Utibron Neohaler Drug Description: Detailed Datasheet
- Generic Name: indacaterol and glycopyrrolate inhalation powder, for oral inhalation use
- Brand Name: Utibron Neohaler
side effects drug center utibron neohaler (indacaterol and glycopyrrolate inhalation powder, for oral inhalation use) drug
Drug Description
What is Utibron Neohaler and how is it used?
Utibron Neohaler is a prescription medicine used to treat the symptoms of Chronic Obstructive Pulmonary Disease (COPD). Utibron Neohaler may be used alone or with other medications.
Utibron Neohaler belongs to a class of drugs called Anticholinergics, Respiratory; Beta2 Agonists; Respiratory Inhalant Combos; COPD Agents.
It is not known if Utibron Neohaler is safe and effective in children.
What are the possible side effects of Utibron Neohaler?
Utibron Neohaler may cause serious side effects including:
- hives,
- difficulty breathing,
- swelling of your face, lips, tongue, or throat,
- shaking,
- fast or irregular heartbeat,
- difficult or painful urination,
- muscle cramps,
- weakness,
- increased thirst,
- increased urination,
- high blood pressure,
- severe wheezing,
- chest pain,
- eye pain, swelling, or redness,
- vision changes,
- blurred vision,
- seeing rainbows around lights,
- rash,
- itching, and
- severe dizziness
Get medical help right away, if you have any of the symptoms listed above.
The most common side effects of Utibron Neohaler include:
- coughing,
- headache, and
- dizziness
Tell the doctor if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of Utibron Neohaler. For more information, ask your doctor or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
WARNING
ASTHMA-RELATED DEATH
Long-acting beta2-adrenergic agonists (LABAs) increase the risk of asthma-related death. Data from a large, placebo-controlled U.S. study that compared the safety of another LABA (salmeterol) or placebo added to usual asthma therapy showed an increase in asthma-related deaths in patients receiving salmeterol. This finding with salmeterol is considered a class effect of all LABAs, including indacaterol, one of the active ingredients in UTIBRON NEOHALER.
The safety and efficacy of UTIBRON NEOHALER in patients with asthma have not been established. UTIBRON NEOHALER is not indicated for the treatment of asthma [see WARNINGS AND PRECAUTIONS].
DESCRIPTION
UTIBRON NEOHALER consists of a dry powder formulation for delivery of a combination of indacaterol and glycopyrrolate to patients by oral inhalation only with the NEOHALER device. The inhalation powder is packaged in hypromellose (HPMC) capsules with yellow transparent cap and uncolored transparent body.
Each capsule contains a dry powder blend of 27.5 mcg/15.6 mcg of indacaterol/glycopyrrolate with approximately 24.9 mg of lactose monohydrate (which contains trace levels of milk protein) and 0.03 mg of magnesium stearate.
One active component of UTIBRON NEOHALER is indacaterol maleate, a (R) enantiomer. Indacaterol maleate is a selective beta2-adrenergic agonist. Its chemical name is (R)-5-[2-(5,6-Diethylindan-2-ylamino)-1-hydroxyethyl]-8- hydroxy-1H-quinolin-2-one maleate; its structural formula is :
 |
Indacaterol maleate has a molecular weight of 508.56, and its empirical formula is C24H28N2O3 • C4H4O4. Indacaterol maleate is a white to very slightly grayish or very slightly yellowish powder. Indacaterol maleate is slightly soluble in ethanol and very slightly soluble in water.
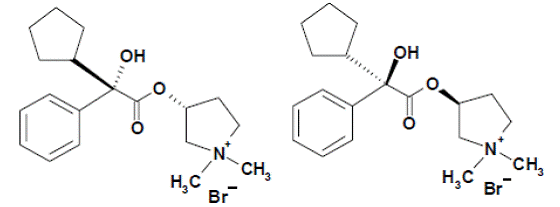
The other active component of UTIBRON NEOHALER is glycopyrrolate, which is chemically described as (3RS)-3[(2SR)-(2-cyclopentyl-2-hydroxy-2-phenylacetyl) oxy]-1,1-dimethylpyrrolidinium bromide. This synthetic quaternary ammonium compound acts as a competitive antagonist at muscarinic acetylcholine receptors, also referred to as anticholinergic. Glycopyrrolate, C19H28BrNO3, is a white powder that is freely soluble in water and sparingly soluble in absolute ethanol. It has a molecular mass of 398.33. The structural formula is:
 |
The NEOHALER device is a plastic inhalation device used to inhale the dry powder within the UTIBRON capsule. The amount of drug delivered to the lung will depend on patient factors, such as inspiratory flow rate and inspiratory time.
Under standardized in vitro testing at a fixed flow rate of 90 L/min for 1.3 seconds, the NEOHALER inhaler delivered 20.8 mcg of indacaterol and 12.8 mcg glycopyrrolate for the 27.5 mcg/15.6 mcg dose strength (equivalent to 27.5 mcg/12.5 mcg of indacaterol/glycopyrronium) from the mouthpiece. This in vitro testing revealed that the NEOHALER device had a specific resistance of 0.059 cm H2O½/L/min. Peak inspiratory flow rates (PIFR) achievable through the NEOHALER device were evaluated in 26 adult patients with COPD of varying severity. Mean PIFR was 95 L/min (range 52 to 133 L/min) for adult patients. Twenty-five of 26 patients (96%) in this study generated a PIFR through the device exceeding 60 L/min.
Indications & Dosage
INDICATIONS
UTIBRON® NEOHALER® is indicated for the long-term, maintenance treatment of airflow obstruction in patients with chronic obstructive pulmonary disease (COPD).
Limitations Of Use
UTIBRON NEOHALER is not indicated for the relief of acute bronchospasm or for the treatment of asthma [see WARNINGS AND PRECAUTIONS]. The safety and effectiveness of UTIBRON in asthma have not been established.
DOSAGE AND ADMINISTRATION
Administration Overview
For oral inhalation only. Do not swallow UTIBRON capsules, as the intended effects on the lungs will not be obtained. UTIBRON capsules should only be used with the NEOHALER device.
Recommended Dosage For Chronic Obstructive Pulmonary Disease
The recommended dosage is one capsule of UTIBRON (27.5 mcg of indacaterol and 15.6 mcg glycopyrrolate) inhalation powder twice daily by oral inhalation using the NEOHALER device.
UTIBRON NEOHALER should be administered at the same time of the day (1 capsule in the morning and 1 capsule in the evening) every day. More frequent administration or a greater number of inhalations (more than 1 capsule twice daily) of UTIBRON NEOHALER is not recommended.
HOW SUPPLIED
Dosage Forms And Strengths
Inhalation powder: UTIBRON capsules contain 27.5 mcg indacaterol and 15.6 mcg glycopyrrolate in a hypromellose capsule with yellow transparent cap and uncolored transparent body with black logo on the cap and black product code “IGP27.5_15.6” under the black bar on the body.
- UTIBRON NEOHALER contains UTIBRON (27.5 mcg indacaterol and 15.6 mcg glycopyrrolate inhalation powder) in hypromellose (HPMC) capsule with yellow transparent cap and uncolored transparent body, with capsules packaged in aluminum blister cards, one NEOHALER device, and an FDA-approved Patient Information.
- Unit Dose (blister pack), Box of 60 (10 blister cards with 6 yellow transparent capsules each) NDC 63402-681-60
- Unit Dose (blister pack), Box of 6 (1 blister card with 6 yellow transparent capsules each) NDC 63402-681-06
- The NEOHALER device consists of a white protective cap and a base with mouthpiece, capsule chamber and 2 yellow push buttons.
Storage And Handling
Store in a dry place at 77°F (25°C); excursions permitted to 59°F to 86°F (15°C to 30°C) [see USP Controlled Room Temperature].
- UTIBRON capsules should be used with the NEOHALER device only. Do not use the NEOHALER device with any other capsules.
- Store UTIBRON capsules in the blister protected from light and moisture. Remove the UTIBRON capsules from the blister immediately before use.
- Always use the new NEOHALER inhaler provided with each new prescription. Keep out of the reach of children.
Manufactured by: Novartis Pharmaceuticals Corporation East Hanover, New Jersey 07936. Revised: Jul 2021
Side Effects
The following clinically significant adverse reactions are described in greater detail in other sections:
- Serious Asthma-Related Events - Hospitalizations, Intubations, Death [see WARNINGS AND PRECAUTIONS]
- Paradoxical Bronchospasm [see WARNINGS AND PRECAUTIONS].
- Hypersensitivity Reactions, including Anaphylaxis [see WARNINGS AND PRECAUTIONS].
- Cardiovascular Effects [see WARNINGS AND PRECAUTIONS].
- Worsening of Narrow-Angle Glaucoma [see WARNINGS AND PRECAUTIONS].
- Worsening of Urinary Retention [see WARNINGS AND PRECAUTIONS].
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, the adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in clinical trials of another drug and may not reflect the rates observed in clinical practice.
The UTIBRON NEOHALER safety database included 2654 subjects with COPD in two 12-week lung function trials and one 52-week long-term safety study. A total of 712 subjects received treatment with UTIBRON NEOHALER 27.5 mcg/15.6 mcg twice daily. The safety data described below are based on the two 12-week trials and the one 52-week trial.
12-Week Trial
The incidence of adverse reactions associated with UTIBRON NEOHALER in Table 1 is based on two 12-week, placebo-controlled trials (Trials 1 and 2; N = 1,001 and N = 1,042, respectively). Of the 2040 subjects, 63% were male and 91% were Caucasian. They had a mean age of 63 years and an average smoking history of 47 pack-years, with 52% identified as current smokers. At screening, the mean post-bronchodilator percent predicted forced expiratory volume in 1 second (FEV1) was55%(range: 29% to 79%), the mean post-bronchodilator FEV1/forced vitalcapacity (FVC) ratio was 50% (range: 19% to 71%), and the mean percent reversibility was 23% (range: 0% to 144%).
The most common adverse reaction (incidence greater than or equal to 2% and higher than placebo) was nasopharyngitis and hypertension.
The proportion of patients who discontinued treatment due to adverse reactions was 2.95% for the UTIBRON NEOHALER treated patients and 4.13% for placebo-treated patients.
Subjects received 1 dose twice daily of the following: UTIBRON NEOHALER 27.5 mcg/15.6 mcg, indacaterol 27.5 mcg, glycopyrrolate 15.6 mcg, or placebo.
