Xyntha Drug Description: Detailed Datasheet
- Generic Name: antihemophilic factor
- Brand Name: Xyntha
side effects drug center xyntha (antihemophilic factor) drug
Drug Description
What is XYNTHA and how is it used?
XYNTHA is an injectable medicine that is used to help control and reduce bleeding in people with hemophilia A. Hemophilia A is also called classic hemophilia. Your healthcare provider may give you XYNTHA when you have surgery.
XYNTHA is not used to treat von Willebrand's disease.
What are the possible side effects of XYNTHA?
Call your healthcare provider or go to the emergency department right away if you have any of the following symptoms because these may be signs of a serious allergic reaction:
- wheezing
- difficulty breathing
- chest tightness
- turning blue (look at lips and gums)
- fast heartbeat
- swelling of the face
- faintness
- rash
- hives
Common side effects of XYNTHA are
- headache
- joint pain
- fever
- cough
- vomiting
- diarrhea
- weakness
Your body can make antibodies against XYNTHA (called “inhibitors”) that may stop XYNTHA from working properly. Your healthcare provider may need to take blood tests from time to time to monitor for inhibitors.
Talk to your healthcare provider about any side effect that bothers you or that does not go away. You may report side effects to FDA at 1-800-FDA-1088.
DESCRIPTION
The active ingredient in XYNTHA, Antihemophilic Factor (Recombinant), is a recombinant antihemophilic factor (rAHF), also called coagulation factor VIII, which is produced by recombinant DNA technology. It is secreted by a genetically engineered Chinese hamster ovary (CHO) cell line. The cell line is grown in a chemically defined cell culture medium that contains recombinant insulin, but does not contain any materials derived from human or animal credits.
The rAHF in XYNTHA is a purified glycoprotein, with an approximate molecular mass of 170 kDa consisting of 1,438 amino acids, which does not contain the B-domain.13 The amino acid sequence of the rAHF is comparable to the 90 + 80 kDa form of human coagulation factor VIII.
The purification process uses a series of chromatography steps, one of which is based on affinity chromatography using a patented synthetic peptide affinity ligand.14 The process also includes a solvent-detergent viral inactivation step and a virus-retaining nanofiltration step.
The potency expressed in International Units (IU) is determined using the chromogenic assay of the European Pharmacopoeia. The Wyeth manufacturing reference standard for potency has been calibrated against the World Health Organization (WHO) International Standard for factor VIII activity using the one-stage clotting assay. The specific activity of XYNTHA is 5,500 to 9,900 IU per milligram of protein.
XYNTHA is formulated as a sterile, nonpyrogenic, no preservative, lyophilized powder preparation for intravenous injection. Each singleuse vial contains nominally 250, 500, 1000, or 2000 IU of XYNTHA. Upon reconstitution, the product is a clear to slightly opalescent, colorless solution that contains sodium chloride, sucrose, L-histidine, calcium chloride, and polysorbate 80.
REFERENCES
13. Sandberg H, Almstedt A, Brandt J, Castro VM, Gray E, Holmquist L, et al. Structural and Functional Characterization of B-Domain Deleted Recombinant Factor VIII. Sem Hematol. 2001;38 (Suppl. 4):4–12.
14. Kelley BD, Tannatt M, Magnusson R, Hagelberg S. Development and Validation of an Affinity Chromatography Step Using a Peptide Ligand for cGMP Production of Factor VIII. Biotechnol Bioeng. 2004;87(3):400–412.
Indications & Dosage
INDICATIONS
XYNTHA, Antihemophilic Factor (Recombinant), is indicated for use in adults and children with hemophilia A (congenital factor VIII deficiency) for:
- On-demand treatment and control of bleeding episodes
- Perioperative management
- Routine prophylaxis to reduce the frequency of bleeding episodes
XYNTHA does not contain von Willebrand factor, and therefore is not indicated in patients with von Willebrand's disease.
DOSAGE AND ADMINISTRATION
For intravenous use after reconstitution only.
Dose
- Dosage and duration of treatment depend on the severity of the factor VIII deficiency, the location and extent of bleeding, and the patient's clinical condition. Titrate the administered doses to the patient's clinical response.
- One International Unit (IU) of factor VIII activity corresponds approximately to the quantity of factor VIII in one milliliter of normal human plasma. The calculation of the required dosage of factor VIII is based upon the empirical finding that, on average, 1 IU of factor VIII per kg body weight raises the plasma factor VIII activity by approximately 2 IU/dL.2
The expected in vivo peak increase in factor VIII level expressed as IU/dL (or % of normal) can be estimated using the following formulas:
Dosage (International Units) = body weight (kg) x desired factor VIII rise (IU/dL or % of normal) x 0.5 (IU/kg per IU/dL)
or
IU/dL (or % of normal) = Total Dose (IU)/body weight (kg) x 2 [IU/dL]/[IU/kg]
On-Demand Treatment And Control Of Bleeding Episodes
A guide for dosing XYNTHA for on-demand treatment and control of bleeding episodes is provided in Table 1. Maintain the plasma factor VIII activity at or above the levels (in % of normal or in IU/dL) outlined in Table 1 for the indicated period.
Table 1: Dosing for On-demand treatment and Control of Bleeding Episodes
| Type of Bleeding Episode | Factor VIII Level Required (ILT/dL or % of normal) | Frequency of Doses (hours) | Duration of Therapy |
| Minor | |||
| Early hemarthrosis, minor muscle or oral bleeds. | 20-10 | 12-24 | At least 1 day, depending upon the severity of the bleeding episode. |
| Moderate | |||
| Bleeding into muscles. Mild head trauma. Bleeding into the oral cavity. | 30-60 | 12-24 | 3-4 days or until adequate local hemostasis is achieved. |
| Major | |||
| Gastrointestinal bleeding. Intracranial, intra-abdominal or intrathoracic bleeding. Fractures. | 60-100 | 8-24 | Until bleeding is resolved. |
Perioperative Management
A guide for dosing XYNTHA during surgery (perioperative management) is provided in Table 2. Maintain the plasma factor VIII activity level at or above the level (in % of normal or in IU/dL) outlined in Table 2 for the indicated period. Monitor the replacement therapy by means of plasma factor VIII activity.
