Hypertensive intracerebral hemorrhage
- Causes and Pathophysiology of Hypertensive Intracerebral Hemorrhage (ICH)
- Clinical Syndromes of Hypertensive Intracerebral Hemorrhage
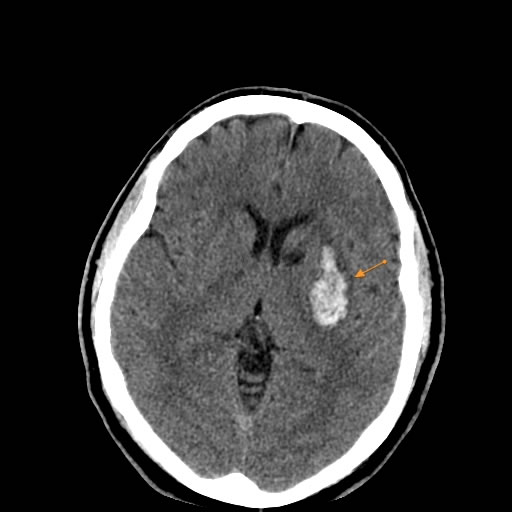
- Putaminal Hypertensive Intracerebral Hemorrhages
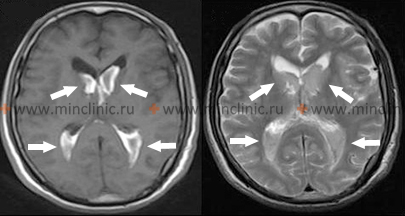
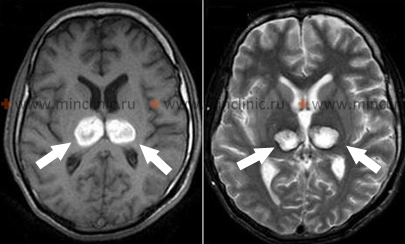
- Thalamic Hypertensive Intracerebral Hemorrhages
- Pontine Hypertensive Intracerebral Hemorrhages
- Cerebellar Hypertensive Intracerebral Hemorrhages
- Hypertensive Intracerebral Hemorrhages Diagnosis (Laboratory and Imaging Evaluation)
- Hypertensive Intracerebral Hemorrhages Treatment
Causes and Pathophysiology of Hypertensive Intracerebral Hemorrhage (ICH)
Hypertensive intracerebral hemorrhage (ICH) typically occurs in specific deep brain locations due to the underlying vascular pathology associated with chronic high blood pressure [1, 2]. Common sites include:
- Putamen (part of the basal ganglia) – the most frequent location (approx. 50%), often with extension into the adjacent internal capsule and surrounding central white matter [1, 2].
- Thalamus [1, 2].
- Pons (part of the brainstem) [1, 2].
- Cerebellum [1, 2].
Primary hemorrhages deep within the lobar white matter are less common causes of hypertensive ICH compared to these deep structure locations [1]. Chronic hypertension predominantly affects small penetrating arteries (arterioles) that branch off larger vessels like the middle cerebral artery, basilar artery, or arteries of the Circle of Willis to supply these deep brain structures [1, 3]. Over time, hypertension induces pathological changes in these small vessels, most notably lipohyalinosis [1, 3]. This condition involves segmental damage to the vessel wall with fibrinoid necrosis, thickening of the wall by connective tissue, and deposition of lipid material, leading to luminal narrowing and weakening of the vessel wall, making it prone to rupture under pressure [1, 3].