Table 1: Adverse Reactions with UTIBRON NEOHALER (greater than or equal to 1% incidence and higher than placebo) in COPD Patients
| Adverse Reaction | UTIBRON NEOHALER 27.5/15.6 mcg twice daily (N = 508) n (%) |
Indacaterol 27.5 mcg twice daily (N = 511) n (%) |
Glycopyrrolate 15.6 mcg twice daily (N = 513) n (%) |
Placebo (N = 508) n (%) |
| Nasopharyngitis | 21 (4.1) | 13 (2.5) | 12 (2.3) | 9 (1.8) |
| Hypertension | 10 (2.0) | 5 (1.0) | 3 (0.6) | 7 (1.4) |
| Back pain | 9 (1.8) | 7 (1.4) | 2 (0.4) | 3 (0.6) |
| Oropharyngeal pain | 8 (1.6) | 4 (0.8) | 8 (1.6) | 6 (1.2) |
Other adverse reactions occurring more frequently with UTIBRON NEOHALER than with placebo, but with an incidence of less than 1% include dyspepsia, gastroenteritis, chest pain, fatigue, peripheral edema, rash/pruritus, insomnia, dizziness, bladder obstruction/urinary retention, atrial fibrillation, palpitations, tachycardia.
52-Week Trial
In a long-term safety trial, 614 subjects were treated for up to 52 weeks with indacaterol/glycopyrrolate 27.5 mcg/15.6 mcg twice daily, indacaterol/glycopyrrolate 27.5/31.2 mcg twice daily or indacaterol 75 mcg once daily. The demographic and baseline characteristics of the long-term safety trial were similar to those of the placebo-controlled efficacy trials described above. The adverse reactions reported in the long-term safety trial were consistent with those observed in the placebo-controlled trials of 12 weeks. Additional adverse reactions that occurred with a frequency greater than or equal to 2% in the group receiving indacaterol/glycopyrrolate 27.5 mcg/15.6 mcg twice daily that exceeded the frequency of indacaterol 75 mcg once daily in this trial were upper and lower respiratory tract infection, pneumonia, diarrhea, headache, gastroesophageal reflux disease, hyperglycemia, rhinitis.
Postmarketing Experience
The following additional adverse reactions of angioedema and dysphonia have been identified during worldwide post-approval use of indacaterol/glycopyrrolate at higher than the recommended dose. Because this reaction is reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate the frequency or establish a causal relationship to drug exposure.
Drug Interactions
Adrenergic Drugs
If additional adrenergic drugs are to be administered by any route, they should be used with caution because the sympathetic effects of indacaterol, a component of UTIBRON NEOHALER, may be potentiated [see WARNINGS AND PRECAUTIONS].
Xanthine Derivatives, Steroids, Or Diuretics
Concomitant treatment with xanthine derivatives, steroids, or diureticsmay potentiateany hypokalemic effect of beta2adrenergic agonists, such as indacaterol, a component of UTIBRON NEOHALER [see WARNINGS AND PRECAUTIONS].
Non-Potassium-Sparing Diuretics
The electrocardiographic (ECG) changes and/or hypokalemia that may result from the administration of non-potassiumsparing diuretics (such as loop or thiazide diuretics) can be acutely worsened by beta-agonists, such as indacaterol, a component of UTIBRON NEOHALER, especially when the recommended dose of the beta-agonist is exceeded.
Although the clinical relevance of these effects is not known, caution is advised in the coadministration of UTIBRON NEOHALER with non-potassium-sparing diuretics.
Monoamine Oxidase Inhibitors, Tricyclic Antidepressants, QTc-Prolonging Drugs
Indacaterol, one ofthecomponents of UTIBRON NEOHALER, as with other beta 2-agonists, should be administered with extreme caution to patients being treated with monoamine oxidase inhibitors, tricyclic antidepressants, or other drugs known to prolong the QTc interval because the action of adrenergic agonists on the cardiovascular system may be potentiated by these agents. Drugs that are known to prolong the QTc interval may have an increased risk of ventricular arrhythmias.
Beta-Blockers
Beta-adrenergic receptor antagonists (beta-blockers) and UTIBRON NEOHALER may interfere with the effect of each other when administered concurrently. Beta-blockers not only block the therapeutic effects of beta-agonists, but may produce severe bronchospasm in COPD patients. Therefore, patients with COPD should not normally be treated with beta-blockers. However, under certain circumstances, e.g., as prophylaxis after myocardial infarction, there may be no acceptable alternatives to the use of beta-blockers in patients with COPD. In this setting, cardioselective beta-blockers could be considered, although they should be administered with caution.
Anticholinergics
There is potential for an additive interaction with concomitantly used anticholinergic medicines. Therefore, avoid coadministration of UTIBRON NEOHALER with other anticholinergic-containing drugs as this may lead to an increase in anticholinergic adverse effects [see WARNINGS AND PRECAUTIONS, ADVERSE REACTIONS].
Inhibitors Of Cytochrome P450 3A4 And P-gp Efflux Transporter
Drug interaction studies with indacaterol, a component of UTIBRON NEOHALER, were carried out using potent and specific inhibitors of CYP3A4 and P-gp (i.e., ketoconazole, erythromycin, verapamil, and ritonavir). The data suggest that systemic clearance of indacaterol is influenced by modulation of both P-gp and CYP3A4 activities and that the 2-fold area under the curve (AUC) increase caused by the strong dual inhibitor ketoconazole reflects the impact of maximal combined inhibition. Indacaterol was evaluated in clinical trials for up to 1 year at doses up to 600 mcg. Inhibition of the key contributors of indacaterol clearance, CYP3A4 and P-gp, has no impact on safety of therapeutic doses of indacaterol. Therefore, no dose adjustment is warranted at the recommended 27.5/15.6 mcg twice-daily dose for UTIBRON NEOHALER when administered concomitantly with inhibitors of CYP3A4 and P-gp [see CLINICAL PHARMACOLOGY].
Warnings & Precautions
WARNINGS
Included as part of the PRECAUTIONS section.
PRECAUTIONS
Serious Asthma-Related Events – Hospitalizations, Intubations, Death
The safety and efficacy of UTIBRON NEOHALER in patients with asthma have not been established. UTIBRON NEOHALER is not indicated for the treatment of asthma [see CONTRAINDICATIONS].
Use of LABA as monotherapy [without inhaled corticosteroids (ICS)] for asthma is associated with an increased risk of asthma-related death. Available data from controlled clinical trials also suggest that use of LABA as monotherapy increases the risk of asthma-related hospitalization in pediatric and adolescent patients. These findings are considered a class effect of LABA monotherapy. When LABA are used in a fixed-dose combination with ICS, data from large clinical trials do not show a significant increase in the risk of serious asthma-related events (hospitalizations, intubations, death) compared with ICS alone.
A 28-week, placebo-controlled U.S. study comparing the safety of another LABA (salmeterol) with placebo, each added to usual asthma therapy, showed an increase in asthma-related deaths in patients receiving salmeterol (13/13,176 in patients treated with salmeterol versus 3/13,179 in patients treated with placebo; RR 4.37, 95% CI 1.25, 15.34). The increased risk of asthma-related death is considered a class effect of the LABAs, including indacaterol, one of the ingredients in UTIBRON NEOHALER.
No study adequate to determine whether the rate of asthma-related death is increased in patients treated with UTIBRON NEOHALER has been conducted.
Available data do not suggest an increased risk of death with use of LABA in patients with COPD.
Deterioration Of Disease and Acute Episodes
UTIBRON NEOHALER should not be initiated in patients with acutely deteriorating or potentially life-threatening episodes of COPD. UTIBRON NEOHALER has not been studied in patients with acutely deteriorating COPD. The initiation of UTIBRON NEOHALER in this setting is not appropriate.
UTIBRON NEOHALER should not be used for the relief of acute symptoms, i.e., as rescue therapy for the treatment of acute episodes of bronchospasm. UTIBRON NEOHALER has not been studied in the relief of acute symptoms, and extra dosesshould not beused for that purpose. Acutesymptomsshould betreated with an inhaled, short-acting beta2-agonist.
When beginning UTIBRON NEOHALER, patients who have been taking oral or inhaled, short-actingbeta 2-agonists on a regular basis (e.g., 4 times a day) should be instructed to discontinue the regular use of these drugs and use them only for symptomatic relief of acute respiratory symptoms. When prescribing UTIBRON NEOHALER, the healthcare provider should also prescribe an inhaled, short-acting beta2-agonist and instruct the patient on howitshould be used. Increasing inhaled beta2-agonist use is a signal of deteriorating disease for which promptmedical attention is indicated.
COPD may deteriorate acutely over a period of hours or chronically over several days or longer. If UTIBRON NEOHALER no longer controls thesymptoms of bronchoconstriction;the patient’s inhaled, short-acting beta2-agonist becomes less effective; or the patient needs moreinhalation ofshort-acting beta2-agonist thanusual, these may be markers of deterioration of disease. In this setting, a re-evaluation of the patient and the COPD treatment regimen should be undertaken at once. Increasing the daily dose of UTIBRON NEOHALER beyond the recommended dose is not appropriate in this situation.
Avoid Excessive Use Of UTIBRON NEOHALER And Avoid Use With Other Long-Acting Beta2-Adrenergic Agonists
As with otherinhaleddrugs containing beta 2-adrenergics, UTIBRON NEOHALER should not be used moreoften than recommended, at higher doses than recommended, or in conjunction with other medications containing LABAs, as an overdose may result. Clinically significant cardiovascular effects and fatalities have been reported in association with excessive use of inhaled sympathomimetic drugs. Patients using UTIBRON NEOHALER should not use another medicine containing a LABA for any reason [see DRUG INTERACTIONS].
Paradoxical Bronchospasm
As with other inhaled medicines, UTIBRON NEOHALER can produce paradoxical bronchospasm that may be life-threatening. If paradoxical bronchospasm occurs following dosing with UTIBRON NEOHALER, it should be treated immediately with an inhaled, short-acting bronchodilator; UTIBRON NEOHALER should be discontinued immediately and alternative therapy instituted.
Hypersensitivity Reactions, Including Anaphylaxis
Immediate hypersensitivity reactions have been reported after administration of indacaterol or glycopyrrolate, the components of UTIBRON NEOHALER. If signs suggesting allergic reactions occur, in particular, angioedema (including difficulties in breathing or swallowing, swelling of tongue, lips, and face), anaphylaxis, urticaria, or skin rash, UTIBRON NEOHALER should be discontinued immediately and alternative therapy instituted. UTIBRON NEOHALER should be used with caution in patients with severe hypersensitivity to milk proteins.