Table 2: Dosing for Perioperative Management
| Type of Surgery | Factor VIII Level Required (ILT/dL or % of normal) | Frequency of Doses (hours) | Duration of Therapy (days) |
| Minor | |||
| Minor operations, including tooth extraction. | 30-60 | 12-24 | 3-4 days or until adequate local hemostasis is achieved. For tooth extraction, a single infusion plus oral antifibrinolytic therapy within 1 hour may be sufficient. |
| Major | |||
| Major operations. | 60-100 | 8-24 | Until threat is resolved, or in the case of surgery, until adequate local hemostasis and wound healing are achieved. |
Routine Prophylaxis
- Adults and adolescents (≥12 years): The recommended starting regimen is 30 IU/kg of XYNTHA administered 3 times weekly.
- Children (<12 years): The recommended starting regimen is 25 IU/kg of XYNTHA administered every other day. More frequent or higher doses may be required in children <12 years of age to account for the higher clearance in this age group [see CLINICAL PHARMACOLOGY].
- Adjust the dosing regimen (dose or frequency) based on the patient's clinical response.
Preparation And Reconstitution
Preparation
- Always wash hands before performing the following procedures.
- Use aseptic technique during the reconstitution procedures.
- Use all components for the reconstitution and administration of this product as soon as possible after opening their sterile containers to minimize unnecessary exposure to the atmosphere.
Note:
- If the patient uses one vial of XYNTHA with one XYNTHA SOLOFUSE for the infusion, reconstitute the vial and the syringe according to the instructions for that respective product kit. Use a separate 10 milliliter or larger luer lock syringe (not included in this kit) to draw back the reconstituted contents of the vial and the syringe. [see Use of a XYNTHA Vial Kit with a XYNTHA SOLOFUSE Kit]
- If the patient uses multiple XYNTHA SOLOFUSE syringes for the infusion, reconstitute each syringe according to the instructions below. Use a separate 10 milliliter or larger luer lock syringe (not included in this kit) to draw back the reconstituted contents of each syringe. [see Use of Multiple XYNTHA SOLOFUSE Kits]
Reconstitution
1. Allow the XYNTHA SOLOFUSE Kit to reach room temperature.
2. Remove the contents of the XYNTHA SOLOFUSE Kit and place on a clean surface, making sure you have all the supplies you will need.
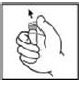
3. Grasp the plunger rod as shown in the following diagram. Avoid contact with the shaft of the plunger rod. Screw the plunger rod firmly into the opening in the finger rest of the XYNTHA SOLOFUSE by pushing and turning firmly until resistance is felt (approximately 2 turns).
 |
Note: Once the white tamper-evident seal is removed it is important to keep the XYNTHA SOLOFUSE in the upright position throughout the reconstitution process to prevent possible leakage.
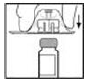
4. Holding the XYNTHA SOLOFUSE upright, remove the white tamper-evident seal by bending the seal right to left (or a gentle rocking motion) to break the perforation of the cap and expose the grey rubber tip cap of the XYNTHA SOLOFUSE.
 |
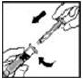
5. Remove the protective blue vented sterile cap from its package. While holding the XYNTHA SOLOFUSE upright, remove the grey rubber tip cap and replace it with the protective blue vented cap (prevents pressure build-up). Avoid touching the open end of both the syringe and the protective blue vented cap.
 |
6. Gently and slowly advance the plunger rod by pushing until the two stoppers inside the XYNTHA SOLOFUSE meet, and all of the diluent is transferred to the chamber containing the XYNTHA powder. Note: To prevent the escape of fluid from the tip of the syringe, the plunger rod should not be pushed with excessive force.
 |
7. With the XYNTHA SOLOFUSE remaining upright, swirl gently several times until the powder is dissolved.
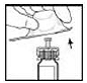 |
Note: The final solution should be inspected visually for particulate matter before administration. The solution should be clear to slightly opalescent and colorless. If it is not, discard the solution and use a new kit.
8. Holding the XYNTHA SOLOFUSE in an upright position, slowly advance the plunger rod until most, but not all, of the air is removed from the drug product chamber.
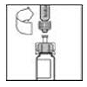 |
Note:
- If the solution is not to be used immediately, store the syringe upright, leaving the protective blue vent cap on the XYNTHA SOLOFUSE until ready to infuse.
- Store the reconstituted solution at room temperature prior to administration, but use within 3 hours after reconstitution or after removal of the grey rubber tip cap.
- XYNTHA, when reconstituted, contains polysorbate 80, which is known to increase the rate of di-(2-ethylhexyl) phthalate (DEHP) extraction from polyvinyl chloride (PVC). This should be considered during the preparation and administration of XYNTHA, including storage time elapsed in a PVC container following reconstitution. The tubing of the infusion set included with this kit does not contain DEHP.
Administration
For intravenous infusion after reconstitution only.
Inspect the final XYNTHA solution visually for particulate matter and discoloration prior to administration. The solution should be clear to slightly opalescent and colorless. If it is not, discard the solution and use a new kit.
Administer XYNTHA solution using the infusion set included in the kit. Do not administer reconstituted XYNTHA in the same tubing or container with other medicinal products.
1. After removing the protective blue vented cap, firmly attach the intravenous infusion set provided in the kit onto the XYNTHA SOLOFUSE.
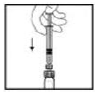 |
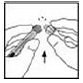
2. Apply a tourniquet and prepare the injection site by wiping the skin well with an alcohol swab provided in the kit.
3. Remove the protective needle cover and perform venipuncture. Insert the needle on the infusion set tubing into the vein, and remove the tourniquet. Verify proper needle placement.
4. Inject the reconstituted XYNTHA intravenously over several minutes. The rate of administration should be determined by the patient's comfort level.
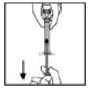 |
5. After infusing XYNTHA, remove and discard the infusion set. The amount of drug product left in the infusion set will not affect treatment. Note: Dispose of all unused solution, the empty XYNTHA SOLOFUSE, and other used medical supplies in an appropriate container.
Use Of A XYNTHA Vial Kit With A XYNTHA SOLOFUSE Kit
These instructions are for the use of only one XYNTHA vial kit with one XYNTHA SOLOFUSE Kit.
