clot) in a brain artery, is a leading cause of ischemic stroke [1, 2]. The heart is the most frequent source of these emboli (cardioembolism) [1, 3]. Arterio-arterial embolism, where emboli originate from atherosclerotic plaques (often with associated thrombus) within the large arteries supplying the brain (such as the carotid or vertebrobasilar systems), is another significant, though perhaps less common overall, cause [1, 4]. Other potential causes, such as thrombosis originating in the pulmonary veins, fat embolism (often after long bone fractures or orthopedic surgery), tumor embolism (fragments of a tumor entering the bloodstream), marantic endocarditis (non-bacterial vegetations on heart valves, typically in chronically ill or cancer patients), paradoxical embolism (venous clots crossing from the right to the left side of the heart through a defect like a patent foramen ovale), and complications following neck or chest surgery, are considered relatively rare [1, 2]. Despite extensive investigation, a significant proportion of embolic strokes occur without a clearly identifiable embolic source; these are termed cryptogenic strokes [1, 5].
Common etiologies (causes) of cerebral embolism can be broadly categorized [1, 2, 3, 4]:
- Cardioembolic Sources (Emboli originating from the heart):
- Atrial fibrillation (AFib) and other cardiac arrhythmias (often associated with underlying conditions like rheumatic heart disease, atherosclerosis, hypertension, or congenital heart defects). AFib is a major cause.
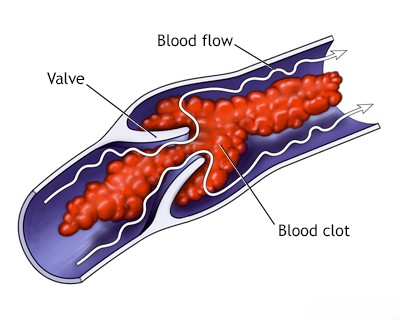
- Myocardial infarction (heart attack) leading to the formation of a mural thrombus (clot attached to the heart wall).
- Acute and subacute bacterial endocarditis (infection of heart valves leading to septic emboli).
- Valvular heart disease even without significant arrhythmias or obvious mural thrombus (e.g., severe mitral stenosis, mechanical prosthetic valves).
- Complications of cardiac surgery.
- Prosthetic heart valves (mechanical valves carry higher thrombotic risk than bioprosthetic valves).
- Non-bacterial thrombotic endocarditis (NBTE or marantic endocarditis - sterile vegetations on valves, often associated with malignancy or chronic inflammation).
- Paradoxical embolism through intracardiac shunts (like Patent Foramen Ovale - PFO, or Atrial Septal Defect - ASD) allowing venous clots to reach the arterial circulation.
- Less common cardiac sources like atrial myxoma (heart tumor) or cardiomyopathies.
- Non-Cardioembolic Sources:
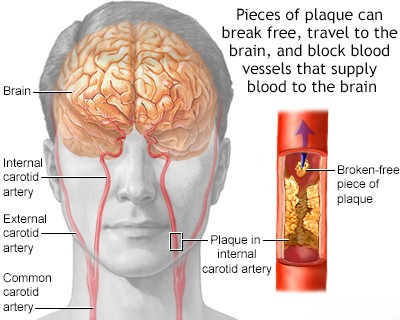
- Atherosclerosis of the aorta (especially the aortic arch) or carotid arteries (leading to mural thrombus formation or release of atheromatous debris - atheroembolism). This is often termed arterio-arterial embolism.
- While less common as a *primary* embolic source, *in situ* thrombosis (clot formation directly within) of cerebral arteries (e.g., vertebral, middle cerebral) can occur, though this is technically thrombosis, not embolism unless a piece breaks off.
- Pulmonary vein thrombosis (rare).
- Fat embolism syndrome (usually after trauma).
- Tumor embolism (fragments from primary or metastatic tumors).
- Air embolism (e.g., during certain medical procedures, diving accidents).
- Complications of neck and chest surgery or trauma involving major vessels.
- Septic emboli from non-cardiac infection sources (rare).
- Cryptogenic Sources (Source remains undetermined after standard investigation) [5].
Cryptogenic cerebral embolisms (or cryptogenic strokes, many presumed embolic) present a significant diagnostic challenge [5]. Patients might have underlying hypercoagulable states (conditions increasing clot formation risk, e.g., related to certain medications like oral contraceptives, underlying chronic inflammatory diseases, inherited thrombophilias, or metastatic malignancy) that predispose them to sudden embolic events [1]. Furthermore, transient cardiac events that could generate emboli, such as brief episodes of paroxysmal atrial fibrillation or fleeting valve abnormalities (like mitral stenosis clicks mentioned in the original, though clicks themselves aren't embolic), might be missed during routine examination or standard short-term heart monitoring [1, 5]. Consequently, a substantial number of patients, particularly younger individuals (e.g., aged 20-50 years), experience embolic-appearing strokes without a readily discernible cause identified through initial workup [1, 5].