Cardiovascular Effects
Indacaterol, like other beta2-agonists, can produce a clinically significant cardiovasculareffect insome patients as measured by increases in pulse rate, systolic or diastolic blood pressure, or symptoms. If such effects occur, UTIBRON NEOHALER may need to be discontinued. In addition, beta-adrenergic agonists have been reported to produce ECG changes, such as flattening of the T-wave, prolongation of the QTc interval, and ST segment depression, although the clinical significance of these findings is unknown. Therefore, UTIBRON NEOHALER should be used with caution in patients with cardiovascular disorders, especially coronary insufficiency, cardiac arrhythmias, and hypertension.
Coexisting Conditions
UTIBRON NEOHALER, like all medicines containing sympathomimetic amines, should be used with caution in patients with convulsive disorders or thyrotoxicosis, and in patients who are unusually responsive to sympathomimetic amines. Doses of the related beta2-agonistalbuterol, when administered intravenously, have been reported toaggravate preexisting diabetes mellitus and ketoacidosis.
Worsening Of Narrow-Angle Glaucoma
UTIBRON NEOHALER should be used with caution in patients with narrow-angle glaucoma. Prescribers and patients should be alert for signs and symptoms of acute narrow-angle glaucoma (e.g., eye pain or discomfort, blurred vision, visual halos or colored images in association with red eyes from conjunctival congestion and corneal edema). Instruct patients to consult their healthcare provider immediately should any of these signs or symptoms develop.
Worsening Of Urinary Retention
UTIBRON NEOHALER should be used with caution in patients with urinary retention. Prescribers and patients should be alert for signs and symptoms of urinary retention (e.g., difficulty passing urine, painful urination), especially in patients with prostatic hyperplasia or bladder-neck obstruction. Instruct patients to consult their healthcare provider immediately should any of these signs or symptoms develop.
Hypokalemia And Hyperglycemia
Beta2-adrenergic agonistsmay produce significant hypokalemiain some patients, which hasthe potentialto produce adverse cardiovascular effects [see CLINICAL PHARMACOLOGY]. The decrease in serum potassium is usually transient, not requiring supplementation. Inhalation of high dosesof beta 2-adrenergic agonists may produceincreases inplasma glucose.
In patients with severe COPD, hypokalemia may be potentiated by hypoxia and concomitant treatment [see DRUG INTERACTIONS], which may increase the susceptibility for cardiac arrhythmias.
In 2 clinical trials of 12-weeks duration evaluating UTIBRON NEOHALER in subjects with COPD, there was no evidence of a treatment effect on serum glucose or potassium.
Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (PATIENT INFORMATION and Instructions for Use).
Serious Asthma-Related Events
Inform patients that LABAs, such as indacaterol, one of the active ingredients in UTIBRON NEOHALER, when used as monotherapy [without an inhaled corticosteroid] increase the risk of serious asthma-related events, including asthma-related death. UTIBRON NEOHALER is not indicated for the treatment of asthma [see CONTRAINDICATIONS, WARNINGS AND PRECAUTIONS].
Not For Acute Symptoms
Inform patients that UTIBRON NEOHALER is not meant to relieve acute symptoms of COPD and extra doses should not be used for that purpose. Advise them to treat acute symptoms with a rescue inhaler, such as albuterol. Provide patients with such medicine and instruct them on how it should be used [see WARNINGS AND PRECAUTIONS].
Instruct patients to seek medical attention immediately if they experience any of the following:
- Symptoms get worse
- Need for more inhalations than usual of their rescue inhaler
Patients should not stop therapy with UTIBRON NEOHALER without physician/healthcare provider guidance since symptoms may recur after discontinuation.
Do Not Use Additional Long-Acting Beta2-Agonists
Instruct patients to not use other medicines containing a LABA. Patients should not use more than the recommended twice daily dose of UTIBRON NEOHALER [see WARNINGS AND PRECAUTIONS].
Instruct patients who have been taking inhaled, short-acting beta2-agonists on a regular basisto discontinue the regular use of these products and use them only for the symptomatic relief of acute symptoms.
Paradoxical Bronchospasm
Inform patients that UTIBRON NEOHALER can produce paradoxical bronchospasm. Advise patients that if paradoxical bronchospasm occurs, patients should discontinue UTIBRON NEOHALER.
Risks Associated With Beta2-Agonist Therapy
Inform patients of adverse effects associated with beta2-agonists, such as palpitations, chest pain, rapid heartrate, tremor, or nervousness. Instruct patients to consult their healthcare provider immediately should any of these signs or symptoms develop [see WARNINGS AND PRECAUTIONS].
Worsening Of Narrow-Angle Glaucoma
Instruct patients to be alert for signs and symptoms of acute narrow-angle glaucoma (e.g., eye pain or discomfort, blurred vision, visual halos or colored images in association with red eyes from conjunctival congestion and corneal edema). Instruct patients to consult their healthcare provider immediately should any of these signs or symptoms develop [see WARNINGS AND PRECAUTIONS].
Worsening Of Urinary Retention
Instruct patients to be alert for signs and symptoms of urinary retention (e.g., difficulty passing urine, painful urination). Instruct patients to consult their healthcare provider immediately should any of these signs or symptoms develop [see WARNINGS AND PRECAUTIONS].
Instructions For Administering UTIBRON NEOHALER
It is important for patients to understand how to correctly administer UTIBRON capsules using the NEOHALER device [see Instructions for Use]. Instruct patients that UTIBRON capsules should only be administered via the NEOHALER device and the NEOHALER device should not be used for administering other medications. Remind patients that the contents of UTIBRON capsules are for oral inhalation only and must not be swallowed.
Instruct patients always to store UTIBRON capsules in sealed blisters and to only remove 1 UTIBRON capsule immediately before use or it may not be as effective. Instruct patients to discard unused additional UTIBRON capsules that are exposed to air (i.e., not intended for immediate use).
Inform patients to use 1 inhalation of UTIBRON NEOHALER orally twice daily (1 capsule in the morning and 1 capsule in the evening) at the same time every day.
Inform patients that if they miss a dose of UTIBRON NEOHALER, they should use their next capsule at the usual time. Instruct patients to not use 2 capsules at one time and to not use more than 2 capsules in a day.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment Of Fertility
No studies of carcinogenicity, mutagenicity, or impairment of fertility were conducted with UTIBRON NEOHALER; however, studies are available for the individual components, indacaterol and glycopyrrolate, as described below.
Indacaterol
Indacaterol produced no treatment-related increases in the incidence of tumors in a 2-year inhalation study in Wistar rats at inhaled doses up to 2.09 mg/kg/day (approximately 110 times the MRHD on an AUC basis). A 26-week oral (gavage) study in CB6F1/TgrasH2 hemizygous mice with indacaterol at doses up to 600 mg/kg/day did not show any evidence of tumorigenicity.
Indacaterol tested negative in the following genotoxicity assays: the in vitro Ames test, in vitro chromosomal aberration test in V79 Chinese hamster cells, and in vivo rat bone marrow micronucleus test.
Indacaterol had no effects on fertility and reproductive performance in male and female rats at subcutaneous doses up to 2 mg/kg/day (approximately 890 and 670 times in males and females, respectively, the MRHD on an AUC basis).
Glycopyrrolate
Glycopyrrolate produced no treatment-related increases in the incidence of tumors in a 2-year inhalation study in Wistar rats at inhaled doses up to 0.56 mg/kg/day (approximately 170 times the MRHD on an AUC basis). No evidence of tumorigenicity occurred in a 26-week oral (gavage) study in male and female TgrasH2 mice that received glycopyrrolate at doses up to 93.8 and 125.1 mg/kg/day, respectively.
Glycopyrrolate tested negative in the following genotoxicity assays: the in vitro Ames assay, in vitro human lymphocyte chromosomal aberration assay, and in vivo rat bone marrow micronucleus assay.
Impairment of fertility was observed in male and female rats at a subcutaneous glycopyrrolate dose of 1.88 mg/kg/day (approximately 1,900 and 1,100 times, respectively, the MRHD on an AUC basis) based upon findings of decreased corpora lutea, implantation sites and corresponding reduction of live fetuses. No effects on fertility and reproductive performance occurred in male and female rats at a subcutaneous glycopyrrolate dose of 0.63 mg/kg/day, approximately 350 times the MRHD on an AUC basis.
Use In Specific Populations
Pregnancy
Risk Summary
There are no adequate and well-controlled studies with UTIBRON NEOHALER or its individual components, indacaterol and glycopyrrolate, in pregnant women. Women should be advised to contact their healthcare provider if they become pregnant while taking UTIBRON NEOHALER. Animal reproduction studies were conducted with individual components, indacaterol and glycopyrrolate.
Indacaterol
In animal reproduction studies, there was no evidence of fetal harm or structural abnormalities following subcutaneous administration of indacaterol maleate to pregnant Wistar rats and New Zealand White rabbits during the period of organogenesis at exposures approximately 340 and 770 times, respectively, the maximum recommended human dose (MRHD of 55 mcg) on an exposure (AUC) basis (see Data).
Glycopyrrolate
In animal reproduction studies, there was no evidence of fetal harm or structural abnormalities in Wistar rats or New Zealand White rabbits at inhaled doses approximately 1400 and 530 times, respectively, the MRHD (31.2 mcg) on an AUC basis (see Data).
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Clinical Considerations
Labor Or Delivery
There are no adequate and well-controlled human trials that have investigated the effects of UTIBRON NEOHALER during labor and delivery. Because beta-agonists may potentially interfere with uterine contractility, UTIBRON NEOHALER should be used during labor only if the potential benefit justifies the potential risk.
Data
Animal Data
Indacaterol: In embryo-fetal development studies, pregnant Wistar rats received indacaterol maleate, during the period of organogenesis from Gestation Days 6 to 17, at exposures up to 340 times the MRHD (on an AUC basis with maternal subcutaneous doses up to 1 mg/kg/day) and pregnant New Zealand White rabbits received indacaterol maleate, during the period of organogenesis from Gestation Days 7 to 20, at exposures up to 1900 times the MRHD (on an AUC basis with maternal subcutaneous doses up to 3 mg/kg/day). Indacaterol maleate produced no evidence of structural abnormalities in rats and rabbits at exposures up to 340 and 770 times, respectively, the MRHD. Rabbit fetuses were observed with increased incidences of full supernumerary rib, a structural variation, at an exposure approximately 1900 times the MRHD.
