The visual pathway and its disorders
- Understanding the Visual Pathway and its Disorders
- Specific Visual Field Defects and Their Implications
- Functional Diagnostics of Anterior Visual Pathway Lesions
- General Principles of Diagnosis and Management
- Differential Diagnosis of Common Visual Field Defects
- When to Consult a Specialist (Ophthalmologist, Neurologist, Neuro-ophthalmologist)
- References
Understanding the Visual Pathway and its Disorders
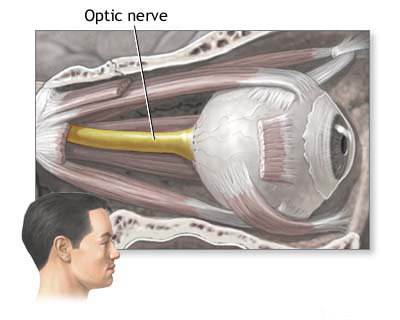
Visual disorders in humans can arise from damage to any part of the complex visual pathway, which extends from the eyes to the visual processing centers in the brain. Understanding the anatomy of this pathway is crucial for localizing lesions and diagnosing the cause of visual impairment.
Anatomical Structures of the Visual Pathway
The primary anatomical structures involved in transmitting and processing visual information include:
- Retina: The light-sensitive neural tissue lining the back of the eye, containing photoreceptor cells (rods and cones) that convert light into electrical signals.
- Optic Nerve (Cranial Nerve II): Formed by axons of retinal ganglion cells, it transmits visual information from the retina to the brain.
- Optic Chiasm: The point where fibers from the nasal (medial) halves of each retina cross over to the opposite side of the brain. Fibers from the temporal (lateral) halves remain uncrossed.
- Optic Tract: Bundles of nerve fibers posterior to the chiasm, each carrying information from the contralateral (opposite) visual field of both eyes.
- Lateral Geniculate Nucleus (LGN) of the Thalamus: The primary relay station for visual information from the optic tracts to the visual cortex.
- Optic Radiations (Geniculocalcarine or Geniculo-occipital Tract): Nerve fibers projecting from the LGN to the primary visual cortex. These include Meyer's loop (carrying inferior retinal fibers through the temporal lobe) and fibers carrying superior retinal information through the parietal lobe.
- Primary Visual Cortex (Occipital Cortex): Located in the occipital lobes of the cerebral hemispheres (specifically around the calcarine fissure/sulcus), where conscious perception of vision occurs.
Visual Field Defects and Lesion Localization
Damage at different points along the visual pathway produces characteristic patterns of visual field loss, which are critical for neurological localization:
Affected Area (Numbered as in Diagram) |
Resulting Visual Field Defect |
Schematic Representation of Field Defect |
|---|---|---|
| 1. Unilateral lesion of the optic nerve (pre-chiasmal) | Blindness or significant vision loss in the affected eye (monocular vision loss). |  |
| 2. Lesion in the optic chiasm (chiasmal lesion) | Bitemporal hemianopsia (loss of the outer/temporal halves of the visual field in both eyes, resembling "blinders"). |  |
| 3. Unilateral lesion of the optic tract (post-chiasmal) | Contralateral homonymous hemianopsia (loss of the same half - left or right - of the visual field in both eyes, opposite to the side of the lesion). |  |
| 4. Unilateral lesion of optic radiations in Meyer's loop (anterior temporal lobe, carrying inferior retinal fibers) | Contralateral superior quadrantanopsia ("pie in the sky" defect - loss of the upper quadrant of the visual field on the opposite side). | 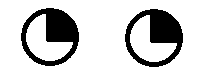 |
| 5. Unilateral lesion of the medial part of optic radiations (parietal lobe, carrying superior retinal fibers) | Contralateral inferior quadrantanopsia (loss of the lower quadrant of the visual field on the opposite side). | 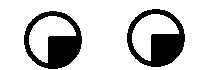 |
| 6. Complete lesion of the occipital lobe (visual cortex on one side) | Contralateral homonymous hemianopsia, often with macular sparing (preservation of central vision due to dual blood supply or bilateral representation of the macula). |  |
| 7. Lesion of the occipital pole (cortical zones representing the macula) | Contralateral homonymous hemianopic central or paracentral scotoma (loss of central vision in the corresponding half-fields of both eyes). |  |
Specific Visual Field Defects and Their Implications
Retinal Lesions and Scotomas (Arcuate, Central, Centrocecal)
Lesions affecting the retina cause various types of scotomas (areas of partial or complete vision loss within the visual field).