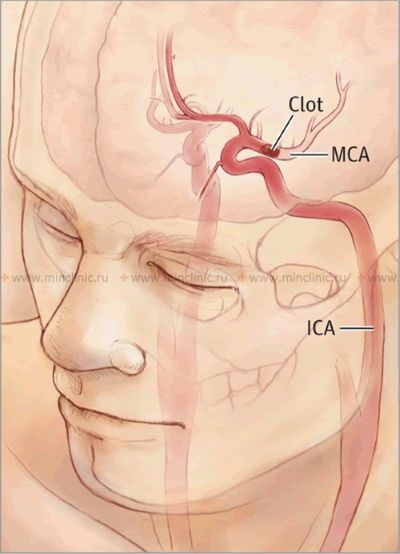
The clinical outcome depends heavily on the embolus itself and the patient's vascular anatomy [1]. The size, location, and composition (e.g., clot, fat, tumor) of the embolus determine which cerebral artery is occluded and thus the extent and location of the resulting cerebral infarction (stroke) [1, 2]. Large emboli (e.g., 2-3 mm or larger) are more likely to occlude major vessels like the trunk of the middle cerebral artery (MCA), potentially leading to extensive infarction involving the cerebral cortex, underlying white matter, and deep gray matter structures (basal ganglia, thalamus) [1, 2]. Smaller emboli may travel further distally, occluding smaller perforating branches of the MCA or basilar artery, often resulting in smaller, deep infarcts known as lacunar infarcts (though lacunar strokes are more classically associated with small vessel disease/lipohyalinosis rather than embolism) [1, 6].
Emboli, particularly those composed of platelet and fibrin (blood clots), are not always static. They can migrate further downstream, undergo spontaneous thrombolysis (dissolution by the body's own clot-busting mechanisms), or fragment within the bloodstream [1]. This dynamic process can lead to fluctuating neurological symptoms or, in some fortunate cases, early reperfusion and complete recovery from ischemia-induced deficits [1]. The final location and size of the cerebral infarction are also critically influenced by the patient's individual capacity for collateral blood flow – the ability of unaffected arteries to supply blood to the ischemic territory via alternative pathways (like the Circle of Willis or leptomeningeal anastomoses) [1, 7].
For example, adequate collateral blood flow through the Circle of Willis or from the vertebral arteries might prevent or minimize ischemia or infarction even when an embolus obstructs a major proximal vessel like the internal carotid artery, the A1 segment of the anterior cerebral artery, or the proximal vertebral artery [7]. Similarly, emboli lodging in cortical branches or even the trunk of the middle cerebral artery might result in relatively limited cortical or subcortical infarcts if robust collateral flow exists from adjacent anterior or posterior cerebral artery territories via leptomeningeal connections over the brain surface [7]. These principles of collateral supply are also highly relevant in determining the consequences of embolism to the posterior circulation, affecting the brainstem and cerebellum [1, 7].
When emboli obstruct vessels, subsequent reperfusion (restoration of blood flow, either spontaneously or via treatment) into the damaged, ischemic brain tissue can sometimes lead to secondary bleeding [1, 8]. This often manifests initially as petechial hemorrhages (small, spot-like bleeds) within the infarcted area, termed hemorrhagic transformation or hemorrhagic infarction [8]. Less commonly, these small hemorrhages can coalesce and expand, forming a larger, space-occupying hemorrhagic mass [1, 8]. Significant hemorrhagic transformation is considered more likely following occlusion of large vessels like the MCA trunk, leading to extensive infarction (particularly involving deep gray and white matter structures), especially if reperfusion occurs after a delay [1, 8].
When the heart is definitively identified as the embolic source, studies suggest a pattern of distribution within the brain. Approximately 80% of cardiac emboli tend to lodge within the territory of the middle cerebral artery (due to it receiving the largest proportion of carotid blood flow), around 10-11% may travel to the posterior cerebral artery territory (via the posterior circulation), and the remainder distribute to the anterior cerebral artery territory or directly into the vertebral or basilar arteries or their branches [1, 2].
Cerebral embolism arising from cardiac conditions is often further categorized based on the underlying heart problem, typically distinguishing between [1, 3]:
- Arrhythmic causes (e.g., atrial fibrillation)
- Structural heart disease causes (e.g., valvular disease, myocardial infarction with thrombus, PFO)
Atrial Fibrillation (AFib) as a Major Cause of Cardioembolic Stroke
Cardiac arrhythmias (irregular heart rhythms) of various types can be associated with symptomatic cerebral embolism (stroke or TIA) and systemic embolism (emboli traveling to other parts of the body) [1, 3]. Particular attention should be paid to the significantly high incidence of embolism in patients with conditions like sick sinus syndrome (a disorder of the heart's natural pacemaker), atrial flutter, and especially atrial fibrillation (AFib) [3, 9]. The risk associated with AFib is further elevated when it occurs in the context of rheumatic valvular heart disease (valvular AFib) [9]. Because of this high risk, long-term anticoagulation therapy, traditionally with warfarin sodium or more commonly now with direct oral anticoagulants (DOACs), is generally indicated to prevent clot formation in the left atrium and subsequent embolism in eligible patients with AFib [9, 10]. Clinically apparent embolic events are most frequently observed in patients with AFib, irrespective of its underlying cause (valvular vs. non-valvular) [3]. The estimated annual incidence of cerebral embolism (stroke) in patients with untreated non-valvular atrial fibrillation is substantial, often cited as 4-7% or higher depending on other risk factors (like those included in the CHA₂DS₂-VASc score), with even a single stroke frequently resulting in significant long-term disability [9].