In a pre-and postnatal development study, pregnant Wistar rats received indacaterol maleate, throughout the periods of organogenesis and lactation from Gestation Day 6 through Lactation Day 20, at doses up to 175 times the MRHD (on a mg/m² basis with maternal subcutaneous doses up to 1 mg/kg/day). F1 pups received subcutaneous doses of indacaterol maleate up to 1 mg/kg/day from postpartum days 4 through 20. There was no evidence of maternal toxicity with doses up to 175 times the MRHD. There were no effects on the physical or behavioral development of F1 pups with doses up to 55 times the MRHD (on a mg/m² basis with maternal and F1 pup subcutaneous doses up to 0.3 mg/kg/day). There was a decrease in the number of F1 pregnant animals at a dose approximately 175 times the MRHD (on a mg/m² basis with maternal and F1 pup subcutaneous doses of 1 mg/kg/day); however, there were no effects on mating or other parameters of reproductive performance.
Glycopyrrolate: In embryo-fetal development studies, pregnant Wistar rats received daily inhalation doses of glycopyrrolate, during the period of organogenesis from Gestation Days 7 to 17, at exposures up to 1400 times the MRHD (on an AUC basis with maternal inhaled doses up to 3.83 mg/kg) and pregnant New Zealand White rabbits received daily inhalation doses of glycopyrrolate, during the period of organogenesis from Gestation Days 7 to 19, at exposures up to 530 times the MRHD (on an AUC basis with maternal inhaled doses up to 4.4 mg/kg). Glycopyrrolate produced no evidence of teratogenicity in Wistar rats or New Zealand White rabbits at exposures up to approximately 1,400 and 530 times, respectively, the MRHD.
In a pre-and postnatal development study in pregnant Wistar rats, glycopyrrolate was administered subcutaneously daily throughout the periods of organogenesis and lactation from Gestation Day 6 through Lactation Day 23 at exposures up to 1100 times the MRHD (on an AUC basis with maternal subcutaneous doses up to 1.88 mg/kg/day). Glycopyrrolate had no effects on physical, neurological, or reproductive development in rats following exposures up to approximately 1100 times the MRHD.
Lactation
Risk Summary
There is no data on the presence of indacaterol or glycopyrrolate or their metabolites in human milk, the effects on the breastfed infant, or the effects on milk production. However, in studies of lactating rats, indacaterol and glycopyrrolate were present in the milk (see Data). The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for UTIBRON NEOHALER and any potential adverse effects on the breastfed child from UTIBRON NEOHALER or from the underlying maternal condition.
Data
Animal Data
Indacaterol: Indacaterol and its metabolites were detected in the milk within 1 hour following a single subcutaneous 650 mcg radiolabeled indacaterol maleate administered to lactating rats postpartum days 8 and 9.
Glycopyrrolate: Glycopyrrolate and its metabolites were detected in the milk of lactating rats following a single intravenous injection of 4 mg/kg of radiolabeled glycopyrrolate.
Pediatric Use
The safety and effectiveness of UTIBRON NEOHALER in pediatric patients have not been established. UTIBRON NEOHALER is not indicated for use in pediatric patients.
Geriatric Use
Based on available data, no adjustment of UTIBRON NEOHALER dosage in geriatric patients is warranted. UTIBRON NEOHALER can be used at the recommended dosage in elderly patients 75 years of age and older.
Of the total number of subjects in clinical studies of UTIBRON NEOHALER, 45% were aged 65 and older, while 11% were aged 75 and older. No overall differences in safety or effectiveness were observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
Renal Impairment
Based on the pharmacokinetic characteristics of its monotherapy components, UTIBRON NEOHALER can be used at the recommended dose in patients with mild to moderate renal impairment. In patients with severe renal impairment (estimated GFR less than 30 mL/min/1.73 m²) or end-stage renal disease requiring dialysis, use UTIBRON NEOHALER only if the expected benefit outweighs the potential risk since the systemic exposure to glycopyrrolate may be increased in this population [see CLINICAL PHARMACOLOGY].
Hepatic Impairment
Based on the pharmacokinetic characteristics of its monotherapy components, UTIBRON NEOHALER can be used at the recommended dose in patients with mild and moderate hepatic impairment. Studies in subjects with severe hepatic impairment have not been performed [see CLINICAL PHARMACOLOGY].
Overdosage & Contraindications
OVERDOSE
In COPD patients, doses higher than the recommended dosage of UTIBRON NEOHALER were associated with an increase in ventricular ectopies after 14 days of dosing with 300/124.8 mcg and 600/124.8 mcg UTIBRON NEOHALER. In a total of four patients, non-sustained ventricular tachycardia was recorded, with the longest episode recorded being 9 beats (4 seconds).
UTIBRON NEOHALER contains both indacaterol and glycopyrrolate; therefore, the risks associated with overdosage for the individual components described below apply to UTIBRON NEOHALER. Treatment of overdosage consists of discontinuation of UTIBRON NEOHALER together with institution of appropriate symptomatic and/or supportive therapy. The judicious use of a cardioselective beta-receptor blocker may be considered, bearing in mind that such medicine can produce bronchospasm. Cardiac monitoring is recommended in cases of overdosage.
Indacaterol
The potential signs and symptoms associated with overdosage of indacaterol are those of excessive beta-adrenergic stimulation and occurrence or exaggeration of any of the signs and symptoms, e.g., angina, hypertension or hypotension, tachycardia, with rates up to 200 beats per minute, arrhythmias, nervousness, headache, tremor, dry mouth, palpitation, muscle cramps, nausea, vomiting, drowsiness, dizziness, fatigue, malaise, hypokalemia, hyperglycemia, metabolic acidosis, and insomnia. As with all inhaled sympathomimetic medications, cardiac arrest and even death may be associated with an overdose of indacaterol.
In COPD patients, single doses of indacaterol 3,000 mcg were associated with moderate increases in pulse rate, systolic blood pressure, and QTc interval.
Glycopyrrolate
An overdosage of glycopyrrolate may lead to anticholinergic signs and symptoms, such as nausea, vomiting, dizziness, lightheadedness, blurred vision, increased intraocular pressure (causing pain, vision disturbances, or reddening of the eye), obstipation or difficulties in voiding.
CONTRAINDICATIONS
UTIBRON NEOHALER is contraindicated in:
- use of along-acting beta2-adrenergic agonist(LABA), including UTIBRON NEOHALER, without an inhaled corticosteroid in patients with asthma [see WARNINGS AND PRECAUTIONS]. UTIBRON NEOHALER is not indicated for the treatment of asthma.
- in patients who have demonstrated hypersensitivity to indacaterol, glycopyrrolate, or to any of the ingredients [see WARNINGS AND PRECAUTIONS].
Clinical Pharmacology
Mechanism Of Action
UTIBRON NEOHALER contains both indacaterol and glycopyrrolate. The mechanisms of action described below for the individual components apply to UTIBRON NEOHALER. These drugs represent 2 different classes of medications (a LABA and an anticholinergic) that have different and additive effects on clinical and physiological indices.
Indacaterol
Indacaterol is a LABA. When inhaled, indacaterol acts locally in the lung as a bronchodilator. In vitro studies have shown that indacaterol has more than 24-fold greater agonist activity at beta2-receptors compared to beta1-receptors and 20-fold greater agonist activity compared to beta3-receptors. This selectivity profile is similar to formoterol. The clinical significance of these findings is unknown. Although beta2-receptors are the predominant adrenergic receptors in bronchial smooth muscle and beta1-receptors are the predominant receptors inthe heart,there are alsobeta2-adrenergic receptors in the human heart comprising 10% to 50% of the total adrenergic receptors. The precise function of these receptorsis not known, buttheir presence raises the possibility that evenhighly selective beta2-adrenergic agonists may have cardiac effects.
The pharmacological effects of beta2-adrenoceptor agonist drugs, including indacaterol, are at least in part attributable to stimulation of intracellular adenyl cyclase, the enzyme that catalyzes the conversion of adenosine triphosphate (ATP) to cyclic-3’, 5’-adenosine monophosphate (cyclic AMP). Increased cyclic AMP levels cause relaxation of bronchial smooth muscle and inhibition of release of mediators of immediate hypersensitivity from cells, especially from mast cells.
Glycopyrrolate
Glycopyrrolate is a long-acting muscarinic antagonist, which is often referred to as an anticholinergic. It has similar affinity to the subtypes of muscarinic receptors M1 to M5. In the airways, it exhibits pharmacological effects through inhibition of M3 receptor at the smooth muscle leading to bronchodilation. The competitive and reversible nature of antagonism was shown with human and animal origin receptors and isolated organ preparations. In preclinical in vitro as well as in vivo studies, prevention of methacholine and acetylcholine -induced bronchoconstrictive effects was dose-dependent and lasted longer than 24 hours. The clinical relevance of these findings is unknown. The bronchodilation following inhalation of glycopyrrolate is predominantly a site-specific effect.
Pharmacodynamics
Cardiac Electrophysiology
The QTc interval was studied in TQT studies with UTIBRON NEOHALER and with each of the monotherapy components. The TQT studies with indacaterol and glycopyrrolate demonstrated that neither of the compounds had a relevant effect on the corrected QT interval at supratherapeutic and therapeutic doses (for glycopyrrolate only a supratherapeutic dose was tested).
In a randomized, partially-blinded, placebo-and positive-controlled, crossover TQT study in 84 healthy subjects, a supratherapeutic dose of UTIBRON NEOHALER (indacaterol/glycopyrrolate 440/499.2 mcg) was administered. This is a 16/32 dose multiple compared to a single dose of the recommended 27.5/15.6 mcg twice-daily dosage of UTIBRON NEOHALER, which resulted in exposure multiples for mean Cmax of 9.3 for indacaterol and 35.2 for glycopyrrolate compared to steady-state pharmacokinetics of UTIBRON NEOHALER 27.5/15.6 mcg twice daily. The mean maximal change from baseline in QTcI compared to placebo was 8.70 msec (2-sided 90% CI 7.3, 10.1) at 30 minutes after dosing.
Pharmacokinetics
Absorption
Following inhalation of UTIBRON NEOHALER, the median time to reach peak plasma concentrations of indacaterol and glycopyrrolate was achieved rapidly at approximately 15 minutes and 5 minutes, respectively.
The steady-state systemic exposure (AUC0-12h,ss ; Cmax,ss ) to indacaterol and glycopyrrolate is similar after thetwice-daily inhalation of 2 (times 2) capsules of UTIBRON NEOHALER 27.5 mcg/15.6 mcg as compared to the twice-daily inhalation of the monotherapy products indacaterol 27.5 mcg (times 2) alone or glycopyrrolate 15.6 mcg (times 2) alone, respectively.