- Arcuate Scotomas: These are focal, arch-shaped defects in the visual field that correspond to damage to bundles of retinal nerve fibers. These defects typically follow the arcuate course of nerve fibers towards the optic disc or appear to originate from it (e.g., Bjerrum scotoma in glaucoma). Arcuate defects have a distinct border along the horizontal midline (horizontal raphe). If extensive, they can lead to the loss of an entire half (altitudinal hemianopsia) or quadrant of the visual field.
- Central Scotoma: Damage to the papillomacular bundle (nerve fibers connecting the macula to the optic disc), which provides sharp central fixation, leads to a central scotoma (loss of vision at the point of gaze fixation).
- Centrocecal Scotoma: A scotoma involving both the point of gaze fixation (central) and the physiological blind spot (cecal), often merging.
- Macular Lesions: Damage to the macula (the central part of the retina responsible for detailed vision) often results in a small central scotoma. This frequently leads to impaired visual acuity and qualitative visual perception disturbances, characterized by **metamorphopsia** (distortion of the shape and size of visible objects, especially straight lines appearing wavy or bent). Metamorphopsia is a key feature that helps distinguish macular lesions from damage primarily affecting the optic nerve.
Optic Nerve Lesions
A centrocecal scotoma is a frequent and specific sign of optic nerve damage. Causes of such damage can be:
- Intrinsic factors: Demyelinating processes (e.g., optic neuritis in multiple sclerosis), infiltrating processes (e.g., tumors like optic nerve glioma, lymphoma, leukemia), or degenerative processes.
- Extrinsic compression: From aneurysms, tumors (e.g., meningiomas of the optic nerve sheath or sphenoid wing, pituitary adenomas compressing the prechiasmatic nerve).
- Toxic effects: From substances like methyl alcohol, quinine, or certain medications (e.g., some phenothiazine-type tranquilizers, ethambutol).
- Nutritional deficiencies: Leading to "tobacco-alcohol amblyopia" (now more accurately termed toxic/nutritional optic neuropathy) can cause relatively symmetrical bilateral central or centrocecal scotomas.
Progressive, generalized narrowing of the peripheral isopters (constriction of the visual field) with relative preservation of central vision may be a consequence of annular (ring-like) compression of the optic nerve, as seen, for example, in meningioma of the optic nerve sheath. Spiral constriction or "tubular vision" (tunnel vision) of inorganic origin (e.g., in hysteria, malingering) characteristically persists with the same angular diameter when examining vision from any distance. In contrast, with organic lesions causing true tunnel vision, the absolute diameter of the visual field defect will increase as the distance between the eye and the test object increases, while the angular size remains similar.
Chiasmal Lesions (Bitemporal Hemianopsia)
A visual field defect localized to one half of the visual field of each eye, with a clear border along the vertical midline, is called hemianopsia.
Bitemporal hemianopsia (loss of the temporal/outer visual fields in both eyes) indicates a lesion affecting the decussating (crossing) fibers of the nasal part of each retina within the optic chiasm. This is usually due to compression of the chiasm by extrinsic masses such as:
- Pituitary adenomas (most common cause).
- Craniopharyngiomas.
- Meningiomas of the tuberculum sellae or diaphragm sellae.
- Suprasellar aneurysms of the arteries of the Circle of Willis.
- Gliomas of the chiasm.
Retrochiasmal Lesions (Homonymous Hemianopsia)
Homonymous hemianopsia (loss of the same half – left or right – of the visual fields in both eyes) occurs when the visual pathway is affected posterior to (above) the optic chiasm (i.e., in the optic tract, lateral geniculate nucleus, optic radiations, or visual cortex). A complete homonymous hemianopsia does not allow for precise localization within the retrochiasmal pathway based on field defect alone.