1. Reconstitute the XYNTHA vial using the instructions included with the product kit.
2. Detach the empty diluent syringe from the vial adapter by gently turning and pulling the syringe counterclockwise, leaving the contents in the vial and the vial adapter in place.
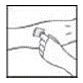 |
3. Reconstitute the XYNTHA SOLOFUSE using the instructions described in Preparation and Reconstitution [see Preparation and Reconstitution]. Remember to remove most, but not all, of the air from the drug product chamber.
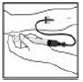 |
4. After removing the protective blue vented cap, connect the XYNTHA SOLOFUSE to the vial adapter by inserting the tip into the adapter opening while firmly pushing and turning the syringe clockwise until secured.
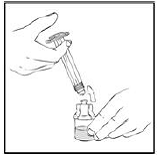 |
5. Slowly depress the plunger rod of the XYNTHA SOLOFUSE until the contents empty into the XYNTHA vial. The plunger rod may move back slightly after release.
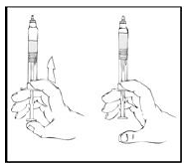 |
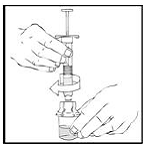
Detach and discard the empty XYNTHA SOLOFUSE from the vial adapter.
Note: If the syringe turns without detaching from the vial adapter, grasp the white collar and turn.
 |
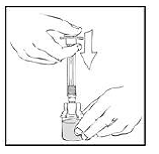
7. Connect a sterile 10 milliliter or larger luer lock syringe to the vial adapter. Inject some air into the vial to make withdrawing the vial contents easier.
 |
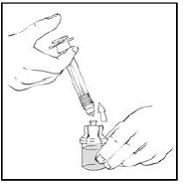
8. Invert the vial and slowly draw the solution into the large luer lock syringe.
 |
9. Detach the syringe from the vial adapter by gently turning and pulling the syringe counterclockwise. Discard the empty XYNTHA vial with the adapter attached.
10. Attach the infusion set to the large luer lock syringe as directed [see Administration].
Use Of Multiple XYNTHA SOLOFUSE Kits
The instructions below are for the use of multiple XYNTHA SOLOFUSE kits with a 10 milliliter or larger luer lock syringe. For further information, please contact the Medical Information Department at Wyeth Pharmaceuticals, 1-800-438-1985.
Note: Luer-to-luer syringe connectors are not provided in these kits. Instruct patients to contact their XYNTHA supplier to order.
1. Reconstitute all XYNTHA SOLOFUSE according to instructions described in Preparation and Reconstitution [see Preparation and Reconstitution].
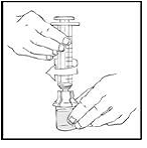
2. Holding the XYNTHA SOLOFUSE in an upright position, slowly advance the plunger rod until most, but not all, of the air is removed from the drug product chamber.
 |
3. Remove the luer-to-luer syringe connector from its package.
4. After removing the protective blue vented cap, connect a sterile 10 milliliter or larger luer lock syringe to one opening (port) in the syringe connector and the XYNTHA SOLOFUSE to the remaining open port on the opposite end.
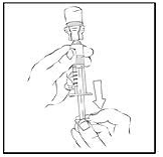 |
5. With the XYNTHA SOLOFUSE on top, slowly depress the plunger rod until the contents empty into the large luer lock syringe.
 |
6. Remove the empty XYNTHA SOLOFUSE and repeat procedures 3 and 4 above for any additional reconstituted XYNTHA SOLOFUSE.
7. Remove the luer-to-luer syringe connector from the large luer lock syringe and attach the infusion set as directed [see Administration].
HOW SUPPLIED
Dosage Forms And Strengths
XYNTHA SOLOFUSE is available as a white to off-white lyophilized powder in the following nominal dosages:
- 250 International Units
- 500 International Units
- 1000 International Units
- 2000 International Units
- 3000 International Units
Each XYNTHA SOLOFUSE has the actual recombinant factor VIII (rFVIII) potency in International Units stated on the label.
Each XYNTHA SOLOFUSE Kit contains: one plunger rod for assembly, one sterile infusion set, two alcohol swabs, one bandage, one gauze pad, one vented sterile cap, and one package insert. The drug product, diluents for injection and the rest of components included within the XYNTHA 250, 500, 1000, 2000, or 3000 International Units kit are free from natural rubber and natural rubber latex.
XYNTHA SOLOFUSE is supplied in a kit that includes the XYNTHA lyophilized powder containing nominally 250, 500, 1000, 2000 or 3000 IU and 4 mL 0.9 % Sodium Chloride solution for reconstitution in a prefilled dual-chamber syringe:
XYNTHA SOLOFUSE Kit
| Nominal Strength | Fill Size Color Indicator | Kit NDC # |
| 250 International Units | Yellow | 58394-022-03 |
| 500 International Units | Blue | 58394-023-03 |
| 1000 International Units | Green | 58394-024-03 |
| 2000 International Units | Red | 58394-025-03 |
| 3000 International Units | Gray | 58394-016-03 |
Actual factor VIII activity in International Units is stated on the label of each XYNTHA SOLOFUSE.
Storage And Handling
Product As Packaged For Sale
- Store XYNTHA SOLOFUSE under refrigeration at a temperature of 2° to 8°C (36° to 46°F) for up to 36 months from the date of manufacture until the expiration date stated on the label.
- Within the expiration date, XYNTHA SOLOFUSE also may be stored at room temperature not to exceed 25°C (77°F) for up to 3 months.
- Clearly record the starting date at room temperature storage in the space provided on the outer carton. At the end of the 3-month period, immediately use or discard the product. Do not put the product back into the refrigerator.
- Do not use XYNTHA SOLOFUSE after the expiration date stated on the label or after 3 months when stored at room temperature, whichever is earlier.
- Do not freeze. (Freezing may damage the XYNTHA SOLOFUSE.)
- During storage, avoid prolonged exposure of XYNTHA SOLOFUSE to light.
- Store the reconstituted solution at room temperature prior to administration. Administer XYNTHA SOLOFUSE within 3 hours after reconstitution or after removal of the grey rubber tip cap from the product.
REFERENCES
1. Nilsson IM, Berntorp EE and Freiburghaus C. Treatment of patients with factor VIII and IX inhibitors. Thromb Haemost. 1993;70(1):56–59.