Indacaterol
Absolute bioavailability of indacaterol after an inhalation dose was on average 43% to 45%. Systemic exposure results from a composite of pulmonary and intestine absorption. Indacaterol serum concentrations increased with repeated once daily administration. Steady-state was achieved within 12 to 15 days. The mean accumulation ratio of indacaterol, i.e., AUC over the 24-hour dosing interval on Day 14 or Day 15 compared to Day 1, was in the range of 2.9 to 3.8 for once daily inhaled doses between 75 mcg and 600 mcg.
Glycopyrrolate
Following repeated once daily inhalation in patients with COPD, pharmacokinetic steady-state of glycopyrrolate was reached within 1 week of treatment. There was no indication that the glycopyrrolate pharmacokinetics changes over time. With once daily doses of 124.8 mcg and 249.6 mcg, steady-state exposure to glycopyrrolate (AUC over the dosing interval) was about 1.4-to 1.7-fold higher than after the first dose.
Distribution
Indacaterol
After intravenous infusion,the volume ofdistribution (Vz) ofindacaterol was 2361 to 2557 L,indicating an extensive distribution. The in vitro human serum and plasma protein binding was 94.1% to 95.3% and 95.1% to 96.2%, respectively.
Glycopyrrolate
After intravenousadministration,the steady-state volume of distribution (Vss) of glycopyrrolate was 83 L and the volume of distribution in theterminal phase (Vz) was 376 L. The in vitro human plasma protein binding of glycopyrrolate was 38% to 41% at concentrations of 1 to 10 ng/mL.
Elimination
Indacaterol
In clinical studies which included urine collection, the amount of indacaterol excreted unchanged via urine was generally lower than 2% of the dose. Renal clearance of indacaterol was, on average, between 0.46 L/h and 1.20 L/h. When compared with the serum clearance of indacaterol of 18.8 L/h to 23.3 L/h, it is evident that renal clearance plays a minor role (about 2% to 6% of systemic clearance) in the elimination of systemically available indacaterol.
In a human ADME study where indacaterol was given orally, the fecal route of excretion was dominant over the urinary route. Indacaterol was excreted into human feces primarily as unchanged parent drug (54% of the dose) and, to a lesser extent, hydroxylated indacaterol metabolites (23% of the dose). Mass balance was complete with greater than or equal to 90% of the dose recovered in the excreta.
Indacaterol serum concentrations declined in a multi-phasic manner with an average terminal half-life ranging from 45.5 to 126 hours. The effective half-life, calculated from the accumulation of indacaterol after repeated dosing, ranged from 40 to 56 hours, which is consistent with the observed time to steady-state of approximately 12 to 15 days.
Glycopyrrolate
Renal elimination of parent drug accounts for about 60% to 70% of total clearance of systemically available glycopyrrolate whereas non-renal clearance processes account for about 30% to 40%. Biliary clearance contributes to the non-renal clearance, but the majority of non-renal clearance is thought to be due to metabolism.
Following inhalation of single and repeated once daily doses between 62.4 mcg and 249.6 mcg glycopyrrolate by healthy volunteers and patients with COPD, mean renal clearance of glycopyrrolate was in the range of 17.4 L/h and 24.4 L/h. Active tubular secretion contributes to the renal elimination of glycopyrrolate.
Glycopyrrolate plasma concentrations declined in a multi-phasic manner. The mean terminal elimination half-life of glycopyrrolate was much longer after inhalation (33 to 53 hours) than after intravenous (6.2 hours) and oral (2.8 hours) administration.
Metabolism
Indacaterol
In vitro investigations indicated that UGT1A1 is the only UGT isoform that metabolized indacaterol to the phenolic O-glucuronide. The oxidative metabolites were found in incubations with recombinant CYP1A1, CYP2D6, and CYP3A4. CYP3A4 is concluded to be the predominant isoenzyme responsible for hydroxylation of indacaterol. In vitro investigations further indicated that indacaterol is a low affinity substrate for the efflux pump P-gp.
After oral administration of radiolabelled indacaterol in a human absorption, distribution, metabolism, excretion (ADME) study, unchanged indacaterol was the main component in serum, accounting for about one-third of total drug-related AUC over 24 hours. A hydroxylated derivative was the most prominent metabolite in serum. Phenolic O-glucuronides of indacaterol and hydroxylated indacaterol were further prominent metabolites. A diastereomer of the hydroxylated derivative, a N-glucuronide of indacaterol, and C-and N-dealkylated products were further metabolites identified.
Glycopyrrolate
In vitro metabolism studies show glycopyrrolate hydroxylation resulting in a variety of mono-and bishydroxylated metabolites and direct hydrolysis resulting in the formation of a carboxylic acid derivative (M9). Further in vitro investigations showed that multiple CYP isoenzymes contribute to the oxidative biotransformation of glycopyrrolate and the hydrolysis to M9 is likely to be catalyzed by members from the cholinesterase family pre-systemically and/or via first pass metabolism from the swallowed dose fraction of orally inhaled glycopyrrolate. Glucuronide and/or sulfate conjugates of glycopyrrolate were found in urine of humans after repeated inhalation, accounting for about 3% of the dose.
Drug Interaction Studies
There is no pharmacokinetic drug-drug interaction resulting from the concomitant administration of inhaled glycopyrrolate and inhaled indacaterol based on steady-state exposure data.
No specific drug-drug interaction studies were conducted with UTIBRON NEOHALER. Information on the potential for interactions for UTIBRON NEOHALER is based on the potential for each of its 2 monotherapy components.
Effect Of Coadministered Drugs On Indacaterol And Glycopyrrolate Exposure
Indacaterol
Inhibitors of Cytochrome P450 3A4 and P-gp Efflux Transporter: Drug interaction studies were carried out using potent and specific inhibitors of CYP3A4 and P-gp (i.e., ketoconazole, erythromycin, verapamil and ritonavir). Coadministration of indacaterol 300 mcg (single dose) with verapamil (80 mg 3 times a day for 4 days) showed 2-fold increase in indacaterol AUC0-24h , and 1.5-fold increase in indacaterolCmax . Coadministration of indacaterol inhalation powder 300 mcg (single dose) with erythromycin (400 mg 4 times a day for 7 days) showed a 1.4-fold increase in indacaterol AUC024h, and 1.2-fold increase in indacaterol Cmax . Coadministration ofindacaterol inhalation powder 300 mcg (single dose) with ketoconazole (200 mg twicedailyfor 7 days)caused a 1.9-fold increase in indacaterol AUC0-24h , and 1.3-fold increase in indacaterol Cmax . Coadministration of indacaterol 300 mcg (single dose) with ritonavir (300 mg twice daily for 7.5 days) resulted in a 1.7-fold increase in indacaterol AUC0-24h whereas indacaterol Cmax was unaffected [see DRUG INTERACTIONS].
Glycopyrrolate
Cimetidine or Other Inhibitors of Organic Cationic Transport: In a clinical study in healthy volunteers, cimetidine, an inhibitor of organic cation transport that is thought to contribute to the renal excretion of glycopyrrolate, increased total exposure (AUC) to glycopyrrolate by 22% and decreased renal clearance by 23%.
Effect Of UTIBRON On Coadministered Drugs Exposure
In vitro studies show that UTIBRON NEOHALER is not likely to inhibit or induce the metabolism of other drugs, nor processes involving drug transporters.
Indacaterol: In vitro investigations indicated that indacaterol has negligible potential to cause metabolic interactions with medications (by inhibition or induction of cytochrome P450 enzymes, or induction of UGT1A1) at the systemic exposure levels achieved in clinical practice. In vitro investigation furthermore indicated that, in vivo, indacaterol is unlikely to significantly inhibit transporter proteins, such as P-gp, MRP2, BCRP, the cationic substrate transporters hOCT1 and hOCT2, and the human multidrug and toxin extrusion transporters hMATE1 and hMATE2K, and that indacaterol has negligible potential to induce P-gp or MRP2.
Glycopyrrolate: In vitro inhibition studies demonstrated that glycopyrrolate has no relevant capacity to inhibit CYP1A2, CYP2A6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, CYP2E1 or CYP3A4/5, the efflux transporters MDR1, MRP2 or MXR, and the uptake transporters OATP1B1, OATP1B3, OAT1, OAT3, OCT1 or OCT2. In vitro enzyme induction studies did not indicate a clinically relevant induction by glycopyrrolate for any of the cytochrome P450 isoenzymes tested as well as for UGT1A1 and the transporters MDR1 and MRP2.
Specific Populations
Population pharmacokinetic analysis showed no evidence of a clinically significant effect of age (40 to 85 years), body weight (45 kg to 120 kg), gender, smoking status, and baseline FEV1 on systemic exposure of either indacaterol or glycopyrrolate following inhalation of UTIBRON NEOHALER.
Similarly no relevant covariate effect (of age,body weight, gender, smoking status, and baseline FEV1) was observed following the inhalation of the 2 components indacaterol and glycopyrrolate separately.
Patients With Renal Impairment
Indacaterol
Due to the very low contribution of the urinary pathway to total body elimination of indacaterol, a study in renally impaired subjects was not performed.
Glycopyrrolate
Renal impairment has an impact on the systemic exposure to glycopyrrolate. A moderate mean increase in total systemic exposure (AUC last) of up to 1.4-fold was seen in subjects with mild and moderate renal impairment [estimated glomerular filtration rate (GFR) greater than or equal to 30 mL/min/1.73m²] and up to 2.2-fold in subjects with severe renal impairment and end stage renal disease [estimated GFR less than 30 mL/min/1.73m²] [see Use In Specific Populations].
Patients With Hepatic Impairment
Based on the clinical pharmacokinetic characteristics of its monotherapy components, UTIBRON NEOHALER can be used at the recommended dose in patients with mild and moderate hepatic impairment. UTIBRON NEOHALER has not been evaluated in subjects with severe hepatic impairment.
Indacaterol
Patients with mild and moderate hepatic impairment showed no relevant changes in Cmax or AUC of indacaterol, nor did protein binding differ between mild and moderate hepatic impaired subjects and their healthy controls. Studies in subjects with severe hepatic impairment were not performed [see Use In Specific Populations].
Glycopyrrolate
Clinical studies in patients with hepatic impairment have not been conducted. Glycopyrrolate is cleared predominantly from the systemic circulation by renal excretion.
Racial Or Ethnic Groups
There was no evidence of a clinically significant ethnic/race effect (across Caucasian, Chinese, and Japanese subjects) on the systemic exposure to indacaterol and glycopyrrolate following inhalation of UTIBRON NEOHALER.