When a weakened penetrating artery ruptures due to hypertension, the resulting hemorrhage initially presents as a small, often oval-shaped collection of blood [1]. This hematoma typically expands over minutes to hours, dissecting through brain tissue along paths of least resistance, thereby compressing and displacing surrounding brain structures [1]. Hypertensive hemorrhages frequently rupture into the adjacent ventricular system, filling parts of the ventricles with blood [1, 4]. Extension directly through the cortex to the subarachnoid space is relatively rare for deep hypertensive bleeds [1]. Small hypertensive hemorrhages (e.g., 1-2 cm³ volume) might remain localized within the deep gray or white matter without significant mass effect or rupture into the cerebrospinal fluid (CSF) pathways [1]. However, massive hypertensive hemorrhages can cause severe compression of adjacent structures like the ventricles or brainstem, leading to significant midline shift, elevated intracranial pressure, stupor, coma, and potentially death [1]. Most hypertensive hemorrhages develop relatively rapidly, often within minutes to the first hour [1]. Some may show gradual enlargement over several hours (e.g., up to 24-48 hours), particularly in patients taking anticoagulants [5]. Unlike bleeding from a ruptured saccular aneurysm which can re-rupture, hypertensive hemorrhages typically cease bleeding spontaneously, likely due to tamponade effect from surrounding tissue pressure and clot formation [1]. Following the initial bleed, edema (swelling) develops in the compressed brain tissue surrounding the intracerebral hematoma [1, 4]. This perifocal edema contributes significantly to the overall mass effect and increase in intracranial pressure, often causing neurological worsening in the days following the hemorrhage [1]. The resolution process begins within about 48 hours, as macrophages infiltrate the hematoma and start engulfing the extravasated blood [1]. Over the subsequent weeks to months (typically 1-6 months), the hematoma is gradually resorbed, usually evolving into a slit-like cavity surrounded by a glial scar containing residual iron deposits (hemosiderin) and macrophages [1, 4].
Clinical Syndromes of Hypertensive Intracerebral Hemorrhage
Hypertensive intracerebral hemorrhage can occur in any individual with hypertension, but it is most frequently encountered in those with a history of chronic, often poorly controlled, essential hypertension [1, 2]. The hemorrhage typically occurs while the patient is awake and active, although it is not necessarily precipitated by strenuous physical exertion [1]. In contrast to the often instantaneous onset of symptoms seen with embolic stroke or subarachnoid hemorrhage, hypertensive hemorrhagic stroke symptoms usually develop smoothly over a period of minutes (e.g., 5-30 minutes), although sometimes progression can occur over several hours [1]. The specific symptoms and their severity are determined by the location and size of the hemorrhage within the brain [1, 2].
Putaminal Hypertensive Intracerebral Hemorrhage
Hemorrhage originating in the putamen (part of the basal ganglia) is the most common type associated with hypertension [1, 2]. The bleeding often extends medially to involve the adjacent internal capsule (a critical white matter pathway) and laterally into the external capsule or surrounding white matter [1].
Extensive putaminal hemorrhages can cause immediate loss of consciousness leading to coma, accompanied by contralateral hemiplegia (paralysis on the opposite side of the body) [1, 2]. More typically, patients experience a progressively worsening headache, followed within minutes by evolving neurological signs such as contralateral facial drooping, slurred speech (dysarthria), confusion, or language difficulties (aphasia if the dominant hemisphere is affected) [1]. Progressive weakness of the contralateral limbs develops [1]. A characteristic sign is conjugate gaze deviation (eyes forced towards the side of the hemorrhage) contralateral to the hemiparesis (i.e., looking away from the weak side) [1]. The relatively smooth progression of these symptoms over 5-30 minutes is highly suggestive of intracerebral hemorrhage [1].
Limb weakness often progresses from paresis (weakness) to plegia (complete paralysis) [1]. Patients may become unresponsive to painful stimuli on the affected side. A positive Babinski sign (upgoing toe) is typically present contralaterally [1]. If the dominant hemisphere is involved, global aphasia (severe impairment of both language production and comprehension) may occur [1]. Drowsiness often progresses to stupor [1]. Severe symptoms developing rapidly indicate compression of the upper brainstem structures [1]. Progression to deep coma is often accompanied by signs of advanced brainstem dysfunction, including irregular breathing patterns, dilation of the pupil ipsilateral (same side) to the hemorrhage with loss of light reactivity (due to third nerve compression), bilateral Babinski signs, and decerebrate posturing (abnormal extension of limbs) [1]. Neurological worsening occurring 12-72 hours after the initial hemorrhage is usually attributed to the development of perifocal edema increasing the mass effect, rather than re-bleeding (re-rupture) [1].