2. Hoyer LW. Hemophilia A. N Engl J Med. 1994;330:38–47.
3. Juhlin F. Stability and Compatibility of Reconstituted Recombinant Factor VIII SQ, 250 IU/ml, in a System for Continuous Infusion. Pharmacia Document 9610224, 1996.
15. Mann KG and Ziedens KB. Overview of Hemostasis. In: Lee CA, Berntorp EE and Hoots WK, eds. Textbook of Hemophilia. USA, Blackwell Publishing; 2005:1–4.
Manufactured by: Wyeth Pharmaceuticals LLC, A subsidiary of Pfizer Inc., Philadelphia, PA 19101. Revised: Aug 2020
Side Effects & Drug Interactions
SIDE EFFECTS
The most common adverse reactions (≥10%) with XYNTHA in adult and pediatric previously treated patients (PTPs) were headache, arthralgia, pyrexia, and cough.
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
XYNTHA was evaluated in five completed clinical studies (N=178), comprising four studies with adult and pediatric PTPs.
The safety and efficacy of XYNTHA was evaluated in two completed pivotal studies. In the first study (n=94), safety and efficacy were examined in PTPs with severe to moderately severe hemophilia A (factor VIII activity in plasma [FVIII:C] ≤2%) who received XYNTHA for routine prophylaxis and ondemand treatment. Ninety-four subjects received at least one dose of XYNTHA, resulting in a total of 6,775 infusions [see Clinical Studies]. The second study (n=30) examined the use of XYNTHA for surgical prophylaxis in PTPs with severe to moderately severe hemophilia A (FVIII:C ≤2%) who required elective major surgery and were expected to receive XYNTHA replacement therapy for at least 6 days post-surgery. All subjects received at least one dose of XYNTHA, resulting in 1,161 infusions. One subject received XYNTHA for a pre-surgery pharmacokinetic assessment only and did not undergo surgery [see Clinical Studies].
Across all studies, safety was evaluated in 72 pediatric PTPs <17 years of age (46 subjects, <6 years of age (4 subjects were 0 to <2 years of age), 4 subjects 6 to <12 years of age, and 22 adolescents, 12 to <17 years of age). A total of 13,109 infusions of XYNTHA were administered with a median dose per infusion of 28 IU/kg (min-max: 6-108 IU/kg).
Across all studies, the most common adverse reactions (≥10%) with XYNTHA in adult and pediatric PTPs were headache (24%), arthralgia (23%), pyrexia (23%), and cough (12%). Other adverse reactions reported in ≥5% of subjects were: diarrhea (8%), vomiting (8%), and asthenia (6%).
Immunogenicity
There is a potential for immunogenicity with therapeutic proteins. The development of factor VIII inhibitors with XYNTHA was evaluated in 167 adult and pediatric PTPs with at least 50 exposure days (EDs). Laboratory-based assessments for FVIII inhibitor (partial Nijmegen modification of the Bethesda inhibitor assay) were conducted in the clinical studies. The criterion for a positive FVIII result test result was ≥0.6 BU/mL. Across all studies, 4 subjects developed factor VIII inhibitors (2.4%).
The completed clinical studies for XYNTHA examined 178 subjects (30 for surgical prophylaxis) who had previously been treated with factor VIII (PTPs). In the first safety and efficacy study, factor VIII inhibitors were detected in two of 89 subjects (2.2%) who completed ≥50 EDs. In a Bayesian statistical analysis, results from this study were used to update PTP results from a prior supporting study using XYNTHA manufactured at the initial facility (with one de novo and two recurrent inhibitors observed in 110 subjects) and the experience with predecessor product (with one inhibitor observed in 113 subjects). The Bayesian analysis indicated that the population inhibitor rate for XYNTHA, an estimate of the 95% upper limit of the true inhibitor rate, was 4.17%.
None of the PTPs developed anti-CHO (Chinese hamster ovary) or anti-TN8.2 antibodies. One PTP developed anti-FVIII antibodies; but, this subject did not develop an inhibitor.
In the surgery study, one low titer persistent inhibitor and one transient false-positive inhibitor were reported. In this study, one surgical subject developed anti-CHO cell antibodies with no associated allergic reaction. One subject developed anti-FVIII antibodies; but, this subject did not develop an inhibitor.
Across all studies, immunogenicity was evaluated in 64 pediatric PTPs <17 years of age with at least 50 EDs (43 children <6 years of age, 4 subjects 6 to <12 years of age, and 17 adolescents, 12 to <17 years of age). Of these, 2 pediatric subjects developed an inhibitor.
The detection of antibody formation is highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of antibody, including neutralizing antibody, positivity in an assay may be influenced by several factors, including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparisons of the incidence of antibodies to XYNTHA with the incidence of antibodies to other products may be misleading.
Postmarketing Experience
Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
The following postmarketing adverse reaction has been reported for XYNTHA:
Inadequate therapeutic response
DRUG INTERACTIONS
No Information provided
Warnings & Precautions
WARNINGS
Included as part of the PRECAUTIONS section.
PRECAUTIONS
Hypersensitivity Reactions
Allergic type hypersensitivity reactions, including anaphylaxis, are possible with XYNTHA. Inform patients of the early signs or symptoms of hypersensitivity reactions (including hives [rash with itching], generalized urticaria, chest tightness, wheezing, and hypotension) and anaphylaxis. Discontinue XYNTHA if hypersensitivity symptoms occur and administer appropriate emergency treatment.
XYNTHA contains trace amounts of hamster proteins. Patients treated with this product may develop hypersensitivity to these non-human mammalian proteins.
Neutralizing Antibodies
Inhibitors have been reported following administration of XYNTHA. Monitor patients for the development of factor VIII inhibitors by appropriate clinical observations and laboratory tests. If expected factor VIII activity plasma levels are not attained, or if bleeding is not controlled with an appropriate dose, perform an assay that measures factor VIII inhibitor concentration to determine if a factor VIII inhibitor is present [see WARNINGS AND PRECAUTIONS]. 4,5,6,7,8,9,10,11,12
Monitoring Laboratory Tests
- Use individual factor VIII values for recovery and, if clinically indicated, other pharmacokinetic characteristics to guide dosing and administration.
- Monitor plasma factor VIII activity levels by the one-stage clotting assay to confirm that adequate factor VIII levels have been achieved and are maintained, when clinically indicated [see DOSAGE AND ADMINISTRATION].