Similarly, no relevant ethnic effect was observed following the inhalation of the 2 components indacaterol and glycopyrrolate separately.
Pharmacogenomics
Indacaterol
The pharmacokinetics of indacaterol were prospectively investigated in subjects with the UGT1A1 (TA)7/(TA)7 genotype (low UGT1A1 expression; also referred to as *28) and the (TA)6, (TA)6 genotype. Steady-state AUC and Cmax of indacaterol were 1.2-fold higher in the [(TA)7, (TA)7] genotype, suggesting no relevant effect of UGT1A1 genotype of indacaterol exposure.
Glycopyrrolate
The effects of pharmacogenomic variants on the pharmacokinetics of glycopyrrolate have not been investigated.
Clinical Studies
The safety and efficacy of UTIBRON NEOHALER were evaluated in a clinical development program that included 3 dose-ranging trials, 2 lung function trials of 12-weeks duration (placebo-controlled and active-controlled) and a 12-month long-term safety trial. The efficacy of UTIBRON NEOHALER is based primarily on the dose-ranging trials in 562 subjects with COPD or asthma and the 2 placebo-and active-controlled confirmatory trials in 2043 subjects with additional support from one active-controlled 12-month trial in 615 subjects with COPD.
Dose-Ranging Trials
Dose selection for UTIBRON NEOHALER for COPD was based on data for the individual components, indacaterol and glycopyrrolate.
Indacaterol
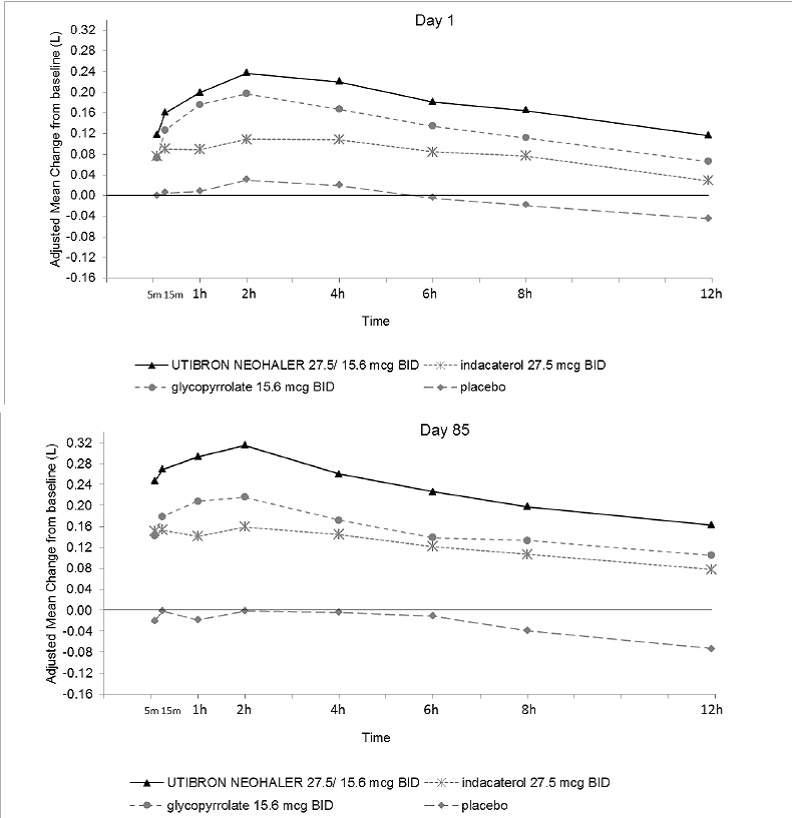
 Indacaterol dose selection in UTIBRON NEOHALER is based on the registered dose of 75 mcg once daily and is also supported by a single-dose, multicenter, randomized, double-blind, placebo-controlled, crossover study in asthma evaluating 5 doses of indacaterol in 91 subjects, (37.5 mcg, 55 mcg, 75 mcg, and 150 mcg once daily, and 27.5 mcg twice daily). A dose-related increase in FEV1 AUC0-24h compared with placebo was observed. The differences in change from baseline in FEV 1 AUC0-24h after single dosing for the indacaterol 37.5 mcg, 55 mcg, 75 mcg, and 150 mcg once daily and the 27.5 mcg twice daily doses compared to placebo were 0.099 L (95% CI: 0.069, 0.128), 0.132 L (0.103, 0.162), 0.143 L (0.114, 0.171), 0.187 L (0.157, 0.216), and 0.121 L (0.092, 0.151), respectively. These results supported the evaluation of indacaterol 27.5 mcg twice daily in the confirmatory COPD trials. UTIBRON NEOHALER is not indicated for asthma.
Figure 1: Adjusted Mean Change From Baseline inFEV 1(L) Over 24 Hourson Day 1
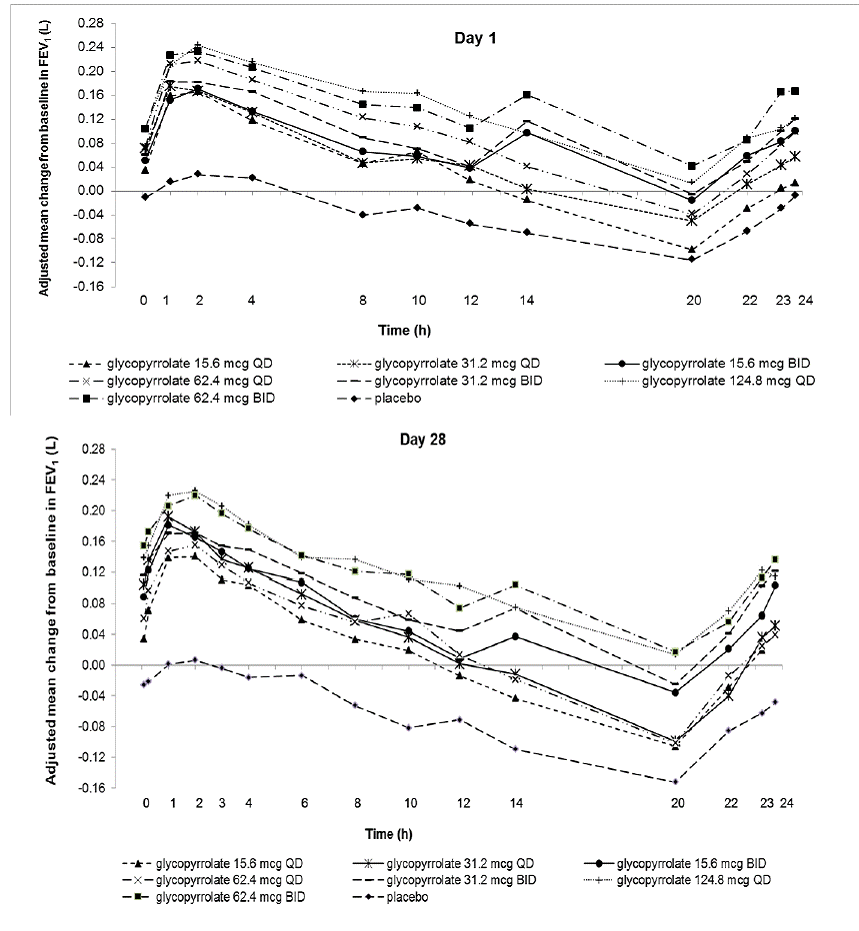 |
Glycopyrrolate
Dose selection for glycopyrrolate in UTIBRON NEOHALER in COPD was supported by a 28-day, randomized, double-blind, placebo-controlled, 2-period, crossover study evaluating 7 doses of glycopyrrolate (15.6 mcg, 31.2 mcg, 62.4 mcg, and 124.8 mcg once daily and 15.6 mcg, 31.2 mcg, and 62.4 mcg twice daily) or placebo in 388 subjects with COPD. The differences in trough FEV1 from baseline after 28 days compared to placebo for the 15.6 mcg, 31.2 mcg, 62.4 mcg, and 124.8 mcg once daily and for 15.6 mcg, 31.2 mcg, and 62.4 mcg twice-daily doses were 0.083 L (95% CI: 0.030, 0.136), 0.098 L (0.048, 0.148), 0.090 L (0.038, 0.142), 0.176 L (0.132, 0.220), 0.139 L (0.089, 0.189), 0.167 L (0.115, 0.219), and 0.177 L (0.132, 0.222), respectively. The dose-ranging results supported the evaluation of glycopyrrolate 15.6 mcg twice daily in the confirmatory COPD trials.
Figure 2: Adjusted Mean Change From Baseline in FEV1 (L) Over 24 Hours on Days 1 and 28
 |
Based on the findings from dose-ranging studies of the individual components, a twice daily dose of indacaterol/glycopyrrolate 27.5 mcg/15.6 mcg was evaluated in the confirmatory COPD trials.
Confirmatory Trials
The clinical development program for UTIBRON NEOHALER included two (Trial 1 and Trial 2) 12-week, randomized, double-blinded, placebo-and active-controlled, parallel-group trials in subjects with COPD designed to evaluate the efficacy and safety of UTIBRON NEOHALER; and one 12-month, randomized, double-blind, active-controlled trial (Trial 3) that evaluated bronchodilation and effects on long-term safety.
The 12-week trials evaluated the efficacy of 2038 subjects that had a clinical diagnosis of COPD, were 40 years of age or older, had a history of smoking greater than 10 pack-years, had a post-albuterol FEV1 greater than or equal to 30% and less than 80% of predicted normalvalues, had aratio of FEV1/FVC ofless than 0.7, and were symptomatic as determined by a Modified Medical Research Council (mMRC) score greater than or equal to 2. Of the 2038 subjects included in the efficacy analysis, 63% were male and 91% were Caucasian. They had a mean age of 63 years and an average smoking history of 47 pack-years, with 52% identified as current smokers, and 46% used concomitant inhaled corticosteroids. At screening, the mean post-bronchodilator percent predicted FEV1 was 55% (range: 29% to 79%), the mean postbronchodilator FEV1/FVC ratio was 50% (range: 19%to 71%), and themean percentreversibility was 23% (range:0% to 144%).
