Basilar artery atherothrombosis
- Basilar Artery Anatomy and Stroke Pathophysiology
- Basilar Artery Occlusion: Clinical Symptoms and Syndromes
- Transient Ischemic Attacks (TIAs) in the Basilar Artery Territory
- Ischemic Stroke Patterns in the Basilar Artery Territory
- Superior Cerebellar Artery (SCA) Stroke
- Anterior Inferior Cerebellar Artery (AICA) Stroke
- Diagnosis and Imaging for Basilar Artery Stroke
- Treatment Strategies for Basilar Artery Ischemic Stroke
Basilar Artery Anatomy and Stroke Pathophysiology
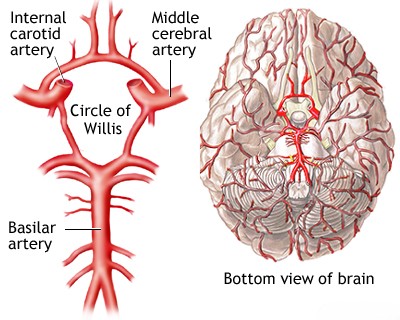
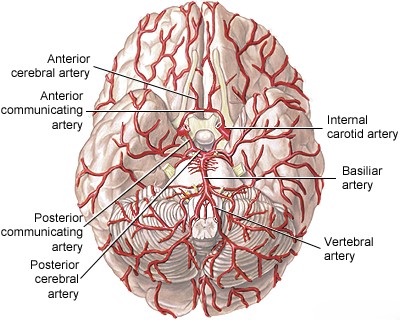
The basilar artery is a vital blood vessel located at the base of the brain [1, 2]. It is typically formed by the convergence of the two vertebral arteries at the junction between the medulla oblongata and the pons [1, 2]. It ascends along the anterior surface of the pons within the pontine cistern and terminates, usually in the interpeduncular fossa, by bifurcating into the two posterior cerebral arteries (PCAs), which supply the occipital lobes and parts of the temporal lobes and thalamus [1, 2].
Branches arising from the basilar artery supply crucial structures including the pons and the superior and anterior portions of the cerebellum [1, 2]. These branches can be categorized into three main groups [1, 2]:
- Paramedian pontine arteries: Typically 7-10 small branches supplying wedge-shaped territories on either side of the midline pons.
- Short circumferential arteries: Usually 5-7 branches supplying the lateral two-thirds of the pons and the middle and superior cerebellar peduncles.
- Long circumferential arteries: Primarily the bilateral Superior Cerebellar Arteries (SCA) and Anterior Inferior Cerebellar Arteries (AICA), which course around the pons to supply the cerebellar hemispheres and parts of the brainstem.
Atherosclerotic plaques can develop anywhere along the basilar artery trunk, but they are most commonly found in the proximal segment (near its origin from the vertebral arteries) and the distal segment (near the bifurcation into the PCAs) [1, 3]. The distal segments of the vertebral arteries are also frequent sites of disease [1]. Atherothrombosis (atherosclerosis complicated by clot formation) can lead to stenosis (narrowing) or complete occlusion of the basilar artery, often involving the proximal basilar and/or one or both distal vertebral arteries [1, 3].
The clinical consequences of basilar artery occlusion vary significantly depending on the location of the blockage, the speed of occlusion, and crucially, the adequacy of collateral blood flow [1, 4]. Retrograde flow via the posterior communicating arteries (PComAs), connecting the internal carotid system to the posterior cerebral arteries, can sometimes compensate for proximal basilar occlusion [1, 4].
Basilar artery occlusion can result from local atherothrombosis or from an embolus [1, 3]. Emboli may originate from the heart (cardioembolism), the aortic arch, or more proximally located atherosclerotic plaques in the vertebral or basilar arteries themselves (artery-to-artery embolism) [1, 3]. Emboli are a more frequent cause of occlusion of the *distal* basilar artery ("top of the basilar" syndrome) or its branches (including the PCAs) [1, 3].