- Monitor for development of factor VIII inhibitors. Perform assay to determine if factor VIII inhibitor is present when expected factor VIII activity plasma levels are not attained, or when bleeding is not controlled with the expected dose of XYNTHA. Use Bethesda Units (BU) to titer inhibitors.
Patient Counseling Information
Advise patients to:
- read the FDA-approved patient labeling (Patient Information and Instructions for Use).
- report any adverse reactions or problems that concern them when taking XYNTHA to their healthcare provider.
- discontinue use of the product, call their healthcare provider, and go to the emergency department if any allergic-type hypersensitivity reactions occur. Inform patients of the early signs of hypersensitivity reactions (including hives [rash with itching], generalized urticaria, tightness of the chest, wheezing, hypotension) and anaphylaxis.
- contact their healthcare provider if they experience a lack of a clinical response to factor VIII replacement therapy, as this may be a manifestation of an inhibitor.
- notify their healthcare provider if they become pregnant or intend to become pregnant during therapy, or if they are breastfeeding.
- Local irritation may occur when infusing XYNTHA SOLOFUSE.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment Of Fertility
No studies have been conducted with XYNTHA to assess its mutagenic or carcinogenic potential. XYNTHA has been shown to be comparable to the predecessor product with respect to its biochemical and physicochemical properties, as well as its nonclinical in vivo pharmacology and toxicology. By inference, predecessor product and XYNTHA would be expected to have equivalent mutagenic and carcinogenic potential. The predecessor product has been shown to be nongenotoxic in the mouse micronucleus assay. No studies have been conducted in animals to assess impairment of fertility or fetal development.
Use In Specific Populations
Pregnancy
Risk Summary
It is not known whether XYNTHA can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Animal reproduction studies have not been conducted with XYNTHA.
There is no information available on the effect of factor VIII replacement therapy on labor and delivery. XYNTHA should be used only if clinically indicated.
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2–4% and 15– 20%, respectively.
Lactation
Risk Summary
There is no information regarding the presence of XYNTHA in human milk, the effect on the breastfed infant, or the effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for XYNTHA and any potential adverse effects on the breastfed child from XYNTHA or from the underlying maternal condition.
Pediatric Use
Safety and efficacy with XYNTHA were evaluated in clinical studies in 68 pediatric subjects <17 years of age (18 subjects aged 12 to <17 years, 50 subjects aged ≤12 years). There were no apparent differences in the efficacy and safety in pediatric subjects as compared to adults [see ADVERSE REACTIONS and Clinical Studies].
In comparison to the pharmacokinetic parameters reported in adults, children have shorter half-lives, larger volumes of distribution and lower recovery of factor VIII after XYNTHA administration. The clearance (based on per kg body weight) is approximately 40% higher in children. Higher or more frequent doses may be required to account for the observed differences in pharmacokinetic parameters [see CLINICAL PHARMACOLOGY].
Geriatric Use
Clinical studies of XYNTHA did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function and of concomitant disease or other drug therapy.
REFERENCES
4. Ehrenforth S, Kreuz W, Scharrer I, et al. Incidence of development of factor VIII and factor IX inhibitors in hemophiliacs. Lancet. 1992;339:594–598.
5. Lusher J, Arkin S, Abildgaard CF, Schwartz RS, the Kogenate PUP Study Group. Recombinant factor VIII for the treatment of previously untreated patients with hemophilia A. N Engl J Med. 1993;328:453–459.
6. Bray GL, Gomperts ED, Courter S, et al. A multicenter study of recombinant factor VIII (Recombinate): safety, efficacy, and inhibitor risk in previously untreated patients with hemophilia A. Blood. 1994;83(9):2428–2435.
7. Kessler C, Sachse K. Factor VIII:C inhibitor associated with monoclonal-antibody purified FVIII concentrate. Lancet. 1990;335:1403.
8. Schwartz RS, Abildgaard CF, Aledort LM, et al. Human recombinant DNA-derived antihemophilic factor (factor VIII) in the treatment of hemophilia A. N Engl J Med. 1990;323:1800–1805.
9. White GC II, Courter S, Bray GL, et al. A multicenter study of recombinant factor VIII (Recombinate™) in previously treated patients with hemophilia A. Thromb Haemost. 1997;77(4):660–667.
10. Gruppo R, Chen H, Schroth P, et al. Safety and immunogenicity of recombinant factor VIII (Recombinate™) in previously untreated patients: A 7.3 year update. Haemophilia. 1998;4:228 (Abstract No. 291, XXIII Congress of the WFH, The Hague).
11. Scharrer I, Bray GL, Neutzling O. Incidence of inhibitors in haemophilia A patients - a review of recent studies of recombinant and plasma-derived factor VIII concentrates. Haemophilia. 1999;5:145–154.
12. Abshire TC, Brackmann HH, Scharrer I, et al. Sucrose formulated recombinant human antihemophilic Factor VIII is safe and efficacious for treatment of hemophilia A in home therapy: Results of a multicenter, international, clinical investigation. Thromb Haemost. 2000;83(6):811-816.
Overdosage & ContraindicationsOVERDOSE
No Information provided
CONTRAINDICATIONS
XYNTHA is contraindicated in patients who have manifested life-threatening immediate hypersensitivity reactions, including anaphylaxis, to the product or its components, including hamster proteins.
Clinical Pharmacology
Mechanism Of Action
XYNTHA temporarily replaces the missing clotting factor VIII that is needed for effective hemostasis.
Pharmacodynamics
The activated partial thromboplastin time (aPTT) is prolonged in patients with hemophilia. Determination of aPTT is a conventional in vitro assay for biological activity of factor VIII. Treatment with XYNTHA normalizes the aPTT over the effective dosing period.
Pharmacokinetics
The pharmacokinetic parameters of XYNTHA in 30 PTPs 12 to 60 years old, who received a single infusion of 50 IU/kg XYNTHA are summarized in Table 3.
In addition, 25 of the same subjects later received a single infusion of 50 IU/kg of XYNTHA for a 6-month follow-up pharmacokinetic study. The parameters were comparable between baseline and 6 months, indicating no time-dependent changes in the pharmacokinetics of XYNTHA.