Another significant source of cardiac emboli is the formation of a mural thrombus (a blood clot attached to the inner wall of a heart chamber). This is relatively common in patients with atherosclerotic cardiovascular disease, particularly following a myocardial infarction (heart attack) that damages the heart wall, creating a surface where clots can form [1, 3]. The risk exists regardless of the presence of associated complications like papillary muscle dysfunction, congestive heart failure, or the formation of a ventricular aneurysm (a bulge in the weakened heart wall) [1, 3].
For patients with non-valvular atrial fibrillation who are considered high-risk for stroke but have contraindications to long-term anticoagulation (or a strong preference for an alternative), left atrial appendage (LAA) closure devices offer a mechanical alternative [10, 11]. The LAA is a small pouch in the left atrium where most stroke-causing clots form in AFib patients [11]. Devices implanted via catheterization effectively seal off the LAA, preventing clot migration into the circulation and thereby reducing the risk of ischemic stroke [11].
Cardiac Surgery, Other Systemic Causes, and Paradoxical Embolism
Certain medical procedures carry a risk of embolism [1]. Heart valve replacement surgery, particularly with mechanical valves, is associated with a relatively high risk of thromboembolism if anticoagulation is inadequate [1, 3]. Less common procedural causes of cerebral embolism include manipulation during chest surgery (potentially leading to pulmonary venous embolism, although rare) and interventions involving the head, neck, aorta, or carotid arteries (which can dislodge atheromatous debris or thrombus, resulting in arterio-arterial emboli) [1]. Fat embolism (globules of fat entering the circulation) or air embolism can result from trauma like long bone fractures, extensive soft tissue injury, certain surgical procedures (especially orthopedic or cardiac), and rarely during diagnostic angiography or other procedures involving intravascular contrast administration or catheterization [1]. These conditions (fat/air embolism) can potentially lead to multiple, small embolic events, sometimes associated with petechial hemorrhages in the brain [1]. The use of artificial hearts or ventricular assist devices (VADs) also carries a significant inherent risk of cerebral embolism originating from the device surfaces or related thrombus formation [3].
Congenital heart defects that create a communication between the right and left sides of the heart (e.g., Patent Foramen Ovale - PFO, Atrial Septal Defect - ASD, Ventricular Septal Defect - VSD) can allow a venous thromboembolus (a clot typically originating in the leg veins, DVT) to bypass the pulmonary circulation and enter the systemic arterial circulation, causing a paradoxical embolism to the brain or other organs [1, 3, 12]. Tumor emboli (fragments of cardiac tumors like atrial myxoma, or more rarely metastatic tumors) can occur [1]. Additionally, thrombotic, infectious (septic), or sterile (marantic/NBTE) vegetations can form on the endocardial surfaces (inner lining) of the heart chambers or, more commonly, the heart valves, and subsequently embolize [1, 3]. Valvular lesions seen in rheumatic heart disease or non-bacterial thrombotic (marantic) endocarditis typically affect the aortic and mitral valves and can be associated with systemic or cerebral embolism; diagnosis relies on integrating patient history, physical examination findings (e.g., murmurs), imaging (echocardiography), and relevant laboratory results [1, 3]. Libman-Sacks endocarditis, characterized by sterile valvular vegetations particularly on the mitral and aortic valves, is a known manifestation of systemic lupus erythematosus (SLE) and can be a source of cerebral emboli [1, 3]. Similarly, bacterial endocarditis (infection of the heart valves) is a critical consideration [1, 3, 13].
Infective (bacterial) endocarditis can lead to the formation of thrombotic, infected vegetations that shed septic emboli [1, 13]. These septic emboli can cause large cerebral infarcts, often appearing similar on imaging to bland (non-infectious) embolic infarcts resulting from major cerebral artery occlusion [13]. However, septic emboli can also lead to the formation of brain abscesses or multiple small microabscesses [13]. A serious complication is the development of mycotic aneurysms (weakening and ballooning of a cerebral artery wall due to infection lodged there by a septic embolus), which carry a high risk of rupture leading to subarachnoid and/or intracerebral hemorrhage [1, 13]. Due to these severe potential consequences, infective endocarditis should always be considered and diligently ruled out in patients presenting with stroke symptoms, especially if fever or other signs of infection are present [13].
Primary cardiac tumors like atrial myxomas, though rare, can fragment and lead to tumor embolism; these emboli originate from deposits on the tumor surface itself [1, 3]. Clues suggesting myxoma in the differential diagnosis might include evidence of pulmonary hypertension, an elevated erythrocyte sedimentation rate (ESR), and systemic constitutional symptoms like fever or malaise [1]. Mitral valve prolapse (MVP), a common condition where the mitral valve leaflets bulge back into the left atrium during systole, has been associated with cerebral embolism in some studies, though the exact mechanisms (e.g., associated atrial fibrillation, thrombus formation on the valve, endothelial dysfunction) are not always fully understood, making the prediction of recurrent embolism risk challenging [1, 3]. Echocardiography is essential for diagnosis and risk stratification in patients with suspected MVP and neurological events [3].
Clinical Syndromes Associated with Cerebral Embolism
Acute ischemic stroke caused by cerebral embolism typically presents with a sudden, abrupt onset of neurological deficits that are often maximal at the very beginning [1, 2]. However, the clinical course can vary. Sometimes the initial deficits may be incomplete and then worsen ("stuttering" or progressive onset), or they may fluctuate significantly after onset, possibly reflecting migration or fragmentation of the embolus with temporary reperfusion [1]. Transient ischemic attacks (TIAs) of embolic origin can manifest as brief episodes of neurological dysfunction (lasting minutes to hours) that resolve completely, sometimes occurring repeatedly before a major stroke [1].