Basilar Artery Occlusion: Clinical Symptoms and Syndromes
The brainstem contains numerous critical neuronal pathways and cranial nerve nuclei packed in close proximity [2]. Ischemia (lack of blood flow) in this region, caused by basilar artery or branch occlusion, can therefore produce a wide array of complex neurological syndromes [1, 3]. Key structures frequently affected include the descending corticospinal tracts (motor control to body), corticobulbar tracts (motor control to face/head), cerebellar pathways (coordination via middle and superior cerebellar peduncles), ascending spinothalamic tracts (pain and temperature sensation), medial lemniscus (touch and proprioception), and various cranial nerve nuclei (III-XII) [1, 2].
While specific symptoms can sometimes suggest involvement of particular branches, it's often challenging clinically to pinpoint the exact site of occlusion (main trunk vs. branch) based solely on initial presentation, especially during transient ischemic attacks [1]. However, recognizing the overall pattern of vertebrobasilar insufficiency or acute occlusion is crucial for timely treatment [1]. A hallmark of significant basilar artery territory ischemia is the combination of [1, 3]:
- Bilateral long tract signs (motor and/or sensory deficits affecting both sides of the body)
- Cranial nerve deficits (e.g., diplopia, facial weakness, dysarthria, dysphagia)
- Cerebellar signs (e.g., ataxia, vertigo, nystagmus)
A devastating consequence of bilateral infarction of the ventral pons is the "locked-in syndrome", where patients are conscious but suffer complete paralysis of all four limbs (quadriplegia) and the muscles controlling speech and swallowing (anarthria), often preserving only vertical eye movements and blinking [1, 3]. This is typically due to bilateral corticospinal and corticobulbar tract damage, while consciousness is maintained because the ascending reticular activating system in the pontine tegmentum may be spared [1].
A primary goal in diagnosis is to identify impending basilar artery occlusion *before* a catastrophic stroke occurs [1]. Prodromal symptoms, such as recurrent, stereotyped transient ischemic attacks (TIAs) or a gradually worsening ("stuttering") stroke pattern, strongly suggest underlying high-grade stenosis or occlusion, often due to atherosclerotic thrombosis in the distal vertebral or proximal basilar arteries, which may be amenable to intervention [1, 3].
Classic Brainstem Vascular Syndromes (Illustrative Examples) [1, 3]:
Note: These are classic descriptions; presentations in practice are often partial or mixed.
| Signs and Symptoms | Structures Typically Involved |
|---|---|
| 1. Medial Medullary Syndrome (Dejerine) (Occlusion of vertebral artery or anterior spinal artery branch) |
|
| Ipsilateral (same side as lesion): | |
| Paralysis and atrophy of half the tongue | Hypoglossal nerve (CN XII) fibers/nucleus |
| Contralateral (opposite side to lesion): | |
| Hemiplegia (arm and leg paralysis), sparing face | Pyramidal tract (before decussation) |
| Impaired proprioception and discriminative touch | Medial lemniscus |
| 2. Lateral Medullary Syndrome (Wallenberg) (Occlusion commonly of PICA or vertebral artery branch) |
|
| Ipsilateral: | |
| Vertigo, nausea, vomiting, nystagmus | Vestibular nuclei |
| Dysphagia, hoarseness, decreased gag reflex | Nucleus ambiguus (CN IX, X fibers) |
| Ipsilateral ataxia (limb and gait) | Inferior cerebellar peduncle and cerebellum |
| Facial pain and temperature loss | Spinal trigeminal nucleus and tract (CN V) |
| Horner’s syndrome (ptosis, miosis, anhidrosis) | Descending sympathetic pathways |
| Loss of taste (less common) | Nucleus solitarius |