In a separate study, 8 of 30 subjects at least 12 years old with hemophilia A undergoing elective major surgery received a single 50 IU/kg infusion of XYNTHA. The pharmacokinetic parameters in these subjects are also summarized in Table 3.
Table 3: Mean ± SD XYNTHA Pharmacokinetic Parameters in Previously Treated Patients with Hemophilia A after Single 50 IU/kg Dose
| Parameter | Initial Visit (n = 30) | Month 6 (n = 25) | Pre-surgery (n=8) |
| Cmax (IU/lllL) | 1.08 ±0.22 | 1.24 ±0.42 | 1.08 ±0.24 |
| AUC∞, (IUhr/mL) | 13.5 ±5.6 | 15.0 ±7.5 | 16.0 ±5.2 |
| t½ (hr) | 11.2 ±5.0 | 11.8 ±6.2* | 16.7 ±5.4 |
| CL (mL/hr/kg) | 4.51 ±2.23 | 4.04 ± 1.87 | 3.48 ± 1.25 |
| Vss (mL/kg) | 66.1 ±33.0 | 67.4 ± 32.6 | 69.0 ±20.1 |
| Recovery (IU/dL per IU/kg) | 2.15 ±0.44 | 2.47 ±0.84 | 2.17 ±0.47 |
| Abbreviations: AUC = area under the plasma concentration-time curve from zero to infinity; C = peak concentration; t = plasma elimination half-life; CL = clearance; n = number of subjects; SD = standard deviation; Vss = volume of distribution at steady-state. *One subject was excluded from the calculation due to lack of a well-defined terminal phase. | |||
Table 4 shows the pharmacokinetic parameters of nine children; four aged 14 or 15 years of age, who are also included in the summary for the adults above, along with five children aged 3.7–5.8 years after single 50 IU/kg doses of XYNTHA. Compared to adults, the half-life of XYNTHA is shorter in children and the clearance (based on per kg body weight) is approximately 40% higher in children.
Table 4: Mean ± SD XYNTHA Pharmacokinetic Parameters in Previously Treated Pediatric Patients with Hemophilia A after Single 50 IU/kg Dose
| Parameter | Young Children (n=5) | Adolescents (n=4) |
| Age (min - max, yr)) | 3.7-5.8 | 14-15 |
| Cmax (IU/lllL) | 0.78 ±0.34 | 0.97 ±0.21 |
| AUC∞, (IU•hr/mL) | 12.2 ±6.50 | 8.5 ±4.0 |
| t½ (hr) | 8.3 ±2.7 | 6.9 ±2.4 |
| CL (mL/hr/kg) | 6.29 ±4.87 | 6.62 ±2.16 |
| Vss (mL/kg) | 66.9 ±55.6 | 67.1 ± 13.6 |
| Recovery (IU/dL per IU/kg) | 1.52 ±0.69 | 1.95 ±0.41 |
| Abbreviations: AUC∞ = area under the plasma concentration-time curve from zero to infinity; Cmax = peak concentration; t½ = plasma elimination half-life; CL = clearance; n = number of subjects; SD = standard deviation; Vss = volume of distribution at steady-state. | ||
Animal Toxicology And/Or Pharmacology
Preclinical studies evaluating XYNTHA in hemophilia A dogs without inhibitors demonstrated safe and effective restoration of hemostasis. XYNTHA demonstrated a toxicological profile that was similar to the toxicological profile observed with the predecessor product. Toxicity associated with XYNTHA was primarily associated with anti-FVIII neutralizing antibody generation first detectable at 15 days of repeat dosing in high (approximately 735 IU/kg/day) level-dosed, non-human primates.
Clinical Studies
Three completed multicenter, open-label studies support the analysis of safety and efficacy of XYNTHA in on-demand treatment and control of bleeding episodes and perioperative management, and routine prophylaxis in PTPs with hemophilia A. These completed clinical studies for XYNTHA examined 174 PTP subjects, 94 from the first study, and 50 from a second study, for on-demand treatment and routine prophylaxis and 30 from a third study for surgical prophylaxis. Subjects with severe to moderately severe hemophilia A (FVIII:C ≤2%) and no history of FVIII inhibitors were eligible for the trials.
On-demand Treatment And Control Of Bleeding Episodes
On-Demand Treatment In Adolescents And Adults
Ninety-four (94) subjects, 12 years of age and older received XYNTHA in a routine prophylaxis treatment regimen with on-demand treatment administered as clinically indicated. All 94 subjects were treated with at least one dose and all are included in the intent-to-treat (ITT) population. Eightynine (89) subjects accrued ≥50 EDs. Median age for the 94 treated subjects was 24 years (mean 28 and min-max: 12–60 years).
Of these 94 subjects, 30 evaluable subjects participated in a randomized crossover pharmacokinetics substudy. Twenty-five (25/30) of these subjects with FVIII:C ≤1% completed both the first (PK1) and the second (PK2) pharmacokinetic assessments [see CLINICAL PHARMACOLOGY].16
Fifty-three subjects (53/94) received XYNTHA on-demand treatment for a total of 187 bleeding episodes. Seven of these bleeding episodes occurred in subjects prior to switching to a prophylaxis treatment regimen. One hundred ten of 180 bleeds (110/180 or 61%) occurred ≤48 hours after the last dose and 39% (70/180 bleeds) occurred >48 hours after the last dose. The majority of bleeds reported to occur ≤48 hours after the last prophylaxis dose were traumatic (64/110 bleeds or 58%). Forty-two bleeds (42/70 or 60%) reported to occur >48 hours after the last prophylaxis dose were spontaneous. The ondemand treatment dosing regimen was determined by the investigator. The median dose for on-demand treatment was 31 IU/kg (min-max: 6–74 IU/kg) and the median exposure per subject was 3 days (min-max: 1–26).
The majority of bleeding episodes (173/187 or 93%) resolved with 1 or 2 infusions (Table 5). One hundred thirty-two of 187 bleeding episodes (132/187 or 71%) treated with XYNTHA were rated excellent or good in their response to initial treatment, 45 (24%) were rated moderate. Five (3%) were rated no response, and 5 (3%) were not rated.