Trial 1 and Trial 2 evaluated UTIBRON NEOHALER (indacaterol/glycopyrrolate) 27.5 mcg/15.6 mcg twice daily, indacaterol 27.5 mcg twice daily, glycopyrrolate 15.6 mcg twice daily, and placebo twice daily. The primary endpoint was the change from baseline in FEV1 AUC0-12h following the morning dose at Day 85 (defined as the mean FEV1 change from baseline over 0 to 12 hours divided by 12 hours) compared with placebo, glycopyrrolate 15.6 mcg twice daily, and indacaterol 27.5 mcg twice daily. The comparison of UTIBRON NEOHALER with indacaterol 27.5 mcg and glycopyrrolate 15.6 mcg was assessed to evaluate the contribution of the individual comparators to UTIBRON NEOHALER. In both trials, UTIBRON NEOHALER demonstrated a larger increase in mean change from baseline in FEV1 AUC0-12h compared to placebo, indacaterol 27.5 mcg twice daily, and glycopyrrolate 15.6 mcg twice daily (see Table 2).
Table 2: Least squares (LS) Mean Change From Baseline in FEV 1 (L) AUC(0-12 h ) at Day 85 in Trials 1 and 2 (Intent-to-Treat Population)
| Treatment | N | FEV 1 (L) AUC0-12h at Week 12 | ||
| Difference from | ||||
| Placebo LS Mean (95% CI) | Indacaterol 27.5 mcg twice daily* LS Mean (95% CI) | Glycopyrrolate 15.6 mcg twice daily* LS Mean (95% CI) | ||
| Trial 1 (N = 996) | ||||
| UTIBRON NEOHALER 27.5 mcg/15.6 mcg twice daily | 249 | N = 246 0.262 L (0.224, 0.300) | N = 251 0.112 L (0.075, 0.149) | N = 250 0.079 L (0.042, 0.116) |
| Trial 2 (N = 1039) | ||||
| UTIBRON NEOHALER 27.5 mcg/15.6 mcg twice daily | 258 | N = 260 0.231 L (0.192, 0.271) | N = 260 0.094 L (0.055, 0.133) | N = 261 0.098 L (0.059, 0.137) |
| N = Number in intent-to-treat population. *The indacaterol and glycopyrrolate comparators used the same inhaler and excipients as UTIBRON NEOHALER. |
||||
With the limited data available, there was no suggestion of a difference in FEV1 AUC0-12h with respect to age, sex, degree of airflow limitation, GOLD stage, smoking status, or inhaled corticosteroid use.
In Trial 1 and Trial 2, serial spirometric evaluations throughout the 12-hour dosing interval were performed in all subjects at Days 1 and 85. The spirometric curves from Trial 1 at Days 1 and 85 are displayed in Figure 3. In Trial 2, the results for UTIBRON NEOHALER in FEV1 AUC0-12h were similar to those observed in Trial 1.
Figure 3: Adjusted Mean Change From Baseline in FEV 1 (L) Over 12 hours on Days 1 and 85 in Trial 1 (All patient Population)
 |
The peak FEV1 was defined as the maximum FEV1 recorded within 4 hours after the morning dose on Days 1 and 85. The mean peak FEV1 improvement from baseline for UTIBRON NEOHALER compared with placebo at Day 1 and at Day 85 was 0.185 L and 0.290 L (Trial 1) and 0.151 L and 0.260 L (Trial 2), respectively. The median time to onset on Day 1, defined as a 100mL increase from baseline in FEV 1, was 12 minutes and 16 minutesin Trials 1 and 2, respectively, in subjects receiving UTIBRON NEOHALER.
In Trials 1 and 2, patients treated with UTIBRON NEOHALER used less daily rescue albuterol compared to patients treated with placebo.
The St. George’s Respiratory Questionnaire (SGRQ) was assessed in Trials 1 and 2. In Trial 2, the SGRQ responder rate (defined as an improvement in score of 4 or more as threshold) was 57%, 46%, 48%, and 39%, for UTIBRON NEOHALER, glycopyrrolate, indacaterol, and placebo, respectively, with odds ratios of 1.6 (95% CI 1.1, 2.3), 1.5 (95% CI 1.1, 2.2), and 2.2 (95% CI 1.5, 3.2), for UTIBRON NEOHALER vs. glycopyrrolate, UTIBRON NEOHALER vs. indacaterol, and UTIBRON NEOHALER vs. placebo, respectively. In Trial 1, the trends were similar, with odds ratios of 1.4 (95% CI 1.0, 2.0), 1.1 (95% CI 0.8, 1.7), and 2.9 (95% CI 1.9, 4.2), for UTIBRON NEOHALER vs. glycopyrrolate, UTIBRON NEOHALER vs. indacaterol, and UTIBRON NEOHALER vs. placebo, respectively.
52-week Effects on Lung Function: At Week 52 (Trial 3), UTIBRON NEOHALER demonstrated a significant treatment effect with an increase of 0.080 L in pre-dose trough FEV 1 compared to indacaterol 75 mcg once daily.
Medication Guide
PATIENT INFORMATION
UTIBRON®
(yoo-TEE-bron) NEOHALER® (indacaterol and glycopyrrolate) inhalation powder, for oral inhalation use
Important: Do not swallow UTIBRON capsules. UTIBRON capsules are used only with the NEOHALER inhaler that comes with UTIBRON NEOHALER. Do not place a capsule in the mouthpiece of the NEOHALER inhaler.
What is UTIBRON NEOHALER?
UTIBRON NEOHALER combines a long-acting beta2 adrenergic agonist (LABA) medicine (indacaterol) and an anticholinergic medicine (glycopyrrolate).
- LABA and anticholinergic medicines help the muscles around the airways in your lungs stay relaxed to prevent symptoms, such as wheezing, cough, chest tightness, and shortness of breath. This makes it hard to breathe.
- UTIBRON NEOHALER is used to treat chronic obstructive pulmonary disease (COPD). COPD is a chronic lung disease that includes chronic bronchitis, emphysema, or both.
- UTIBRON NEOHALER is for long-term use and should be taken, 2 times each day, to improve symptoms of COPD for better breathing.
- UTIBRON NEOHALER is not used to treat sudden symptoms of COPD. Always have a short-acting beta 2 agonist medicine (rescue inhaler) with you to treat sudden symptoms of COPD. If you do not have a rescue inhaler, contact your healthcare provider to have one prescribed for you.
- UTIBRON NEOHALER is not for the treatment of asthma. It is not known if UTIBRON NEOHALER is safe and effective in people with asthma.
- UTIBRON NEOHALER should not be used in children. It is not known if UTIBRON NEOHALER is safe and effective in children.
Do not use UTIBRON NEOHALER if you:
- have asthma.
- are allergic to indacaterol, glycopyrrolate, or any of the ingredients in UTIBRON NEOHALER. Ask your healthcare provider if you are not sure. See “What are the ingredients in UTIBRON NEOHALER?” at the end of this Patient Information leaflet for a complete list of ingredients in UTIBRON NEOHALER.
Before using UTIBRON NEOHALER, tell your healthcare provider about all of your medical conditions,including if you:
- have heart problems
- have high blood pressure
- have seizures
- have thyroid problems
- have diabetes
- have liver problems
- have kidney problems
- have eye problems, such as glaucoma. UTIBRON NEOHALER may make your glaucoma worse.
- have prostate or bladder problems, or problems passing urine. UTIBRON NEOHALER may make these problems worse.
- are pregnant or plan to become pregnant. It is not known if UTIBRON NEOHALER can harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if the medicines in UTIBRON NEOHALER pass into your breast milk and if it can harm your baby. You and your healthcare provider should decide if you will take UTIBRON NEOHALER or breastfeed.
- are allergic to UTIBRON NEOHALER or any of its ingredients, any other medicines, or food products.
UTIBRON NEOHALER contains lactose (milk sugar) and a small amount of milk proteins. It is possible that allergic reactions may happen in people who have a severe milk protein allergy.
Tell your healthcare provider about all the medicines you take, including prescription medicines, over-the-counter medicines, vitamins, and herbal supplements. UTIBRON NEOHALER may affect the way other medicines work, and other medicines can affect how UTIBRON NEOHALER works. Using UTIBRON NEOHALER with other medicines may cause serious side effects.
Know the medicines you take. Keep a list of your medicines with you and show it to your healthcare provider and pharmacist when you get a new medicine.
How should I use UTIBRON NEOHALER?
Read the step-by-step instructions for using UTIBRON NEOHALER at the end of this Patient Information leaflet.
- Do not use UTIBRON NEOHALER unless your healthcare provider has taught you how to use the inhaler and you understand how to use it correctly.
- Use UTIBRON NEOHALER exactly as your healthcare provider tells you to use it. Do not use UTIBRON NEOHALER more often than prescribed for you.
- Do not swallow UTIBRON capsules. Only use UTIBRON capsules with the UTIBRON NEOHALER inhaler.
- Use 1 UTIBRON capsule inhaled through the NEOHALER inhaler 2 times each day (1 capsule in themorning and 1 capsule in the evening).
- To make sure that the full dose has been taken, you should open the inhaler to check that there is no powder left in the capsule. As long as the capsule is empty, you have received 1 full dose.
- If you miss a dose of UTIBRON NEOHALER, take it as soon as you remember. Take your next dose at your usual time.
- Do not use 2 capsules at one time.
- Do not use more than 2 capsules in a day.
- UTIBRON capsules should always be stored in the blister strip and only removed immediately before use. Peel the backing foil away from the blister to open it, do not push the capsule through the foil.
- Always use the new NEOHALER inhaler that is provided with each new prescription.
- UTIBRON NEOHALER does not relieve sudden symptoms of COPD. Always have a rescue inhaler medicine with you to treat sudden symptoms. If you do not have a rescue inhaler medicine, call your healthcare provider to have a rescue inhaler prescribed for you.
- Do not stop using UTIBRON NEOHALER or other medicines to control or treat your COPD unless told to do so by your healthcare provider because your symptoms might get worse. Your healthcare provider will change your medicines as needed.
- Call your healthcare provider or get emergency medical care right away if your breathing problems worsen with UTIBRON NEOHALER, you need to use your rescue medicine more often than usual, or your rescue inhaler medicine does not work as well for you at relieving your symptoms.
What are the possible side effects of UTIBRON NEOHALER?
UTIBRON NEOHALER can cause serious side effects, including:
- people with asthma who take LABA medicines, such as indacaterol (one of the medicines in UTIBRONNEOHALER), without also using a medicine called an inhaled corticosteroid, have an increased risk ofserious problems from asthma, including being hospitalized, needing a tube placed in their airway to helpthem breathe, or death.
- Call your healthcare provider if breathing problems worsen over time while using UTIBRONNEOHALER. You may need a different treatment.
- Get emergency medical care if:
- your breathing problems worsen quickly
- you use your rescue inhaler medicine, but it does not relieve your breathing problems
- COPD symptoms that get worse over time. If your COPD symptoms worsen over time, do not increase your dose of UTIBRON NEOHALER, instead call your healthcare provider.