| Numbness of ipsilateral arm/trunk/leg (less common) | Cuneate/gracile nuclei |
| Contralateral: | |
| Impaired pain and temperature sensation (body, sometimes face) | Spinothalamic tract |
| Signs and Symptoms | Structures Typically Involved |
|---|---|
| 1. Medial Inferior Pontine Syndrome (Foville) (Occlusion of paramedian branch of basilar artery) |
|
| Ipsilateral: | |
| Paralysis of conjugate gaze to side of lesion (lateral gaze palsy) | Paramedian pontine reticular formation (PPRF) or Abducens nucleus (CN VI) |
| Facial nerve palsy (LMN type) | Facial nerve (CN VII) fascicle |
| Contralateral: | |
| Hemiplegia (arm and leg) | Corticospinal tract |
| Impaired proprioception/touch (variable) | Medial lemniscus |
| 2. Lateral Inferior Pontine Syndrome (Occlusion typically of AICA) |
|
| Ipsilateral: | |
| Vertigo, nausea, vomiting, nystagmus | Vestibular nuclei/nerve (CN VIII) |
| Hearing loss, tinnitus | Cochlear nuclei/nerve (CN VIII) |
| Facial paralysis (LMN type) | Facial nucleus/nerve (CN VII) |
| Lateral gaze palsy | PPRF / Abducens nucleus (CN VI) |
| Ataxia | Middle cerebellar peduncle / Cerebellum |
| Facial sensory loss | Spinal trigeminal nucleus/tract (CN V) |
| Horner's syndrome | Descending sympathetic fibers |
| Contralateral: | |
| Impaired pain and thermal sense (body) | Spinothalamic tract |
| 3. Medial Mid-Pontine Syndrome (Occlusion of paramedian branch of mid-basilar artery) |
|
| Ipsilateral: | |
| Ataxia (variable, more prominent if bilateral) | Pontine nuclei / Pontocerebellar fibers |
| Contralateral: | |
| Hemiparesis (face, arm, leg) | Corticospinal and corticobulbar tracts |
| Impaired proprioception/touch (variable) | Medial lemniscus |
| 4. Lateral Mid-Pontine Syndrome (Occlusion of short circumferential artery) |
|
| Ipsilateral: | |
| Ataxia | Middle cerebellar peduncle |
| Paralysis of muscles of mastication | Trigeminal motor nucleus/nerve (CN V) |
| Impaired facial sensation | Trigeminal sensory nucleus/nerve (CN V) |
| Contralateral (Less common): | |
| Impaired pain and temperature sensation (body) | Spinothalamic tract |
| Signs and Symptoms | Structures Typically Involved |
|---|---|
| 1. Medial Midbrain Syndrome (Weber) (Occlusion of paramedian branches from upper basilar/PCA) |
|
| Ipsilateral: | |
| Oculomotor nerve palsy (CN III): eye 'down and out', ptosis, dilated pupil | Oculomotor nerve fascicles |
| Contralateral: | |
| Hemiplegia (face, arm, leg) | Cerebral peduncle (corticospinal/bulbar tracts) |
| 2. Central/Tegmental Midbrain Syndromes (Claude / Benedikt) (Occlusion of penetrating arteries from PCA/upper basilar) |
|
| Ipsilateral: | |
| Oculomotor nerve palsy (CN III) | Oculomotor nerve fascicles / Red nucleus |
| Contralateral: | |
| Ataxia, tremor, choreoathetosis (involuntary movements) | Red nucleus and/or dentatorubrothalamic tract |
Transient Ischemic Attacks (TIAs) in the Basilar Artery Territory
Transient ischemic attacks (TIAs) involving the vertebrobasilar system often precede a major stroke and are frequently manifestations of underlying vertebrobasilar insufficiency (VBI), usually due to atherosclerosis [1, 3]. When TIAs relate to impending occlusion of the proximal basilar artery, symptoms may reflect ischemia in both the pons and medulla [1].
Patients commonly report dizziness or vertigo [1]. This is often described as a sensation of spinning, floating, rocking, tilting, or general instability, sometimes with a feeling of the environment moving ("oscillopsia") [1]. While vertigo is a very characteristic symptom of VBI, it is rarely the *only* symptom when caused by vertebrobasilar ischemia [1]. Typically, it is accompanied by other brainstem or cerebellar symptoms [1].