Thalamic Hypertensive Intracerebral Hemorrhage
Hemorrhage originating in the thalamus, another common site for hypertensive bleeds, typically causes contralateral hemiplegia or hemiparesis due to compression or involvement of the adjacent internal capsule [1, 2]. A prominent feature is often contralateral hemisensory loss, affecting all sensory modalities (pain, temperature, proprioception, tactile sensation), reflecting the thalamus's role as a major sensory relay nucleus [1].
Dominant hemisphere thalamic lesions may result in language disturbances (dysphasia or aphasia), often characterized by fluent speech with paraphasic errors but relatively preserved repetition abilities, sometimes accompanied by memory deficits [1]. Hemorrhage in the non-dominant thalamus may lead to constructional apraxia and spatial neglect (apractoagnosia) [1]. Homonymous visual field defects (affecting the same half of the visual field in both eyes) can occur due to involvement of pathways near the thalamus but may resolve partially [1]. Extension of thalamic hemorrhage inferomedially towards the subthalamic region and upper midbrain frequently results in characteristic oculomotor (eye movement) dysfunctions, including [1]:
- Impaired vertical gaze (especially upward gaze palsy)
- Downward deviation of the eyes at rest ("setting sun sign")
- Pupillary abnormalities: anisocoria (unequal pupils), pupils often small or mid-sized and poorly reactive to light
- Skew deviation (vertical misalignment of the eyes)
- Impaired convergence
- Pseudo-abducens palsy (impaired outward eye movement mimicking sixth nerve palsy due to gaze pathway involvement)
- Retraction nystagmus (eyes pull back into sockets on attempted upgaze)
Other potential signs include ipsilateral ptosis and miosis (part of Horner's syndrome if sympathetic pathways are compressed), eyelid edema, or rarely, mutism (inability to speak) particularly with bilateral or dominant thalamic involvement [1].
Pontine Hypertensive Intracerebral Hemorrhage
Hemorrhage originating within the pons (part of the brainstem) typically results in catastrophic neurological deficits with rapid onset, often leading quickly to deep coma [1, 2]. Characteristic clinical findings include quadriplegia (paralysis of all four limbs), marked decerebrate rigidity (abnormal extensor posturing), and pinpoint pupils (severe miosis, typically 1 mm diameter) that are usually reactive to light initially, though reactivity may be difficult to assess or lost later [1]. Horizontal eye movements are severely impaired or absent; testing the vestibulo-ocular reflex (e.g., "doll's eye" maneuver or caloric testing) typically reveals absent horizontal responses [1]. Vertical eye movements might be preserved initially [1]. Patients often exhibit abnormal respiratory patterns (like hyperventilation or irregular breathing), systemic hypertension (due to autonomic dysregulation), and sometimes hyperhidrosis (excessive sweating) [1]. Pontine hemorrhage carries a very high mortality rate, often resulting in death within hours to days [1, 2].
In rare instances of very small pontine hemorrhages, particularly those limited to the tegmentum (dorsal part), consciousness might be preserved initially [1]. Clinical signs in such cases might indicate focal pontine lesions, such as horizontal gaze palsy, facial weakness, dysarthria, contralateral hemiplegia or hemisensory deficits (alternating syndromes), miosis, other cranial nerve palsies (V, VI, VII), or bilateral pyramidal tract signs [1].