In other scenarios, a patient might initially present with a mild deficit which then progresses over hours to a more extensive infarct involving a major cerebral artery territory, perhaps due to propagation of thrombus distal to the initial embolic occlusion [1]. Regardless of the temporal pattern, the specific neurological deficit produced reflects the vascular territory supplied by the occluded artery, whether it's a large vessel, a smaller branch, or a tiny penetrating artery [1, 2]. The resulting neurological syndrome corresponds to the location and extent of brain tissue affected by the lack of blood flow. As noted before, the size of the embolus generally dictates the diameter of the vessel it is likely to occlude [1].
Certain patterns of neurological deficits strongly suggest cerebral artery embolism as the underlying cause [1, 2]. Syndromes commonly associated with embolism affecting the middle cerebral artery (MCA) territory include:
- Frontal opercular syndrome: Characterized by contralateral facial weakness (sparing the forehead), severe expressive language difficulty (aphasia if dominant hemisphere, or dysprosody if non-dominant), and often severe dysarthria (slurred speech), typically due to occlusion of superior division MCA branches.
- Brachio-predominant paralysis: Weakness predominantly affecting the contralateral arm (brachium), potentially involving the entire arm, forearm, wrist, or hand, sometimes with associated cortical sensory deficits (e.g., impaired stereognosis, graphesthesia) if the parietal sensory cortex is involved.
- Isolated Wernicke's aphasia (impaired language comprehension) or Broca's aphasia (impaired language production) in a patient with a lesion in the dominant (usually left) hemisphere, often resulting from occlusion of specific inferior (Wernicke's) or superior (Broca's) division branches of the MCA.
- Isolated contralateral hemianopia (loss of half the visual field) can occur with lesions affecting the optic radiations or primary visual cortex within the occipital lobe, typically supplied by the posterior cerebral artery (PCA), but sometimes MCA branch occlusions affecting temporal or parietal optic radiations can cause visual field defects.
Other suggestive syndromes include: sudden onset of hemianopia potentially pointing to posterior cerebral artery (PCA) embolism; acute leg weakness (crural paresis) disproportionately affecting the leg more than the arm or face suggesting anterior cerebral artery (ACA) embolism; and acute onset of vertigo, gait instability, nausea/vomiting, and incoordination (ataxia) suggesting embolism to the posterior circulation affecting the cerebellum or its brainstem connections (vertebral or basilar artery branches like PICA, AICA) [1].
Determining the precise cause (embolism, atherothrombosis, or small vessel disease like lipohyalinosis) of small, deep infarcts (lacunar strokes) based solely on clinical presentation can be challenging, as lipohyalinosis is statistically more common for typical lacunar syndromes [1, 6]. However, certain specific lacunar-like syndromes are highly suggestive of embolism to the top of the basilar artery [1]. For example, the acute onset of hypersomnolence (excessive sleepiness), impaired upward gaze (vertical gaze palsy), and bilateral ptosis (drooping eyelids) strongly suggests an embolism lodging at the basilar artery tip, potentially causing infarction via occlusion of the Artery of Percheron [1, 15]. The Artery of Percheron is a variant vessel originating from the PCA that uniquely supplies the medial aspects of both thalami and sometimes the subthalamus bilaterally [15].
Presentations can also be non-focal, especially with certain types of emboli. Septic emboli associated with endocarditis or widespread fat embolism often present with more diffuse neurological symptoms, including disorientation, confusion, agitation, delirium, or fluctuating levels of consciousness, rather than a clear focal deficit [1, 13].
Neurological syndromes observed after cardiac surgery can be varied. Common presentations include delayed awakening from anesthesia, slowed cognitive processing (bradyphrenia), confusion, agitation, memory impairment, and occasionally visual disturbances or hallucinations [1]. While most of these symptoms tend to resolve within days to a week, persistent visual perception deficits might indicate underlying parietal-occipital infarction, often within the MCA territory watershed zones, potentially resulting from perioperative hypotension (low blood pressure) or showers of multiple microemboli (from the heart, aorta, or bypass circuit) [1].
Seizures occurring after a stroke are statistically more common following embolic strokes compared to thrombotic ones, and also perhaps more common with lacunar infarctions affecting deep white matter pathways than purely deep gray matter lacunes [1]. Any cortical supratentorial infarction (affecting the brain surface above the tentorium) carries a risk of causing seizures, but their occurrence is not specific (pathognomonic) for any particular stroke etiology [1]. It's worth noting that new-onset epilepsy in elderly individuals (cryptogenic epilepsy) may sometimes result from previously unrecognized ("silent") chronic cortical infarcts, and these seizures often respond well to standard anticonvulsant therapy like phenytoin [1].
Cerebral Embolism: Clinical Examination and Laboratory/Imaging Studies
Crucially, before initiating anticoagulant therapy (blood thinners) in a patient presenting with acute stroke symptoms suggestive of cerebral embolism, it is essential to obtain urgent brain imaging, typically a non-contrast computed tomography (CT) scan [16]. The primary purpose of this initial scan is to reliably exclude the presence of intracranial hemorrhage (bleeding), as anticoagulants are contraindicated in hemorrhagic stroke and could worsen bleeding [16]. While CT is excellent for detecting acute blood, magnetic resonance imaging (MRI) is more sensitive for detecting early ischemic changes and differentiating acute from chronic hemorrhage or prior infarcts [14, 16].