A combination of transient vertigo with symptoms such as diplopia (double vision), dysarthria (slurred speech), facial or perioral numbness, and/or hemisensory loss strongly suggests a TIA in the vertebrobasilar territory [1]. The presence of hemiparesis (weakness on one side of the body) during a TIA usually indicates involvement extending to the pons or midbrain, implicating the basilar artery itself, rather than just a distal vertebral artery [1].
TIAs warning of impending basilar artery thrombosis are often brief (5-30 minutes), frequent (sometimes multiple times per day), and stereotyped (similar symptoms each time) [1]. This pattern suggests a hemodynamic cause (temporary drop in blood flow past a severe stenosis) rather than recurrent embolism [1]. While TIAs often involve bilateral symptoms (reflecting midline basilar artery involvement), strokes from occlusion of individual branches typically cause unilateral brainstem deficits [1].
Ischemic Stroke Patterns in the Basilar Artery Territory
Completed ischemic stroke due to atherothrombotic occlusion of the basilar artery trunk typically results in bilateral neurological deficits reflecting widespread brainstem damage [1, 3]. Common presentations include combinations of:
- Bilateral motor weakness (hemiparesis progressing to quadriparesis)
- Bilateral sensory deficits
- Gaze palsies or complex ophthalmoplegia (impaired eye movements)
- Bulbar palsy (dysarthria, dysphagia)
- Altered consciousness (ranging from drowsiness to coma, especially with upper brainstem involvement)
- Combinations of cranial nerve palsies and long tract signs (motor/sensory)
In contrast, occlusion of individual branches of the basilar artery (paramedian, short circumferential, or long circumferential like SCA/AICA) usually leads to unilateral deficits [1]. These often manifest as specific, well-defined clinical syndromes (some described above and below), frequently classified as "lacunar strokes" if resulting from occlusion of small penetrating arteries supplying deep brainstem structures [1, 3].
Superior Cerebellar Artery (SCA) Stroke
Occlusion of the Superior Cerebellar Artery (SCA), a long circumferential branch of the distal basilar artery, typically causes infarction in the superior cerebellum, cerebellar peduncles, and sometimes parts of the upper lateral pons and midbrain [1, 3]. Key clinical features include [1]:
- Ipsilateral cerebellar ataxia: Marked incoordination of limbs (especially arm) and gait disturbance (falling towards the side of the lesion), due to damage to the superior cerebellar peduncle and cerebellar hemisphere.
- Nausea and vomiting
- Dysarthria: Often "scanning" or explosive speech.
- Contralateral loss of pain and temperature sensation: Affecting the limbs, trunk, and sometimes face (due to spinothalamic and trigeminothalamic tract involvement). Proprioception and touch are usually spared.
- Other potential signs: Partial hearing loss, ipsilateral Horner's syndrome, atactic tremor, palatal myoclonus.
Complete SCA territory infarction is less common than partial syndromes due to variability in arterial anatomy and collateral supply [1].
Anterior Inferior Cerebellar Artery (AICA) Stroke
Occlusion of the Anterior Inferior Cerebellar Artery (AICA), usually arising from the lower or mid-basilar artery, leads to infarction in the lateral pons, middle cerebellar peduncle, and anterior inferior cerebellum [1, 3]. The clinical syndrome can be variable because AICA's size and territory often vary inversely with those of the Posterior Inferior Cerebellar Artery (PICA, typically a branch of the vertebral artery) [1].
Classic signs of AICA syndrome include [1]:
- Ipsilateral hearing loss and tinnitus: Due to infarction of the cochlear nuclei or internal auditory artery (often arises from AICA).
- Ipsilateral facial weakness: Lower motor neuron type (affecting forehead and lower face) due to facial nerve nucleus/fascicle involvement.
- Vertigo, nausea, vomiting, nystagmus: Due to vestibular nuclei involvement.
- Ipsilateral cerebellar ataxia: Affecting limbs and gait.