- using too much of a LABA medicine may cause:
- chest pain
- increased blood pressure
- fast and irregular heartbeat
- headache
- tremor
- nervousness
- sudden shortness of breath immediately after use of UTIBRON NEOHALER. Sudden shortness of breathmay be life-threatening. If you have sudden breathing problems immediately after inhaling your medicine, stop taking UTIBRON NEOHALER and call your healthcare provider or go to the nearest hospital emergency room right away.
- serious allergic reactions. Stop using UTIBRON NEOHALER and call your healthcare provider or get emergency medical care right away if you get any of the following symptoms of a serious allergic reaction:
- rash
- hives
- swelling of the tongue, lips, and face
- difficulty breathing or swallowing
- effects on your heart
- fast or irregular heartbeat, awareness of a heartbeat
- increased blood pressure
- chest pain
- new or worsened eye problems, including acute narrow-angle glaucoma. Acute narrow-angle glaucoma can cause permanent loss of vision if not treated. Symptoms of acute narrow-angle glaucoma may include:
- eye pain or discomfort
- nausea or vomiting
- blurred vision
- red eyes
- seeing halos or bright colors around lights
If you have these symptoms, stop taking UTIBRON NEOHALER and call your healthcare provider right away before taking another dose.
- new or worsened urinary retention. People who use UTIBRON NEOHALER may develop new or worse urinary retention. Urinary retention can be caused by a blockage in your bladder. Urinary retention can also happen in men who have a larger than normal prostate. Symptoms of urinary retention may include:
- difficulty urinating
- painful urination
- urinating frequently
- urination in a weak stream or drips
If you have these symptoms, stop taking UTIBRON NEOHALER and call your healthcare provider right away before using another dose.
- changes in laboratory blood levels, including high levels of blood sugar (hyperglycemia) and low levels of potassium (hypokalemia) which may cause symptoms of muscle spasm, muscle weakness or abnormal heart rhythm.
Common side effects of UTIBRON NEOHALER include sore throat and runny nose, high blood pressure, and back pain.
Tell your healthcare provider about any side effect that bothers you or that does not go away. These are not all of the possible side effects of UTIBRON NEOHALER. For more information, ask your healthcare provider or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store UTIBRON NEOHALER?
- Store UTIBRON NEOHALER (inhaler and blister-packaged capsules) at room temperature between 68°F to 77°F (20°C to 25°C).
- Do not remove UTIBRON capsules from the blister card it comes in until you are ready to use a dose of UTIBRON NEOHALER.
- Do not store UTIBRON capsules in the NEOHALER inhaler.
- Keep UTIBRON NEOHALER in a dry place away from light and moisture.
Keep UTIBRON NEOHALER and all medicines out of the reach of children.
General information about the safe and effective use of UTIBRON NEOHALER.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use UTIBRON NEOHALER for a condition for which it was not prescribed. Do not give UTIBRON NEOHALER to other people, even if they have the same symptoms you have. It may harm them.
You can ask your healthcare provider or pharmacist for information that is written for healthcare professionals.
For more information about UTIBRON NEOHALER or to report side effects, go to www.Utibron.com or call 1-888-6696682
What are the ingredients in UTIBRON NEOHALER?
Active ingredients: indacaterol maleate, glycopyrrolate
Inactive ingredients: lactose monohydrate (contains milk proteins) and magnesium stearate.
This Patient Information has been approved by the U.S. Food and Drug Administration. Revised: 07/2021
Instructions for Use
UTIBRON®
(yoo-TEE-bron)
NEOHALER®
(indacaterol and glycopyrrolate) inhalation powder, for oral inhalation use
For Oral Inhalation Only
Do not swallow UTIBRON capsules.
 |
Follow the instructions below for using UTIBRON NEOHALER. You will breathe-in (inhale) the medicine in the UTIBRON capsules from the NEOHALER inhaler. If you have any questions, ask your healthcare provider or pharmacist.
Your UTIBRON NEOHALER
UTIBRON NEOHALER consists of both the inhaler and the blister-packaged capsules. Each package contains UTIBRON capsules and a NEOHALER inhaler.
- (1) NEOHALER inhaler which consists of a cap and a base (see figure below)
- UTIBRON capsules come in blister cards to be used only in the NEOHALER inhaler (see figure below)
Your inhaler is made to give you the medicine contained in the capsules.
 |
Only use the NEOHALER inhaler contained in this pack. Do not use UTIBRON NEOHALER capsules with any other inhaler, do not use NEOHALER inhaler to take any other capsule medicine.
How to use your inhaler:
Step 1. Pull off cap.
 |
Step 2. Open inhaler:
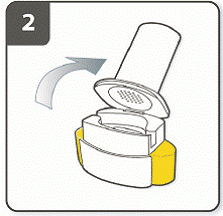
Hold the base of the inhaler firmly and tilt the mouthpiece to open the inhaler.
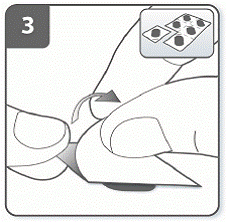 |
Step 3. Prepare capsule:
Separate 1 of the blisters from the blister card by tearing along the perforation.
Take 1 blister and peel away the protective backing to expose the capsule.
Do not push the capsule through the foil to remove it from the blister.
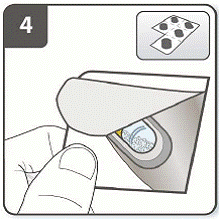 |
Step 4. Remove a UTIBRON capsule:
Capsules should always be stored in the blister and only removed immediately before use.
With dry hands, remove 1 capsule from the blister.
Do not swallow the UTIBRON capsule.
 |
Step 5. Insert capsule:
Place the capsule into the capsule chamber.
Do not place a capsule directly into the mouthpiece.
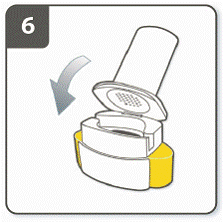 |
Step 6. Close the inhaler:
Close the inhaler fully. You should hear a ‘click’ as it fully closes.
 |
Step 7. Pierce the capsule:
Hold the inhaler upright with the mouthpiece pointing up.
Press both piercing buttons together firmly at the same time. You should hear a ‘click’ as the capsule is being pierced.
Do not press the piercing buttons more than 1 time.
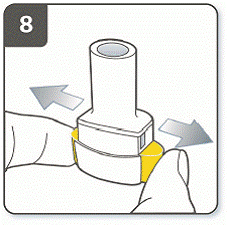 |
Step 8. Release the piercing buttons fully.
 |
Step 9. Breathe out:
Before placing the mouthpiece in your mouth, breathe out fully.
Do not blow into the mouthpiece.
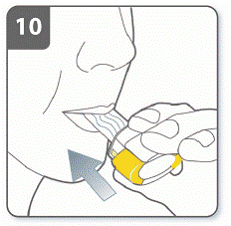 |
Step 10. Inhale the medicine:
Before breathing in:
- Hold the inhaler as shown in the figure for Step 10. Make sure that the piercing buttons are to the left and right of the inhaler (not up and down).
- Place the mouthpiece in your mouth and close your lips firmly around the mouthpiece.
- Breathe in rapidly but steadily, as deeply as you can. Do not press the piercing buttons.
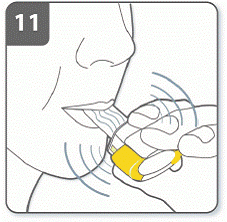 |
Step 11. Note:
As you breathe in through the inhaler, the capsule spins around in the chamber and you should hear a whirring noise. You may experience a sweet flavor as you inhale the medicine.
If you do not hear a whirring noise, the capsule may be stuck in the capsule chamber. If this occurs, open the inhaler and carefully loosen the capsule by tapping the base of the inhaler. Do not press the piercing buttons to loosenthe capsule. (Repeat steps 9 and 10 if necessary.)
 |
Step 12. Hold breath:
Continue to hold your breath for at least 5 to 10 seconds or as long as comfortably possible while removing the inhaler from your mouth. Then breathe out.
Open the inhaler to see if any powder is left in the capsule. If there is powder left in the capsule, close the inhaler and repeat steps 9 to 12. Most people are able to empty the capsule with 1 or 2 inhalations.
Some people may cough soon after inhaling the medicine. If you do, don’t worry, as long as the capsule is empty, you have received 1 full dose.
 |
Step 13. Remove capsule:
After you have finished taking your dose of UTIBRON NEOHALER, open the mouthpiece again, remove the empty capsule by tipping it out of the capsule chamber, and throwing it away. Close the inhaler and replace the cap.
Do not leave the used capsules in the NEOHALER inhaler.
 |
Additional Information:
Occasionally, very small pieces of the capsule can get past the screen and enter your mouth. If this happens, you may be able to feel these pieces on your tongue. It is not harmful if these pieces are swallowed or inhaled. The chances of the capsule shattering will be increased if the capsule is pierced more than once (see Step 7). Therefore, it is recommended that you follow the storage directions, remove the capsule from the blister immediately before use and pierce each capsule only once.
How to clean your inhaler:
Cleaning the inhaler device is not necessary; however, if you want to clean your inhaler, wipe the mouthpiece inside and outside with a clean, dry, lint-free cloth, or a clean, dry, soft brush may be used to wipe the inhaler between uses to remove any powder residue. Keep the inhaler dry.
REMEMBER:
- Do not swallow UTIBRON capsules.
- Only use the NEOHALER inhaler contained in this pack.
- Do not place a UTIBRON capsule directly into the mouthpiece of the NEOHALER inhaler.
- Do not blow into the mouthpiece of the NEOHALER inhaler.
- Always release the piercing buttons before inhalation.
- Do not press the piercing buttons more than once.
- Do not take the NEOHALER inhaler apart.
- Always use the new NEOHALER inhaler that comes with your new UTIBRON NEOHALER medication pack.
- Do not use UTIBRON capsules with inhalers from other medicines.
How should I store UTIBRON NEOHALER?
- Store UTIBRON NEOHALER (inhaler and blister-packaged capsules) at room temperature between 68°F to 77°F (20°C to 25°C).
- Do not remove UTIBRON capsules from the blister card until you are ready to use a dose of UTIBRON NEOHALER.
- Do not store UTIBRON capsules in the NEOHALER inhaler.
- Keep UTIBRON NEOHALER in a dry place away from light and moisture.
Keep UTIBRON NEOHALER and all medicines out of the reach of children.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