Differential Diagnosis of Acute Focal Neurological Deficits (Stroke Mimics) [1, 17]
| Condition | Key Features / Distinguishing Points | Typical Investigations / Findings |
|---|---|---|
| Ischemic Stroke (Embolic/Thrombotic) | Sudden onset focal neurological deficit corresponding to vascular territory. | CT head negative for hemorrhage initially. MRI (DWI) positive for ischemia early. CTA/MRA may show occlusion/stenosis. Identify risk factors/source. |
| Intracerebral Hemorrhage (ICH) | Sudden onset focal neurological deficit, often with headache, vomiting, decreased consciousness, severe hypertension. | Non-contrast CT head shows hemorrhage. |
| Seizure with Todd's Paralysis | Post-ictal focal weakness mimicking stroke. History of seizure. Resolves within hours to 2 days. | History. EEG may show abnormalities. Imaging usually normal unless underlying lesion. Transient nature. |
| Migraine with Aura (esp. Hemiplegic) | Transient neurological symptoms (often spreading gradually) followed by/accompanying headache. History of similar episodes. Full recovery. | Clinical diagnosis. Normal exam between attacks. Imaging usually normal. |
| Hypoglycemia | Can cause focal neurological deficits, confusion, seizures. History of diabetes relevant. | Low blood glucose. Symptoms improve with glucose. |
| Brain Tumor | Can present acutely with seizure or hemorrhage causing focal signs, but often progressive symptoms precede. | MRI with contrast shows mass lesion. |
| Brain Abscess | Subacute onset usually. Focal signs, headache, fever, seizures. History of infection source. | MRI shows ring-enhancing lesion, restricted diffusion. Elevated inflammatory markers. |
| Multiple Sclerosis Relapse | Acute/subacute onset focal deficits. History of prior episodes possible. Usually younger adults. | MRI shows demyelinating lesions. |
| Subdural Hematoma | Can cause focal signs due to compression. Headache, altered mental status. History of trauma (may be minor/remote). | CT/MRI shows subdural collection. |
| Metabolic Encephalopathy | Diffuse brain dysfunction (confusion, lethargy). Focal signs uncommon unless superimposed issue. Identifiable systemic cause. | Specific lab abnormalities. Imaging usually non-specific. |
| Functional Neurological Disorder | Neurological symptoms inconsistent with organic patterns. Positive clinical signs (e.g., Hoover's). | Diagnosis of exclusion after thorough workup. Normal imaging/labs. |
A lumbar puncture (spinal tap) to examine the cerebrospinal fluid (CSF) for red blood cells is generally *not* indicated in the routine evaluation of suspected embolic stroke [1]. Its utility is very limited, primarily considered only in rare situations where subarachnoid hemorrhage is strongly suspected but initial CT scans are negative or equivocal, or perhaps historically when advanced imaging was unavailable and differentiating small brainstem hemorrhages (e.g., pontine) from infarcts was difficult [1]. Bone artifacts on CT scans, particularly in the posterior fossa (brainstem and cerebellum), can sometimes obscure small hemorrhages [14]. Modern magnetic resonance imaging (MRI), especially sequences sensitive to blood products (like gradient echo or susceptibility-weighted imaging), is far superior for differentiating acute from chronic hemorrhage and is highly sensitive for detecting early ischemic changes (infarction) within hours of onset [14, 16].
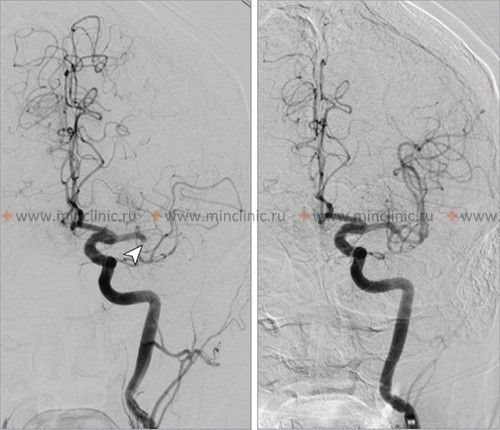
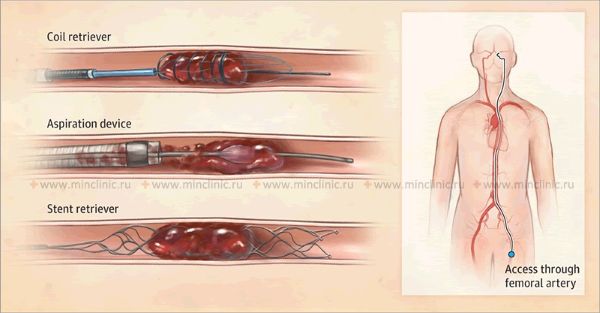
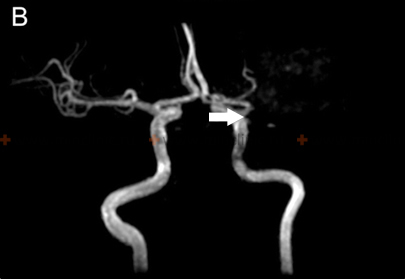
If cerebral artery embolism is suspected, identifying the location of the arterial occlusion and assessing the downstream brain tissue is critical for guiding acute treatment (like thrombectomy) [16, 19]. Non-invasive vascular imaging, such as CT angiography (CTA) or MR angiography (MRA), is typically performed urgently [16]. Conventional catheter-based cerebral angiography (Digital Subtraction Angiography - DSA) provides the highest resolution detail of the vessels but is invasive [14]. It may be performed if endovascular treatment (thrombectomy) is planned or if non-invasive imaging is inconclusive [16, 19]. It's important to note that after several hours or days, an embolus might migrate distally, fragment, or undergo spontaneous lysis (dissolution), meaning that angiography performed later might not show the original occlusion, making the definitive diagnosis of embolic stroke sometimes presumptive based on clinical presentation and infarct pattern [1]. Standard intravenous contrast administration during a routine CT or MRI scan generally lacks the resolution and timing necessary to directly visualize small cerebral emboli within vessels [14].