- Ipsilateral loss of facial pain and temperature: Due to spinal trigeminal tract involvement.
- Contralateral loss of pain and temperature sensation over the body (spinothalamic tract).
- Other potential signs: Ipsilateral Horner's syndrome, ipsilateral gaze palsy.
Proximal AICA occlusion may also involve the corticospinal tract, causing contralateral hemiparesis [1].
As mentioned, occlusion of the smaller paramedian or short circumferential branches causes more restricted infarcts within the pons, leading to various lacunar syndromes [1]. Differentiating these based on clinical signs alone can be challenging, but certain patterns are suggestive [1]:
- Dysarthria-clumsy hand syndrome often points to a pontine base infarct.
- Isolated hemiparesis can occur with pontine infarcts but also with supratentorial lesions (e.g., internal capsule).
- Crossed sensory findings (e.g., ipsilateral face, contralateral body) strongly suggest a brainstem localization. Dissociated sensory loss (pain/temp affected, touch/proprioception spared) is also typical of brainstem (spinothalamic tract) lesions.
- Specific cranial nerve palsies (e.g., CN III, VI, VII) are crucial for localizing the level of the lesion within the brainstem (midbrain, pons, medulla).
(Classic eponyms like Weber, Claude, Benedikt, Foville, Raymond-Cestan, Millard-Gubler refer to specific combinations of these signs correlating with lesions in particular brainstem locations) [1].
Diagnosis and Imaging for Basilar Artery Stroke
Prompt and accurate diagnosis is critical for guiding treatment in suspected vertebrobasilar stroke [1, 5].
Differential Diagnosis of Basilar Artery Stroke Symptoms [1, 6]
| Condition | Key Features / Distinguishing Points | Typical Investigations / Findings |
|---|---|---|
| Basilar Artery Ischemic Stroke (Thrombotic/Embolic) | Sudden or stuttering onset of bilateral motor/sensory signs, cranial nerve palsies (III-XII), cerebellar signs (ataxia, vertigo), altered consciousness (coma, locked-in). | MRI (DWI) confirms acute infarct in pons, midbrain, cerebellum, thalamus, occipital lobes. CTA/MRA/DSA shows basilar/vertebral occlusion or stenosis. |
| Posterior Circulation Hemorrhage (Pons, Cerebellum) | Sudden onset, severe headache, vomiting, rapid decline in consciousness. Pontine: pinpoint pupils, quadriplegia, coma. Cerebellar: ataxia, vomiting, inability to stand, CN palsies, potential hydrocephalus/brainstem compression. Often hypertensive. | Non-contrast CT head shows hemorrhage in pons or cerebellum. MRI provides more detail. Angiography may be needed if underlying vascular lesion suspected. |
| Migraine with Brainstem Aura | Transient brainstem symptoms (vertigo, dysarthria, diplopia, ataxia, bilateral sensory/visual changes) followed by headache. History of migraine. Fully reversible. | Clinical diagnosis. Normal imaging. Normal exam between attacks. |
| Peripheral Vestibulopathy | Acute vertigo, nausea, vomiting, nystagmus. Usually NO other brainstem signs. | Clinical exam (HINTS). Normal imaging. Audiometry if hearing loss. |
| Metabolic/Toxic Encephalopathy | Diffuse brain dysfunction (confusion, altered LOC, coma). May have ataxia, nystagmus, but usually symmetric and accompanied by other systemic signs. | Specific lab abnormalities (glucose, electrolytes, toxins, ammonia, etc.). Imaging usually non-specific. EEG shows diffuse slowing. |
| Wernicke's Encephalopathy | Triad of ophthalmoplegia (often CN VI palsy, gaze palsy), ataxia, confusion. History of malnutrition, alcoholism. Due to thiamine deficiency. | Clinical diagnosis. MRI may show characteristic signal changes (mammillary bodies, thalamus, periaqueductal gray). Response to thiamine administration. |
| Seizure (esp. Generalized or Occipital) | Can cause altered consciousness, post-ictal confusion, visual phenomena, or rarely focal brainstem signs post-ictally. | History of event. EEG. Post-ictal state. Imaging usually normal unless underlying cause. |