However, incomplete homonymous hemianopsia can provide more specific clues about the lesion site:
- If the visual field defects are **congruous** (identical in shape, size, and location in both eyes), it is most likely that the lesion is localized in the occipital cortex (visual cortex in the spur furrow/calcarine sulcus).
- If there is a **mismatch or incongruity** of defects (asymmetry), then it is more likely that the fibers of the optic tract, the lateral geniculate body, or the optic radiations within the parietal or temporal lobe have been damaged. Lesions of the optic tract are characterized by the development of asymmetric (incongruous) homonymous hemianopsia. Chronic damage to the optic tract is often accompanied by an impaired afferent pupillary reaction to light (Wernicke's hemianopic pupil, though difficult to elicit reliably) and "band" or "bow-tie" atrophy of the optic nerve on the side opposite the tract lesion (due to loss of nasal fibers from that eye) and temporal pallor on the ipsilateral side (due to loss of temporal fibers from that eye, a more complex pattern known as transverse atrophy).
In cases of lesions affecting the visual pathway above (posterior to) the lateral geniculate body (i.e., optic radiations or visual cortex), pupillary light reflexes are preserved because the afferent pupillary pathway diverges before the LGN.
Nerve fibers from the lower quadrants of the retina (representing the superior visual fields) project into the temporal lobe (Meyer's loop of the optic radiations). Therefore, damage to the temporal lobe can cause a contralateral superior homonymous quadrantanopsia ("pie in the sky" defect). Lesions of the parietal lobes, which carry fibers from the superior retina (representing inferior visual fields), tend to affect the lower quadrants more than the upper quadrants, potentially leading to a contralateral inferior homonymous quadrantanopsia. Parietal lobe lesions can also lead to hemianopsia due to visual inattention or neglect.
Complete homonymous hemianopsia with destruction of the macular fibers (fibers coming from the macula) develops if the cortical parts of one cerebral hemisphere in the area of the calcarine sulcus are extensively damaged. The phenomenon of "macular sparing" (preservation of central vision despite a homonymous hemianopsia) is often attributed to imperfect fixation during testing, dual vascular supply to the occipital pole, or bilateral cortical representation of the macula.
Cortical Blindness and Other Central Visual Disorders
Bilateral homonymous hemianopsia, resulting in complete cortical blindness, occurs as a result of bilateral lesions of the visual cortex, usually of an ischemic nature (e.g., bilateral posterior cerebral artery infarcts). Persistent cortical blindness may develop. In such patients, **Anton's syndrome** may be observed, characterized by bilateral blindness, denial of vision loss (anosognosia), normal pupillary light reflexes (as the lesion is posterior to the pupillary reflex pathway), and evidence of bilateral infarcts in the occipital-parietal regions.
Other disorders of central vision include various types of visual distortion, in which objects may appear either too small (**micropsia**), excessively large (**macropsia**), or curved/distorted (**metamorphopsia**, as mentioned with macular lesions). With bilateral symptoms of this nature, the temporal lobes are most likely to be affected. In such cases, visual disorders may occur at the time of epileptic seizures (visual aura) and can be accompanied by complex visual hallucinations or other manifestations of temporal lobe epilepsy.
Functional Diagnostics of Anterior Visual Pathway Lesions
In addition to the clinical examination of visual fields (perimetry), functional electrophysiological research methods are used in clinical practice to objectively assess lesions of the anterior segment of the visual pathway (retina and optic nerve):
Electroretinography (ERG and P-ERG)
Electroretinography (ERG) measures the electrical potentials generated by the different cell layers of the retina in response to light stimulation. It allows for the detection of retinal lesions often before changes become apparent on funduscopic examination, such as in early retinitis pigmentosa.
- Full-field ERG (ffERG): Assesses the global function of photoreceptors (a-wave, primarily from cones and rods) and bipolar/Müller cells (b-wave). Different protocols (scotopic for rods, photopic for cones, flicker for cones) help differentiate rod and cone system dysfunction. However, ffERG does not detect changes caused by lesions primarily affecting retinal ganglion cells or more posterior afferent parts of the visual pathway.