Cerebellar Hypertensive Intracerebral Hemorrhage
Hypertensive hemorrhage originating within the cerebellum typically develops over several hours, although onset can sometimes be more abrupt [1, 2]. A key feature distinguishing cerebellar hemorrhage early on is that initial loss of consciousness is rare, unless there is rapid expansion with brainstem compression or extension into the fourth ventricle causing acute hydrocephalus [1]. Patients commonly present with symptoms reflecting cerebellar dysfunction: severe headache (often occipital), dizziness or vertigo, repeated vomiting, and marked inability to stand or walk (truncal ataxia) [1, 2]. These early clinical signs should strongly raise suspicion for cerebellar hemorrhage, prompting urgent neuroimaging and neurosurgical evaluation, as timely surgical intervention (hematoma evacuation) can be life-saving and lead to good outcomes if performed before significant brainstem compression occurs [1, 5].
Neurological examination in the acute phase may initially reveal minimal or subtle focal signs besides the ataxia and vomiting [1]. Appendicular limb ataxia (incoordination of arms/legs) might be present ipsilateral to the hemorrhage [1]. Gaze abnormalities are common: horizontal gaze palsy (inability to look towards the side of the lesion) or forced gaze deviation away from the side of the lesion (contralateral deviation) can occur due to involvement of pontine gaze centers or pathways [1]. Ipsilateral peripheral facial nerve (VII) palsy or abducens nerve (VI) palsy (causing impaired eye abduction) may also be seen due to compression [1].
Other ocular manifestations occasionally observed include blepharospasm (involuntary eyelid closure), unilateral eyelid closure, or skew deviation [1]. Ocular bobbing (spontaneous, brisk downward eye movements followed by slow upward drift), typically associated with severe pontine damage, might occur later if consciousness deteriorates leading to coma due to brainstem compression [1]. Vertical eye movements usually remain preserved until late stages [1]. Pupils typically remain small but reactive to light until significant brainstem compression occurs [1]. Ipsilateral facial weakness (often peripheral type VII nerve palsy) and a decreased corneal reflex on the same side are frequently observed [1].
Contralateral hemiplegia and central-type facial weakness are typically absent in pure cerebellar hemorrhage, helping to distinguish it from supratentorial bleeds [1]. Rarely, large cerebellar hemorrhages compressing the brainstem severely can present with tetraplegia while consciousness is initially preserved, or with spastic paraparesis [1]. Plantar reflexes (Babinski sign) may initially be flexor but can progress to extensor responses as brainstem function deteriorates [1]. The major danger with cerebellar hemorrhage is sudden neurological decline into stupor and coma due to progressive brainstem compression or acute hydrocephalus from fourth ventricle obstruction [1, 5]. Once significant brainstem compression occurs, therapeutic or surgical interventions are often much less effective [1].
Specific patterns of ocular neurological symptoms are particularly useful for helping to localize the site of an intracerebral hemorrhage [1]:
- Putaminal hemorrhage: Conjugate gaze deviation towards the side of the hemorrhage (away from the hemiparesis).
- Thalamic hemorrhage: Downward gaze deviation, impaired vertical gaze, and various pupillary abnormalities (often small, poorly reactive).
- Pontine hemorrhage: Horizontal gaze paralysis (impaired horizontal vestibulo-ocular reflex) with typically pinpoint but reactive pupils.
- Cerebellar hemorrhage: Often ipsilateral gaze palsy or contralateral gaze deviation, but typically without significant contralateral hemiparesis initially.
Headache is not a universal symptom of hypertensive intracerebral hemorrhage, occurring in only about 50% of patients [1]. Vomiting, however, is quite common, especially with posterior fossa (pontine, cerebellar) hemorrhages or large supratentorial bleeds causing increased intracranial pressure [1]. Loss of consciousness progressing to coma is also variable and depends heavily on the size and location of the hematoma; consciousness may be fully preserved initially, particularly with smaller hematomas, even if there is some extension into the ventricular system [1].
Seizures are relatively uncommon at the onset of typical hypertensive intracerebral hemorrhage (occurring in less than 10% of cases), unlike some other stroke types or causes of hemorrhage [1]. The diagnosis is primarily based on the combination of clinical presentation (rapidly evolving neurological deficits) and characteristic findings on neuroimaging [1, 4].