| Multiple Sclerosis (MS) Relapse | Acute/subacute onset of brainstem or cerebellar symptoms (diplopia, vertigo, ataxia, dysarthria). History of prior events possible. | MRI shows demyelinating lesions in posterior fossa +/- enhancement. |
| Posterior Fossa Tumor | Usually progressive symptoms (ataxia, headache, CN palsies). Can present acutely with hemorrhage or hydrocephalus. | MRI with contrast shows mass lesion. |
Neuroimaging:
- Non-Contrast Computed Tomography (CT): This is typically the first imaging study performed in the emergency setting [5]. Its primary role is to rapidly exclude intracranial hemorrhage, which would preclude thrombolytic therapy [5]. While CT can show established infarcts (usually visible as low-density areas after 12-48 hours), it is insensitive for detecting acute ischemia, especially within the first few hours, and visualization of the posterior fossa (brainstem and cerebellum) is often limited by beam-hardening artifacts from surrounding bone [5, 7]. A dense basilar artery sign (hyperdensity within the vessel) can sometimes suggest acute thrombosis but is not reliably present [7].
- Magnetic Resonance Imaging (MRI): MRI, particularly with Diffusion-Weighted Imaging (DWI) sequences, is the most sensitive technique for detecting acute ischemic stroke, often within minutes to hours of onset [5, 7]. It provides excellent visualization of the brainstem and cerebellum without bony artifact [7]. MRI can identify small lacunar infarcts caused by branch occlusions, larger territorial infarcts, and helps differentiate stroke from mimics like tumors (e.g., pontine glioma) or demyelination (e.g., multiple sclerosis plaques) [7]. FLAIR sequences can help estimate stroke age, and Gradient Echo (GRE) or Susceptibility Weighted Imaging (SWI) sequences are sensitive for detecting hemorrhage [7].
Vascular Imaging: Essential for identifying the location and nature of the vascular lesion (stenosis, occlusion, dissection, aneurysm) [5].
- CT Angiography (CTA): Widely available and rapidly performed, often immediately after the non-contrast CT [5]. Involves intravenous contrast injection to visualize arteries. CTA provides good detail of the larger vessels, including the vertebral and basilar arteries, and can detect significant stenosis or occlusion [5]. Relative contraindications include severe contrast allergy and significant renal impairment.
- MR Angiography (MRA): Can be performed without ionizing radiation or iodinated contrast (though gadolinium contrast is often used for better detail, with its own contraindications like severe renal failure) [5, 7]. Provides good visualization of the vertebrobasilar system but may be less accurate than CTA or DSA for quantifying stenosis severity [7].
- Digital Subtraction Angiography (DSA): Considered the gold standard for vessel imaging, providing the highest spatial resolution [5, 7]. It is an invasive procedure involving catheter insertion (usually via the femoral artery) and direct injection of contrast into the target vessels [7]. DSA carries a small but significant risk of complications, including stroke, vessel dissection, or access site issues [1]. It is typically reserved for cases where non-invasive imaging is inconclusive or when endovascular treatment (like thrombectomy or stenting) is planned [5]. Transient cortical blindness or confusion is a rare complication following contrast injection into the posterior circulation [1].
- Transcranial Doppler (TCD) Ultrasound: A non-invasive bedside technique that can assess blood flow velocities and direction within the major intracranial arteries, including the vertebral and basilar arteries [5]. Useful for detecting severe stenosis or occlusion and monitoring for emboli or vasospasm, but provides less anatomical detail than angiography [5].
Other diagnostic tests may include electrocardiogram (ECG) and echocardiography (transthoracic or transesophageal) to investigate for potential cardiac sources of embolism (e.g., atrial fibrillation, valvular disease), and blood tests (including inflammatory markers, lipid profile, glucose, coagulation studies) [1, 5].