Table 5: Summary of Response to Infusions to Treat New Bleeding Episode by Number of Infusions Needed for Resolution
| Number of Infusions (%) | ||||||
| Response to 1st Infusion | 1 | 2 | 3 | 4 | >4 | Total Number of Bleeds |
| Excellent* | 42 (95.5) | 2(4.5) | 0 (0.0) | 0 (0.0) | 0(0.0) | 44 |
| Good† | 69 (78.4) | 16(18.2) | 3 (3.4) | 0 (0.0) | 0(0.0) | 88 |
| Moderate‡ | 24 (53.3) | 16 (35.6) | 2 (4.4) | 0 (0.0) | 3 (6.7) | 45 |
| No Response§ | 0 (0.0) | 0 (0.0) | 2 (40.0) | 2 (40.0) | 1 (20.0) | 5 |
| Not Assessed | 4 (80.0) | 0 (0.0) | 0 (0.0) | 1 (20.0) | 0(0.0) | 5¶ |
| Total | 139 (74.3) | 34(18.2) | 7 (3.7) | 3(1.6) | 4(2.1) | 187 |
| *Excellent: Definite pain relief and/or improvement in signs of bleeding starting within 8 hours after an infusion, with no additional infusion administered. †Good: Definite pain relief and/or improvement in signs of bleeding starting within 8 hours after an infusion, with at least one additional infusion administered for complete resolution of the bleeding episode. ‡ Moderate Probable or slight improvement starting after 8 hours following the infusion, with at least one additional infusion administered for complete resolution of the bleeding episode. § No Response: No improvement at all between infusions or during the 24 hour interval following an infusion, or condition worsens. ¶Includes one infusion with commercial FVIII that occurred before routine prophylaxis began. | ||||||
Of the 94 subjects described above, in the first completed open-label safety and efficacy study of XYNTHA, 18 were adolescent subjects 12 to <17 years of age with severe to moderately severe hemophilia A (FVIII:C ≤2%). Ten (10) of these adolescent subjects, received XYNTHA for the on-demand treatment of 66 bleeding episodes, with the majority of the bleeding episodes (63/66 or 95%) resolving with 1 or 2 infusions. The response to infusion was rated on a pre-specified 4 point hemostatic efficacy scale. Thirty-eight (38) of 66 bleeding episodes (58%) were rated excellent or good in their response to initial treatment, 24 (36%) were rated as moderate, and 4 (6%) were not rated. The median dose per on demand infusion was 47 IU/kg (min–max: 24–74).
On-Demand Treatment In Children
Additional data for 50 subjects are available from a second safety and efficacy study of XYNTHA in children (≤12 years of age) with severe to moderately severe hemophilia A (FVIII:C ≤2%). Of the 50 subjects, 38 subjects received XYNTHA for on-demand and follow-up treatment of 562 bleeding episodes with the majority of the bleeding episodes (518/562 or 92%) resolving with 1 or 2 infusions. Of 559 bleeding episodes treated with XYNTHA with response assessments to the first infusion, 526 (94%) were rated excellent or good in their response to initial treatment and 27 (5%) were rated as moderate. The median dose per on-demand infusion was 28 IU/kg (min-max: 10–92).
Perioperative Management
In a study (n=30) for surgical prophylaxis in subjects with hemophilia A, XYNTHA was administered to 25 efficacy-evaluable PTPs undergoing major surgical procedures (11 total knee replacements, 1 hip replacement, 5 synovectomies, 1 left ulnar nerve transposition release, 1 ventral hernia repair/scar revision, 1 knee arthroscopy, 1 revision and debridement of the knee after a total knee replacement, 1 hip arthroplasty revision, 1 stapes replacement, 1 ankle arthrodesis, and 1 pseudotumor excision).17
The results of the hemostatic efficacy ratings for these subjects are presented in Table 6. Investigator's ratings of efficacy at the end of surgery and at the end of the initial postoperative period were excellent or good for all assessments. Intraoperative blood loss was reported as “normal” or “absent” for all subjects. Thirteen of the subjects (13/25 or 52%) had blood loss in the postoperative period. The postoperative blood loss was rated as “normal” for ten of these cases while three cases were rated “abnormal” (1 due to hemorrhage following surgical trauma to the epigastric artery, 1 due to an 800 mL blood loss after hip replacement surgery, and 1 after an elbow synovectomy where the blood loss could not be measured by the investigator).
Table 6: Summary of Hemostatic Efficacy
| Time of Hemostatic Efficacy Assessment | Excellent* | Good† | Number of subjects |
| End of surgery | 18 (72%) | 7 (28%) | 25 |
| End of initial postoperative period‡ | 23 (92%) | 2 (8%) | 25 |
| *Excellent : Achieved hemostasis comparable to that expected after similar surgery in a patient without hemophilia. †Good: Prolonged time to hemostasis, with somewhat increased bleeding compared with that expected after similar surgery in a patient without hemophilia. ‡End of initial postoperative period is date of discharge or postoperative Day 6, whichever occurs later. | |||
Routine Prophylaxis
One hundred and two (102) subjects (94 subjects ≥12 years of age and 8 subjects <12 years of age) received XYNTHA for routine prophylaxis, for comparison of annualized bleeding rate (ABR) to on-demand treatment alone as a part of 2 completed studies. XYNTHA was administered for routine prophylaxis at a dose of 25 ± 5 IU/kg every other day (in subjects <12 years of age) or 30 ± 5 IU/kg administered 3 times weekly (in subjects 12 years of age or older), with provisions for dose escalation based on pre-specified criteria (over a 4-week period, 2 spontaneous bleeds into a major joint and/or target joint, or 3 or more spontaneous bleeding episodes in any location). Among these 102 subjects, 7 dose escalations were prescribed for 6 subjects.
In subjects ≥12 years, 42 subjects (42/94 or 45%) reported no bleeding while on routine prophylaxis. The mean±SD total ABR during routine prophylaxis was 4.0±6.64 with median (min-max) of 1.9 (0.0-44.2). The mean ABR for subjects during routine prophylaxis was 88% lower than the mean ABR for subjects during on-demand treatment (Table 7).
In subjects <12 years, 4 subjects (4/8 or 50%) reported no bleeding while on routine prophylaxis. The mean±SD total ABR during routine prophylaxis was 1.5±2.2 with median (min-max) of 0.6 (0.0-6.2). The mean ABR for subjects during routine prophylaxis was 97% lower than the mean ABR for subjects during on-demand treatment (Table 7).