When consciousness is preserved, clinically distinguishing between an ischemic stroke and an intracerebral hemorrhage based solely on symptoms can be challenging, although the smoother progression over minutes often favors hemorrhage [1]. Therefore, urgent brain imaging (typically non-contrast CT) is essential in all patients with acute stroke symptoms [5]. Timely neuroimaging is crucial for accurate diagnosis, differentiating hemorrhage from ischemia, localizing the lesion precisely, assessing its size and effect on surrounding structures, and guiding appropriate acute management, particularly important for identifying potentially life-threatening but treatable conditions like large cerebellar hemorrhages [5].
Diagnosis of Hypertensive Intracerebral Hemorrhage (Laboratory and Imaging Evaluation)
Differential Diagnosis of Acute Intracerebral Hemorrhage [1, 4, 5]
| Cause | Typical Location | Key Clinical / Imaging Features |
|---|---|---|
| Hypertensive ICH | Basal ganglia (putamen), thalamus, pons, cerebellum (deep structures). | History of chronic hypertension. Smooth evolution of symptoms over minutes. CT shows deep hematoma, +/- ventricular extension. |
| Lobar ICH (e.g., CAA, AVM, Tumor) | Superficial (cortical/subcortical) within cerebral lobes (frontal, temporal, parietal, occipital). | CAA: Elderly, recurrent lobar bleeds, often microbleeds on MRI (GRE/SWI). AVM/Tumor: Younger age possible, look for underlying lesion on contrast MRI/Angiography. Seizures more common. |
| Ruptured Saccular Aneurysm | Predominantly Subarachnoid Hemorrhage (SAH) in cisterns/sulci. May have associated ICH (often frontal/temporal lobe) or Intraventricular Hemorrhage (IVH). | Sudden "thunderclap" headache. CT shows SAH. CTA/DSA identifies aneurysm. |
| Hemorrhagic Transformation of Ischemic Stroke | Hemorrhage occurs within an area of pre-existing infarct, following vascular territory. | Often occurs days after initial ischemic stroke. Imaging shows blood within established infarct. Higher risk with large infarcts, embolic source, reperfusion therapy. |
| Cavernous Malformation Hemorrhage | Can occur anywhere (supratentorial, infratentorial, spinal cord). Often smaller, contained bleeds. May present with seizures or focal deficits. | MRI shows characteristic "popcorn" lesion with blood products of different ages, hemosiderin rim. Angiographically occult. |
| Anticoagulant-Associated ICH | Can occur anywhere. May be larger or show continued expansion. | History of anticoagulant use. Elevated INR (warfarin) or relevant drug levels/activity assay. Requires specific reversal strategies. |
| Coagulopathy / Hematologic Disorder | Can occur anywhere, may be multiple. Often associated systemic bleeding (petechiae, bruising). | Abnormal blood counts (thrombocytopenia) or coagulation studies (PT/PTT). Underlying diagnosis (leukemia, liver failure, DIC). |
| Traumatic ICH | Typical locations (frontal/temporal poles - contusions), can occur anywhere. May be associated with SDH/EDH/SAH, skull fracture. | Clear history of head trauma. Imaging shows hemorrhage pattern consistent with trauma mechanism. |
Brain computed tomography (CT), specifically non-contrast CT, is a highly reliable and widely available method for diagnosing acute intracerebral hemorrhage [4, 5]. Fresh blood appears hyperdense (bright white) on CT scans, making it readily identifiable [4]. CT can reliably detect hemorrhagic lesions with a diameter of 1 cm or greater within the cerebral hemispheres and cerebellum, especially when performed within the first few days of symptom onset [4]. Over time (typically after one to two weeks), the density (attenuation) of the hematoma gradually decreases as blood products break down, eventually becoming isodense (similar density to brain tissue) and later hypodense (darker than brain tissue) [4]. This means older hemorrhages might be missed on CT if there is no residual perifocal edema or mass effect [4]. In some cases, a ring of contrast enhancement may appear around the resolving hematoma after 2-4 weeks, persisting for several months, which can sometimes mimic a tumor or abscess [4]. As mentioned previously, visualizing small hemorrhages in the pons or other posterior fossa structures can sometimes be challenging with CT due to potential motion artifacts and beam-hardening artifacts from surrounding bone [4].