Treatment Strategies for Basilar Artery Ischemic Stroke
Management depends critically on the time from symptom onset, the severity of the neurological deficit, the imaging findings (presence of hemorrhage, extent of established infarct, location of vascular occlusion/stenosis), and the patient's overall condition [1, 5].
Acute Treatment (within hours of onset): Reperfusion Therapies
- Intravenous Thrombolysis (IV tPA): Alteplase (tPA) can be administered within a specific time window (typically 3 to 4.5 hours) from symptom onset if there is no hemorrhage on imaging and the patient meets eligibility criteria [5, 8]. Its effectiveness may be lower for large clots occluding the basilar artery compared to smaller vessel occlusions [5].
- Endovascular Therapy (EVT) / Mechanical Thrombectomy: This is the primary treatment for acute ischemic stroke caused by large vessel occlusion (LVO), including the basilar artery, in eligible patients [5, 9]. It involves inserting a catheter via an artery (usually femoral) and using devices (stent retrievers or aspiration catheters) to physically remove the clot and restore blood flow [9]. EVT can be effective within an extended time window (up to 24 hours or sometimes longer in selected patients based on advanced imaging showing salvageable brain tissue) [5, 9]. Outcomes are generally better with faster reperfusion [9].
General Acute Management [1, 5]:
- Admission to a specialized stroke unit for close monitoring.
- Supportive care: Airway management (intubation if necessary), blood pressure control (avoiding excessive lowering initially), glucose management, fever control, hydration.
- Antiplatelet therapy (e.g., Aspirin) is typically initiated once hemorrhage is excluded (often 24 hours after IV tPA if given).
- Anticoagulation (e.g., Heparin): Its role in acute atherothrombotic stroke is limited and generally not recommended routinely. It may be considered in specific situations like documented cardioembolism, arterial dissection, or sometimes as a bridge to other treatments, but carries bleeding risks.
- Management of potential complications: Cerebral edema, aspiration pneumonia, deep vein thrombosis (DVT prophylaxis).
Secondary Prevention (Long-term management to prevent recurrence) [1, 10]:
- Antiplatelet Agents: Standard for non-cardioembolic stroke. Options include Aspirin, Clopidogrel, or Aspirin+Dipyridamole. Dual antiplatelet therapy (Aspirin + Clopidogrel) may be used short-term (e.g., 21-90 days) for minor stroke/TIA or after stenting.
- Anticoagulation: Indicated for strokes caused by cardioembolism (e.g., atrial fibrillation). Warfarin or Direct Oral Anticoagulants (DOACs) are used. Long-term anticoagulation is generally *not* first-line for atherosclerotic basilar artery disease unless specific indications exist (e.g., recurrent events despite antiplatelets, though this is controversial).
- Statins: High-intensity statin therapy is recommended for all patients with atherosclerotic stroke, regardless of baseline cholesterol levels, to stabilize plaque and reduce cardiovascular risk.
- Risk Factor Modification: Essential for long-term prevention. This includes:
- Strict blood pressure control.
- Optimal diabetes management.
- Smoking cessation.
- Healthy diet and regular exercise.
- Weight management.
- Intracranial Angioplasty and Stenting: May be considered for patients with symptomatic high-grade (>70%) stenosis of the basilar artery who have recurrent symptoms despite optimal medical therapy [11]. However, this procedure carries significant risks (including periprocedural stroke) and its benefit over aggressive medical management remains debated (informed by trials like SAMMPRIS [11]) [1]. It should only be performed in experienced centers [1].
- Rehabilitation: Physical therapy, occupational therapy, and speech therapy are crucial for maximizing functional recovery after stroke [1].
The prognosis after basilar artery stroke varies widely, ranging from complete recovery to severe disability or death, depending largely on the severity of the initial stroke, the location and extent of infarction, the patient's age and comorbidities, and the timeliness and success of reperfusion therapies [1, 3].