Treatment of Cerebral Embolism
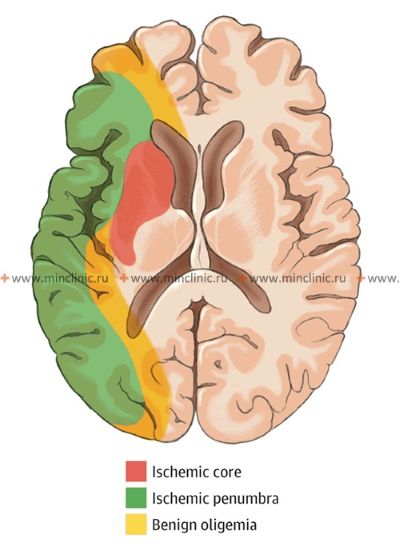
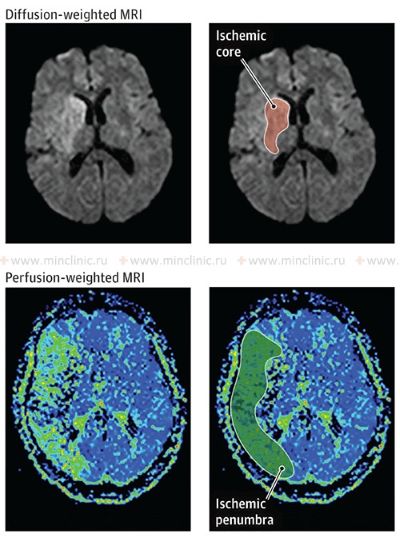
Treatment for stroke caused by cerebral embolism encompasses managing the acute phase to minimize brain damage and implementing strategies for secondary prevention to reduce the risk of future embolic events [1, 16]. In the acute setting (first hours to days), a primary focus is often on maintaining adequate cerebral perfusion pressure to support blood flow to the ischemic penumbra (the area surrounding the core infarct that is at risk but potentially salvageable) [16]. Current guidelines generally recommend permissive hypertension (allowing blood pressure to remain somewhat elevated) unless it exceeds very high thresholds (e.g., >220/120 mmHg, or >185/110 mmHg if thrombolysis is given) or there are specific contraindications [16]. Aggressively lowering blood pressure should generally be avoided unless necessary for specific conditions (e.g., aortic dissection, acute heart failure) [16]. Conversely, significant hypotension (low blood pressure) should be treated promptly but cautiously, as excessive blood pressure elevation might worsen cerebral edema or increase the risk of hemorrhagic transformation [1, 16]. Cerebral edema (swelling) typically develops over 2-3 days following a significant infarction and may peak around day 3-5, potentially persisting for up to 10 days or longer in severe cases [1].
While reperfusion therapies (like intravenous thrombolysis or mechanical thrombectomy) aim to restore blood flow by dissolving or removing the embolus [16, 19], there's a theoretical concern that reperfusion into damaged tissue might increase edema or hemorrhagic transformation [1, 8]. However, the benefits of timely reperfusion in salvaging the penumbra generally outweigh these risks in eligible patients [16, 19]. Symptomatic cerebral edema in embolic infarction tends to follow two main patterns with significant clinical implications [1]:
- Supratentorial Edema: In embolic infarctions affecting the cerebral hemispheres (supratentorial), larger infarct sizes correlate directly with an increased risk and severity of cerebral edema. Occlusion of the MCA trunk by large emboli is particularly prone to causing significant edema ("malignant MCA syndrome"), which can lead to increased intracranial pressure, midline shift, brain herniation (especially temporal lobe herniation), coma, and death if not managed aggressively. Emboli affecting smaller branches typically cause less edema.
- Posterior Fossa Edema: Even relatively small cerebellar infarcts, particularly those affecting the territory of the posterior inferior cerebellar artery (PICA), can cause significant edema within the confined space of the posterior fossa. This can rapidly lead to compression of the fourth ventricle (causing obstructive hydrocephalus) and direct brainstem compression, potentially resulting in sudden neurological deterioration, coma, and respiratory arrest. Urgent neurosurgical consultation for possible surgical decompression (e.g., suboccipital craniectomy) may be necessary in cases of life-threatening posterior fossa swelling.