Brain MRI is generally more sensitive than CT for detecting smaller hematomas, particularly in the brainstem (pons and medulla oblongata), and for characterizing hemorrhages at different stages (hyperacute, acute, subacute, chronic) based on the signal characteristics of blood breakdown products on various sequences (like T1, T2, FLAIR, gradient echo/SWI) [4]. However, differentiating hyperacute hemorrhage (first few hours) from other conditions can sometimes be challenging even with MRI, and CT remains the primary tool for rapid exclusion of hemorrhage in the acute setting [4, 5].
With the accuracy and availability of modern CT and MRI, the need for lumbar puncture (LP) in diagnosing intracerebral hemorrhage has significantly diminished and is generally contraindicated due to the risk of brain herniation [1, 5]. LP might only be considered in very specific circumstances, such as strong suspicion of subarachnoid hemorrhage with negative initial CT scans, or perhaps historically when imaging was limited and differentiating small pontine bleeds causing blood in the CSF from other pathologies was necessary [1]. Performing an LP in the presence of a large intracerebral hematoma, particularly a supratentorial one causing mass effect, carries a significant risk of inducing or worsening transtentorial (temporal lobe) or tonsillar herniation [1].
If imaging (MRI or CT) reveals a hematoma location atypical for hypertensive hemorrhage (e.g., lobar white matter, especially in a younger or non-hypertensive patient) or shows features suggestive of an underlying lesion (like surrounding edema disproportionate to hematoma age, abnormal vessels), further investigation with vascular imaging (CTA, MRA, or DSA) is warranted to look for potential causes like a ruptured aneurysm, arteriovenous malformation (AVM), dural arteriovenous fistula, or tumor [1, 5]. For example, a temporal lobe hematoma located near the Sylvian fissure should raise suspicion for a possible ruptured middle cerebral artery aneurysm [1]. Angiography is often performed in such cases to identify a potential source requiring specific treatment (e.g., clipping or coiling of an aneurysm) [1, 5]. If rapidly evolving edema threatens herniation (e.g., cerebellar herniation), emergent surgical evacuation of the hematoma might be considered even before definitive vascular imaging, depending on the clinical urgency [5].
Angiography performed acutely may not reliably exclude an underlying AVM if it is compressed or obscured by the hematoma itself; repeat angiography after hematoma resorption may sometimes be necessary if clinical suspicion remains high [1]. CT with and without contrast enhancement might sometimes show abnormal vessels suggestive of an AVM as the source of hemorrhage, but a negative contrast CT does not rule it out [4]. MRI/MRA can also be useful in detecting underlying AVMs, sometimes even after hematoma resorption, as the abnormal vessels may still be visible as flow voids (dark structures representing flowing blood) on certain MRI sequences [4].
Other investigations like a Chest X-ray and electrocardiogram (ECG) are standard parts of the evaluation, primarily to look for evidence of end-organ damage from chronic hypertension (e.g., left ventricular hypertrophy on ECG, cardiomegaly on X-ray) or potential cardiac sources of embolism if the diagnosis is uncertain, helping to support the likely etiology of the neurological event [1].
Clinical grading scales are used primarily for subarachnoid hemorrhage (SAH) prognosis, but concepts are relevant. The Hunt and Hess scale, developed in 1968, assesses SAH severity based on clinical presentation and helps predict outcome [10]:
- Asymptomatic or mild headache, slight nuchal rigidity – Prognosis generally good (e.g., ~70% survival reported historically).
- Moderate to severe headache, nuchal rigidity, cranial nerve palsy (e.g., IIIrd nerve) possible, no other major focal deficit – Prognosis fair (e.g., ~60% survival).
- Drowsiness, confusion, or mild focal neurological deficit – Prognosis guarded (e.g., ~50% survival).