References
- Ropper AH, Samuels MA, Klein JP, Prasad S. Adams and Victor's Principles of Neurology. 11th ed. McGraw Hill; 2019. Chapter 34: Cerebrovascular Diseases (Sections on Posterior Circulation Stroke, Basilar Artery Occlusion).
- Blumenfeld H. Neuroanatomy through Clinical Cases. 2nd ed. Sinauer Associates; 2010. Chapter 18: Brainstem III: Vascular Supply.
- Caplan LR. Caplan's Stroke: A Clinical Approach. 5th ed. Cambridge University Press; 2016. Chapter on Posterior Circulation Stroke Syndromes.
- Ropper AH, Samuels MA, Klein JP, Prasad S. Adams and Victor's Principles of Neurology. 11th ed. McGraw Hill; 2019. Chapter 34: Cerebrovascular Diseases, sections on Collateral Circulation.
- Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the Early Management of Patients With Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2019 Dec;50(12):e344-e418.
- Caplan LR. Stroke Mimics. Semin Neurol. 2016 Apr;36(2):203-12.
- Osborn AG, Hedlund GL, Salzman KL. Osborn's Brain: Imaging, Pathology, and Anatomy. 2nd ed. Elsevier; 2017. Section on Stroke and Vascular Disease (Posterior Circulation).
- Hacke W, Kaste M, Bluhmki E, et al; ECASS Investigators. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008 Sep 25;359(13):1317-29.
- Goyal M, Menon BK, van Zwam WH, et al; HERMES collaborators. Endovascular thrombectomy after large-vessel ischaemic stroke: a meta-analysis of individual patient data from five randomised trials. Lancet. 2016 Apr 23;387(10029):1723-31. (And subsequent trials on EVT for posterior circulation).
- Kernan WN, Ovbiagele B, Black HR, et al; American Heart Association Stroke Council, Council on Cardiovascular and Stroke Nursing, Council on Clinical Cardiology, and Council on Peripheral Vascular Disease. Guidelines for the prevention of stroke in patients with stroke and transient ischemic attack: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014 Jul;45(7):2160-236. (Or more recent updates).
- Chimowitz MI, Lynn MJ, Derdeyn CP, et al; SAMMPRIS Trial Investigators. Stenting versus aggressive medical therapy for intracranial arterial stenosis. N Engl J Med. 2011 Sep 15;365(11):993-1003.
See also
- Ischemic stroke, cerebral ischemia
- Vertebrobasilar insufficiency (VBI) with vertigo symptom
- Somatoform autonomic dysfunction
- Dizziness, stuffiness in ear and tinnitus
- Ischemic brain disease:
- Atherosclerotic thrombosis
- Atherothrombotic occlusion of internal carotid artery
- Asymptomatic carotid bifurcation stenosis with noise
- Atherothrombotic occlusion of vertebrobasilar and posterior cerebral arteries
- Atherothrombotic occlusion of posterior cerebral artery
- Atherothrombotic occlusion of vertebral and posterior inferior cerebellar arteries (PICA)
- Atherothrombotic occlusion of basilar artery
- Small-vessel stroke (lacunar infarction)
- Other causes of ischemic stroke (cerebral infarction)
- Cerebral embolism
- Spontaneous intracranial (subarachnoid) and intracerebral hemorrhage:
- Arteriovenous malformations of the brain
- Hypertensive intracerebral hemorrhage
- Cerebral arteries inflammatory diseases (cerebral arteritis)
- Giant intracranial aneurysms
- Other causes of intracerebral hemorrhage
- Lobar intracerebral hemorrhage
- Saccular aneurysm and subarachnoid hemorrhage
- Mycotic intracranial aneurysms
- Repeated cerebral artery aneurysm rupture
- Communicating hydrocephalus after intracerebral hemorrhage with ruptured aneurysm
- Cerebral vasospasm
- Cerebrovascular diseases - ischemic stroke, transient ischemic attack (TIA):
- Transient ischemic attack (TIA)
- Sigmoid sinus suppurative thrombophlebitis with thrombosis