In situations with significant cerebral edema causing increased intracranial pressure, medical management strategies like maintaining head elevation, ensuring adequate oxygenation and ventilation, and using osmotic diuretics are indicated [1, 16]. Intravenous mannitol is commonly used to draw fluid out of the brain tissue by increasing plasma osmolarity (target typically 300-320 mOsm/L), usually administered as intermittent boluses every few hours while monitoring electrolytes and renal function [1].
The decision regarding the initiation and timing of anticoagulant therapy after an embolic stroke depends heavily on the suspected or confirmed embolic source (e.g., cardiac thrombus vs. other/unknown) and the individual patient's risk profile [1, 10, 20]. Concerns about inducing or worsening hemorrhagic transformation within the infarcted area have led to varying historical opinions and practices [1, 20]. Some have recommended delaying anticoagulation for several days to weeks, particularly after large infarcts, citing a potentially low risk of early recurrent embolism in some scenarios [1]. Others argue that clinically significant hemorrhagic transformation is relatively uncommon, typically occurring mainly with very large infarcts involving deep structures (e.g., basal ganglia involvement with MCA trunk emboli), and that the risk of early recurrent embolism (especially from high-risk cardiac sources like AFib or mural thrombus) often outweighs the risk of anticoagulation-related bleeding [1, 20]. Current guidelines generally favor earlier initiation of anticoagulation (often within 4-14 days, depending on infarct size and bleeding risk) for secondary prevention in patients with cardioembolic sources like AFib, unless there are specific contraindications or very large infarcts [10, 20]. However, immediate anticoagulation is strictly contraindicated in the setting of known or suspected septic emboli (e.g., from endocarditis) due to the high risk of inducing hemorrhage from associated mycotic aneurysms [1, 13].
The underlying source dictates long-term secondary prevention [10, 20]. Most significant cardioembolic events (excluding perhaps marantic, septic, and tumor emboli) result from the formation of thrombi within the heart [3]. These can be microthrombi forming within the left atrial appendage (especially common in atrial fibrillation), larger mural thrombi developing on damaged heart muscle after myocardial infarction, or thrombotic masses forming within ventricular aneurysms or on diseased or prosthetic heart valves [3]. The presence of such thrombi generally warrants therapeutic anticoagulation until the underlying condition is resolved or the risk of recurrent embolism is deemed sufficiently low (or indefinitely if the underlying cause persists) [3, 10, 20]. For example, anticoagulation is typically recommended for at least 3-6 months following acute myocardial infarction if a mural thrombus is present, and usually indefinitely for patients with persistent or paroxysmal atrial fibrillation meeting risk criteria [3, 9, 10].
Atrial fibrillation occurring in the context of significant rheumatic valvular heart disease (particularly mitral stenosis) often necessitates lifelong anticoagulation, even without a prior documented embolic event, due to the very high associated stroke risk [9]. The management of asymptomatic atrial fibrillation related to other causes (like coronary artery disease or hypertension) is guided by stroke risk stratification scores (e.g., CHA₂DS₂-VASc); patients exceeding a certain risk threshold generally benefit from anticoagulation [9, 10]. While anticoagulation clearly reduces stroke risk in these populations compared to no treatment or aspirin alone [9], the optimal duration of anticoagulation in some specific scenarios (e.g., after successful AFib ablation) remains an area of ongoing research and debate [10].
In patients experiencing cerebral embolism where the origin remains undetermined (cryptogenic) despite thorough investigation, the decision regarding long-term anticoagulation is complex [5, 20]. If a high-risk source like paroxysmal AFib or a PFO is suspected but not definitively proven, low-dose anticoagulation (e.g., with warfarin targeting a lower INR, or increasingly with DOACs) might be considered, especially in younger patients or those with recurrent events, always weighing the potential benefits against the bleeding risks and considering contraindications [5, 12, 20]. There are no universally agreed-upon guidelines for the optimal duration of anticoagulation in truly cryptogenic embolic stroke; however, empirical treatment for a period (e.g., 6 months to 1 year), particularly in patients under 50, might be considered reasonable by some clinicians, followed by reassessment [1, 5].
References
- Ropper AH, Samuels MA, Klein JP, Prasad S. Adams and Victor's Principles of Neurology. 11th ed. McGraw Hill; 2019. Chapter 34: Cerebrovascular Diseases (Section on Embolic Infarction).
- Grotta JC, Albers GW, Broderick JP, et al. Stroke: Pathophysiology, Diagnosis, and Management. 7th ed. Elsevier; 2021. Chapter on Mechanisms of Ischemic Stroke.
- Hart RG, Halperin JL, Pearce LA, et al. Cardioembolic stroke. Lancet Neurol. 2017 Jun;16(6):489-500. (Or a similar comprehensive review on cardioembolic stroke).
- Caplan LR. Caplan's Stroke: A Clinical Approach. 5th ed. Cambridge University Press; 2016. Chapter on Large Artery Atherosclerosis (for arterio-arterial embolism).
- Hart RG, Catanese L, Perera KS, Ntaios G, Connolly SJ. Embolic strokes of undetermined source: the case for a new clinical construct. Lancet Neurol. 2017 May;16(5):401-411.
- Caplan LR. Caplan's Stroke: A Clinical Approach. 5th ed. Cambridge University Press; 2016. Chapter on Lacunar and Small Vessel Disease Strokes.