- Stupor, moderate to severe hemiparesis, possibly early decerebrate rigidity, vegetative disturbances – Prognosis poor (e.g., ~20% survival).
- Deep coma, decerebrate rigidity, moribund appearance – Prognosis very poor (e.g., ~10% survival).
Prognosis for hypertensive intracerebral hematomas strongly depends on the initial hematoma size, location, presence of intraventricular extension, and the patient's initial level of consciousness (often assessed using the Glasgow Coma Scale and specific ICH Scores) [1, 5]. Large supratentorial hematomas (e.g., volume > 30-60 cm³ or diameter > 5 cm) generally have a guarded prognosis [5]. Pontine hematomas larger than approximately 3 cm in diameter are often associated with very high mortality [1]. The development of significant cerebral edema within the first week also worsens the prognosis [1].
It is important to remember that brain tissue displaced and compressed around an intracerebral hematoma is not necessarily irreversibly infarcted [1]. Significant clinical improvement can occur as the hematoma resolves and the mass effect diminishes [1]. Therefore, careful medical management during the acute phase, focused on preventing secondary brain injury (e.g., controlling blood pressure, managing intracranial pressure, preventing complications), can potentially lead to significant neurological recovery even after a severe initial presentation [5].
Treatment of Hypertensive Intracerebral Hemorrhage
The role of surgical evacuation for acute hypertensive intracerebral hemorrhage remains debated and is generally indicated only in specific situations [5, 11]. For most deep supratentorial hematomas (putaminal, thalamic), surgery is rarely beneficial and may even cause harm compared to medical management alone [5, 11]. However, surgical evacuation of a large supratentorial hematoma might be considered as a life-saving measure in patients who are deteriorating neurologically with signs of impending temporal lobe (uncal) herniation (e.g., progressing coma, pupillary dilation), particularly if they still retain some brainstem reflexes [5, 11]. In contrast, surgical evacuation of an acute cerebellar hematoma causing neurological deterioration or significant brainstem compression (often defined by size > 3 cm or presence of hydrocephalus) is typically considered the preferred treatment and is often life-saving, potentially allowing for excellent functional recovery if performed before irreversible brainstem damage occurs [5, 11].
In patients with small cerebellar hematomas who are alert and without signs of brainstem compression, immediate surgery might be deferred in favor of close observation in an intensive care setting [5]. However, clinical deterioration can occur rapidly, so readiness for emergency surgical intervention is crucial in these cases [5].
Medical management focuses on controlling blood pressure, managing intracranial pressure (ICP) if elevated, and preventing complications [5]. Osmotic diuretics like intravenous mannitol or hypertonic saline are commonly used to reduce cerebral edema and lower ICP [5]. The use of corticosteroids (steroids) has been studied but has shown no benefit and may increase complications (like infection and hyperglycemia), thus they are generally not recommended for ICH [5]. Continuous ICP monitoring via an implanted device may be used in select comatose patients to guide therapy (like osmotic agents) and help prevent secondary injury from excessive blood pressure fluctuations or undetected pressure increases [5]. While intuitively appealing, aggressively lowering blood pressure acutely to "stop the bleeding" has not been proven effective and may risk worsening ischemia in the perihematomal region; most hypertensive hemorrhages have already stopped bleeding by the time the patient is evaluated [1, 5]. Current guidelines typically recommend controlled lowering of systolic blood pressure (e.g., to a target around 140 mmHg) acutely, balancing the risk of re-bleeding/expansion against the risk of cerebral hypoperfusion [5].
Specific underlying conditions contributing to hypertension, such as preeclampsia/eclampsia or malignant hypertension, require prompt diagnosis and careful, specialized treatment, often involving specific antihypertensive agents, while carefully monitoring to avoid excessive or rapid blood pressure fluctuations that could be detrimental [1].