- Pattern Electroretinography (P-ERG): Uses a patterned stimulus (e.g., checkerboard) and the recorded data allow for judgment on the activity of retinal ganglion cells. P-ERG responses decrease or completely disappear with lesions of the optic nerve (e.g., due to retrograde degeneration of ganglion cells in conditions like Leber's hereditary optic neuropathy or demyelinating optic neuritis).
Visual Evoked Potentials (VEP)
Visual Evoked Potentials (VEP), also known as Visual Evoked Responses (VER), characterize the electrical response of the visual cortex, predominantly reflecting macular function, as measured from electrodes placed over the occipital pole of the cerebral cortex. When a patterned stimulus (e.g., checkerboard reversal) is used, VEPs primarily assess the integrity of the visual pathway from the retina to the visual cortex, with a strong emphasis on the optic nerve and macula-cortical projections.
In the absence of significant retinal damage, VEPs make it possible to assess, first and foremost, the functioning of the segment of the visual pathway up to the lateral geniculate body (external cranial body in some older texts), and especially the optic nerve itself. Key parameters measured are the latency (timing) and amplitude of the P100 wave (a major positive component).
- Delayed P100 latency is a hallmark of demyelination of the optic nerve (e.g., in optic neuritis associated with multiple sclerosis).
- Reduced P100 amplitude can indicate axonal loss in the optic nerve.
VEP testing provides significant assistance in the diagnosis of multiple sclerosis, allowing for the detection of an optic nerve lesion (even subclinical, i.e., in the absence of other overt symptoms of visual impairment).
General Principles of Diagnosis and Management
The diagnosis of visual pathway disorders requires a meticulous approach involving detailed history, comprehensive neuro-ophthalmological examination (visual acuity, color vision, pupils, ocular motility, ophthalmoscopy, perimetry), and targeted investigations (electrophysiology, neuroimaging).
Management is entirely dependent on the underlying cause:
- Inflammatory conditions (e.g., optic neuritis): Often treated with corticosteroids.
- Compressive lesions (e.g., tumors, aneurysms): May require surgical decompression or other specific oncological/vascular treatments.
- Ischemic events (e.g., CRAO, AION): Focus on managing vascular risk factors; acute treatments have limited efficacy for established infarction. For arteritic AION (GCA), urgent steroids are vital.
- Degenerative/Hereditary conditions: Often supportive care, genetic counseling, low vision aids. Specific treatments are emerging for some genetic retinal diseases.
- Toxic/Nutritional causes: Removal of toxin, supplementation of deficient nutrients.
Collaboration between ophthalmologists, neurologists, neuro-ophthalmologists, neurosurgeons, and radiologists is often essential for optimal patient care.
Differential Diagnosis of Common Visual Field Defects
| Visual Field Defect | Most Likely Lesion Location | Common Causes |
|---|---|---|
| Monocular Vision Loss / Central Scotoma (one eye) | Retina, Optic Nerve (pre-chiasmal) | Macular degeneration, Optic neuritis, AION, CRAO/BRVO, Compressive optic neuropathy, Glaucoma (advanced). |
| Bitemporal Hemianopsia | Optic Chiasm | Pituitary adenoma, Craniopharyngioma, Meningioma (suprasellar), Aneurysm. |
| Homonymous Hemianopsia (contralateral) | Optic Tract, LGN, Optic Radiations, Visual Cortex (post-chiasmal) | Stroke, Tumor, Trauma, AVM, Demyelination. |
| Homonymous Superior Quadrantanopsia ("Pie in the sky") | Contralateral Temporal Lobe (Meyer's Loop of Optic Radiations) | Stroke, Tumor, Temporal lobe epilepsy surgery. |
| Homonymous Inferior Quadrantanopsia | Contralateral Parietal Lobe (Superior Optic Radiations) or superior occipital cortex. | Stroke, Tumor. |
| Arcuate Scotoma / Nasal Step | Retinal Nerve Fiber Layer / Optic Disc | Glaucoma, Optic disc drusen, Ischemic optic neuropathy. |
| Enlarged Blind Spot | Optic Disc | Papilledema, Optic disc drusen, Glaucoma, Myopia. |
When to Consult a Specialist (Ophthalmologist, Neurologist, Neuro-ophthalmologist)
Any sudden or progressive change in vision, appearance of visual field defects, or unexplained visual disturbances warrants prompt medical evaluation. Key specialists include:
- Ophthalmologist: For comprehensive eye examination, diagnosis of retinal and optic disc diseases, and management of most ocular conditions.