- Ropper AH, Samuels MA, Klein JP, Prasad S. Adams and Victor's Principles of Neurology. 11th ed. McGraw Hill; 2019. Chapter 34: Cerebrovascular Diseases, sections on Collateral Circulation.
- Grotta JC, Albers GW, Broderick JP, et al. Stroke: Pathophysiology, Diagnosis, and Management. 7th ed. Elsevier; 2021. Chapter on Hemorrhagic Transformation of Ischemic Stroke.
- January CT, Wann LS, Calkins H, et al. 2019 AHA/ACC/HRS Focused Update of the 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society. Circulation. 2019 Jul 9;140(2):e125-e151.
- Kernan WN, Ovbiagele B, Black HR, et al; American Heart Association Stroke Council, Council on Cardiovascular and Stroke Nursing, Council on Clinical Cardiology, and Council on Peripheral Vascular Disease. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014 Jul;45(7):2160-236. (Or more recent updates).
- Holmes DR Jr, Reddy VY, Turi ZG, et al; PROTECT AF Investigators. Percutaneous closure of the left atrial appendage versus warfarin therapy for prevention of stroke in patients with atrial fibrillation: a randomised non-inferiority trial. Lancet. 2009 Aug 15;374(9689):534-42. (Example LAA closure trial).
- Saver JL, Carroll JD, Thaler DE, et al; RESPECT Investigators. Long-Term Outcomes of Patent Foramen Ovale Closure or Medical Therapy after Cryptogenic Stroke. N Engl J Med. 2017 Sep 14;377(11):1022-1032. (Example PFO closure trial).
- Baddour LM, Wilson WR, Bayer AS, et al; American Heart Association Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease of the Council on Cardiovascular Disease in the Young, Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and Stroke Council. Infective Endocarditis in Adults: Diagnosis, Antimicrobial Therapy, and Management of Complications: A Scientific Statement for Healthcare Professionals From the American Heart Association. Circulation. 2015 Oct 13;132(15):1435-86.
- Osborn AG, Hedlund GL, Salzman KL. Osborn's Brain: Imaging, Pathology, and Anatomy. 2nd ed. Elsevier; 2017. Section on Stroke and Vascular Disease.
- Lazzaro NA, Wright B, Castillo M, et al. Artery of Percheron infarction: imaging patterns and clinical spectrum. AJNR Am J Neuroradiol. 2010 Aug;31(7):1283-9.
- Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2019 Dec;50(12):e344-e418.
- Caplan LR. Stroke Mimics. Semin Neurol. 2016 Apr;36(2):203-12.
- Grotta JC, Albers GW, Broderick JP, et al. Stroke: Pathophysiology, Diagnosis, and Management. 7th ed. Elsevier; 2021. Chapter on Pathophysiology of Ischemic Stroke (Penumbra).
- Goyal M, Menon BK, van Zwam WH, et al; HERMES collaborators. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016 Apr 23;387(10029):1723-31.
- Paciaroni M, Agnelli G, Falocci N, et al. Early Recurrence and Cerebral Bleeding in Patients With Acute Ischemic Stroke and Atrial Fibrillation: Effect of Anticoagulation and Its Timing: The RAF Study. Stroke. 2015 Aug;46(8):2175-82. (Example study on timing of anticoagulation).
- Nogueira RG, Jadhav AP, Haussen DC, et al; DAWN Trial Investigators. Thrombectomy 6 to 24 Hours after Stroke with a Mismatch between Deficit and Infarct. N Engl J Med. 2018 Jan 4;378(1):11-21. (Extended window thrombectomy trial).
See also
- Ischemic stroke, cerebral ischemia
- Vertebrobasilar insufficiency (VBI) with vertigo symptom
- Somatoform autonomic dysfunction
- Dizziness, stuffiness in ear and tinnitus
- Ischemic brain disease:
- Atherosclerotic thrombosis
- Atherothrombotic occlusion of internal carotid artery
- Asymptomatic carotid bifurcation stenosis with noise
- Atherothrombotic occlusion of vertebrobasilar and posterior cerebral arteries
- Atherothrombotic occlusion of posterior cerebral artery
- Atherothrombotic occlusion of vertebral and posterior inferior cerebellar arteries (PICA)
- Atherothrombotic occlusion of basilar artery
- Small-vessel stroke (lacunar infarction)
- Other causes of ischemic stroke (cerebral infarction)
- Cerebral embolism
- Spontaneous intracranial (subarachnoid) and intracerebral hemorrhage:
- Arteriovenous malformations of the brain
- Hypertensive intracerebral hemorrhage
- Cerebral arteries inflammatory diseases (cerebral arteritis)
- Giant intracranial aneurysms
- Other causes of intracerebral hemorrhage
- Lobar intracerebral hemorrhage
- Saccular aneurysm and subarachnoid hemorrhage
- Mycotic intracranial aneurysms
- Repeated cerebral artery aneurysm rupture
- Communicating hydrocephalus after intracerebral hemorrhage with ruptured aneurysm
- Cerebral vasospasm
- Cerebrovascular diseases - ischemic stroke, transient ischemic attack (TIA):
- Transient ischemic attack (TIA)
- Sigmoid sinus suppurative thrombophlebitis with thrombosis

 in close proximity to the tumor on gadolinium-enhanced T1-weighted MRI sequences.jpg)