References
- Ropper AH, Samuels MA, Klein JP, Prasad S. Adams and Victor's Principles of Neurology. 11th ed. McGraw Hill; 2019. Chapter 34: Cerebrovascular Diseases (Section on Intracerebral Hemorrhage).
- Grotta JC, Albers GW, Broderick JP, et al. Stroke: Pathophysiology, Diagnosis, and Management. 7th ed. Elsevier; 2021. Chapter on Intracerebral Hemorrhage.
- Kumar V, Abbas AK, Aster JC. Robbins & Cotran Pathologic Basis of Disease. 10th ed. Elsevier; 2020. Chapter 28: The Central Nervous System (Section on Hypertensive Cerebrovascular Disease).
- Osborn AG, Hedlund GL, Salzman KL. Osborn's Brain: Imaging, Pathology, and Anatomy. 2nd ed. Elsevier; 2017. Section on Intracranial Hemorrhage.
- Hemphill JC 3rd, Greenberg SM, Anderson CS, et al; American Heart Association Stroke Council; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology. Guidelines for the Management of Spontaneous Intracerebral Hemorrhage: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke. 2015 Jul;46(7):2032-60.
- Greenberg MS. Handbook of Neurosurgery. 9th ed. Thieme; 2019. Chapter 20: Brain Tumors (sections on specific tumor types and complications like hemorrhage).
- Osborn AG, Hedlund GL, Salzman KL. Osborn's Brain: Imaging, Pathology, and Anatomy. 2nd ed. Elsevier; 2017. Section on Vascular Malformations and Intracranial Hemorrhage.
- Greenberg MS. Handbook of Neurosurgery. 9th ed. Thieme; 2019. Chapter 31: Spinal Cord Injury & Chapter 40: Spinal Vascular Malformations.
- Caplan LR. Caplan's Stroke: A Clinical Approach. 5th ed. Cambridge University Press; 2016. Chapter on Intracerebral Hemorrhage.
- Hunt WE, Hess RM. Surgical risk as related to time of intervention in the repair of intracranial aneurysms. J Neurosurg. 1968 Jan;28(1):14-20.
- Mendelow AD, Gregson BA, Rowan EN, Murray GD, Gholkar A, Mitchell PM; STICH II Investigators. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial lobar intracerebral haematomas (STICH II): a randomised trial. Lancet. 2013 Aug 3;382(9890):397-408. (Example of surgical trial for ICH).
See also
- Ischemic stroke, cerebral ischemia
- Vertebrobasilar insufficiency (VBI) with vertigo symptom
- Somatoform autonomic dysfunction
- Dizziness, stuffiness in ear and tinnitus
- Ischemic brain disease:
- Atherosclerotic thrombosis
- Atherothrombotic occlusion of internal carotid artery
- Asymptomatic carotid bifurcation stenosis with noise
- Atherothrombotic occlusion of vertebrobasilar and posterior cerebral arteries
- Atherothrombotic occlusion of posterior cerebral artery
- Atherothrombotic occlusion of vertebral and posterior inferior cerebellar arteries (PICA)
- Atherothrombotic occlusion of basilar artery
- Small-vessel stroke (lacunar infarction)
- Other causes of ischemic stroke (cerebral infarction)
- Cerebral embolism
- Spontaneous intracranial (subarachnoid) and intracerebral hemorrhage:
- Arteriovenous malformations of the brain
- Hypertensive intracerebral hemorrhage
- Cerebral arteries inflammatory diseases (cerebral arteritis)
- Giant intracranial aneurysms
- Other causes of intracerebral hemorrhage
- Lobar intracerebral hemorrhage
- Saccular aneurysm and subarachnoid hemorrhage
- Mycotic intracranial aneurysms
- Repeated cerebral artery aneurysm rupture
- Communicating hydrocephalus after intracerebral hemorrhage with ruptured aneurysm
- Cerebral vasospasm
- Cerebrovascular diseases - ischemic stroke, transient ischemic attack (TIA):
- Transient ischemic attack (TIA)
- Sigmoid sinus suppurative thrombophlebitis with thrombosis