- Neurologist: If visual symptoms are associated with other neurological signs, or if a central nervous system cause (e.g., stroke, tumor, MS) is suspected.
- Neuro-ophthalmologist: A specialist dealing with complex visual problems related to the nervous system, including optic neuropathies, chiasmal disorders, and unexplained vision loss.
Early diagnosis and management are crucial for many conditions affecting the visual pathway to preserve vision and address underlying serious pathologies.
References
- Miller NR, Newman NJ, Biousse V, Kerrison JB. Walsh and Hoyt's Clinical Neuro-Ophthalmology. 6th ed. Lippincott Williams & Wilkins; 2005. (Comprehensive neuro-ophthalmology reference).
- American Academy of Ophthalmology. Basic and Clinical Science Course (BCSC), Section 5: Neuro-Ophthalmology. San Francisco, CA: American Academy of Ophthalmology. (Published annually).
- Kandel ER, Schwartz JH, Jessell TM, Siegelbaum SA, Hudspeth AJ, Mack S. Principles of Neural Science. 6th ed. McGraw Hill; 2021. Chapters on Vision and the Visual System.
- Yanoff M, Duker JS. Ophthalmology. 5th ed. Elsevier; 2019. (Chapters on Retina, Optic Nerve, Neuro-ophthalmology).
- Bowling B, Kanski JJ. Kanski's Clinical Ophthalmology: A Systematic Approach. 9th ed. Elsevier; 2020. (Visual fields and optic nerve disorders).
- Traquair HM. An Introduction to Clinical Perimetry. 6th ed. Henry Kimpton; 1949. (Classic text on visual fields).
- Celesia GG. Visual evoked potentials and electroretinograms. In: Aminoff MJ, ed. Electrodiagnosis in Clinical Neurology. 6th ed. Elsevier Saunders; 2012:chap 18.
- Hoyt WF, Luis O. Visual fiber anatomy in the infrageniculate pathway of the primate. Arch Ophthalmol. 1962;68:94-106. (Anatomical details).
See also
- Anatomy of the nervous system
- Spinal disc herniation
- Pain in the arm and neck (trauma, cervical radiculopathy)
- The eyeball and the visual pathway:
- Anatomy of the eye and physiology of vision
- The visual pathway and its disorders
- Eye structures and visual disturbances that occur when they are affected
- Retina and optic disc, visual impairment when they are affected
- Impaired movement of the eyeballs
- Nystagmus and conditions resembling nystagmus
- Dry Eye Syndrome
- Optic nerve and retina:
- Compression neuropathy of the optic nerve
- Edema of the optic disc (papilledema)
- Ischemic neuropathy of the optic nerve
- Meningioma of the optic nerve sheath
- Optic nerve atrophy
- Optic neuritis in adults
- Optic neuritis in children
- Opto-chiasmal arachnoiditis
- Pseudo-edema of the optic disc (pseudopapilledema)
- Toxic and nutritional optic neuropathy
- Neuropathies and neuralgia:
- Diabetic, alcoholic, toxic and small fiber sensory neuropathy (SFSN)
- Facial nerve neuritis (Bell's palsy, post-traumatic neuropathy)
- Fibular (peroneal) nerve neuropathy
- Median nerve neuropathy
- Neuralgia (intercostal, occipital, facial, glossopharyngeal, trigeminal, metatarsal)
- Post-traumatic neuropathies
- Post-traumatic trigeminal neuropathy
- Post-traumatic sciatic nerve neuropathy
- Radial nerve neuropathy
- Tibial nerve neuropathy
- Trigeminal neuralgia
- Ulnar nerve neuropathy
- Tumors (neoplasms) of the peripheral nerves and autonomic nervous system (neuroma, sarcomatosis, melanoma, neurofibromatosis, Recklinghausen's disease)
- Carpal tunnel syndrome
- Ulnar nerve compression in the cubital canal