Table 7: Summary of Annualized Bleeding Rate During Routine Prophylaxis Treatment with XYNTHA
| Age Category (years) | Number of Subjects | % Reduction from OD | Statistic | Treated Total Routine Prophylaxis ABR | Treated Spontaneous Routine Prophylaxis ABR | Treated Traumatic Routine Prophylaxis ABR |
| 0 to <12 | 8 | 97% | Mean SD | 1.5 ± 2.20 | 0.6 ± l.31 | 0.9 ± l.30 |
| Median (Min-Max) | 0.6 (0.0-6.2) | 0.0 (0.0-3.7) | 0.0 (0.0-3.2) | |||
| ≥12 | 94* | 88% | Mean SD | 4.0 ± 6.64 | 2.0 ± 4.25 | 2.0 ± 4.10 |
| Median (Min-Max) | 1.9 (0.0-14.2) | 0.0 (0.0-32.1) | 0.0 (0.0-23.3) | |||
| 12 to <17 | 18† | 84% | Mean SD | 7.3 ± 11.37 | 3.3 ± 7.73 | 4.0 ± 5.94 |
| Median (Min-Max) | 3.0 (0.0-14.2) | 0.0 (0.0-32.1) | 1.9 (0.0-19.6) | |||
| ≥17 | 76 | 89% | Mean SD | 3.2 ± 4.70 | 1.6 ± 2.88 | 1.6 ± 3.42 |
| Median (Min-Max) | 1.9 (0.0-23.3) | 0.0 (0.0-13.7) | 0.0 (0.0-23.3) | |||
| OD = on demand; ABR = annualized bleeding rate; SD = standard deviation, Min = minimum, Max = maximum. *The treated total ABR mean ± SD during prophylaxis for the 93 subjects aged ≥12 years (outlier removed), was 3.6 ± 5.18 with median (min–max) of 1.9 (0.0–23.3). The spontaneous treated ABR mean ± SD was 1.6 ± 2.87 with median (min–max) of 0.0 (0.0–13.7). The traumatic ABR mean ± SD was 1.9 ± 3.99 with median (min–max) of 0.0 (0.0–23.3). †The treated total ABR mean ± SD during prophylaxis for the 17 adolescents (outlier removed), was 5.2 ± 6.90 with median (min–max) of 2.0 (0.0–21.4). The spontaneous ABR mean ± SD was 1.6 ± 2.94 with median (min–max) of 0.0 (0.0–11.6). The traumatic ABR mean ± SD was 3.5 ± 5.77 with median (min–max) of 1.9 (0.0–19.6). | ||||||
REFERENCES
16. Recht M, Nemes L, Matysiak M et al. Clinical Evaluation of Moroctocog Alfa (AF-CC), a New Generation of B-Domain Deleted Recombinant Factor VIII (BDDrFVIII) for Treatment of Haemophilia A: Demonstration of Safety, Efficacy and Pharmacokinetic Equivalence to Full-length Recombinant Factor VIII. Haemophilia 2009; 1–12.
17. Windyga J, Rusen L, Gruppo R, et al. BDDrFVIII (Moroctocog alfa [AF-CC]) for surgical haemostasis in patients with haemophilia A: results of a pivotal study. Haemophilia. 2010 Sep 1;16(5):731.
Medication Guide
PATIENT INFORMATION
XYNTHA® SOLOFUSE® /ZIN-tha/
[Antihemophilic Factor (Recombinant)]
Please read this patient information carefully before using XYNTHA and each time you get a refill. There may be new information. This leaflet does not take the place of talking with your healthcare provider about your medical problems or your treatment.
What is XYNTHA?
XYNTHA is an injectable medicine that is used to help control and reduce bleeding in people with hemophilia A. Hemophilia A is also called classic hemophilia. Your healthcare provider may give you XYNTHA when you have surgery.
XYNTHA is not used to treat von Willebrand's disease.
What should I tell my healthcare provider before using XYNTHA?
Tell your healthcare provider about all of your medical conditions, including if you:
- have any allergies, including allergies to hamsters.
- are pregnant or planning to become pregnant. It is not known if XYNTHA may harm your unborn baby.
- are breastfeeding. It is not known if XYNTHA passes into your milk and if it can harm your baby.
Tell your healthcare provider about all of the medicines you take, including all prescription and non-prescription medicines, such as over-the-counter medicines, supplements, or herbal remedies.
How should I infuse XYNTHA?
Step-by-step instructions for infusing with XYNTHA SOLOFUSE are provided at the end of this leaflet.
The steps listed below are general guidelines for using XYNTHA SOLOFUSE. Always follow any specific instructions from your healthcare provider. If you are unsure of the procedures, please call your healthcare provider before using.
Call your healthcare provider right away if bleeding is not controlled after using XYNTHA.
Call your healthcare provider right away if you take more than the dose you should take.
Talk to your healthcare provider before traveling. Plan to bring enough XYNTHA SOLOFUSE for your treatment during this time.
What are the possible side effects of XYNTHA?
Call your healthcare provider or go to the emergency department right away if you have any of the following symptoms because these may be signs of a serious allergic reaction:
- wheezing
- difficulty breathing
- chest tightness
- turning blue (look at lips and gums)
- fast heartbeat
- swelling of the face
- faintness
- rash
- hives
Common side effects of XYNTHA are
- headache
- joint pain
- fever
- cough
- vomiting
- diarrhea
- weakness
Your body can make antibodies against XYNTHA (called “inhibitors”) that may stop XYNTHA from working properly. Your healthcare provider may need to take blood tests from time to time to monitor for inhibitors.
Talk to your healthcare provider about any side effect that bothers you or that does not go away. You may report side effects to FDA at 1-800-FDA-1088.
How should I store XYNTHA SOLOFUSE?
Store in the refrigerator at 36° to 46°F (2° to 8°C).
Do not freeze.
Protect from light.
XYNTHA SOLOFUSE can last at room temperature (below 77°F) for up to 3 months. If you store XYNTHA SOLOFUSE at room temperature, carefully write down the date you put XYNTHA SOLOFUSE at room temperature, so you will know when to throw it away. There is a space on the carton for you to write the date.
Throw away any unused XYNTHA SOLOFUSE after the expiration date.
Infuse within 3 hours after reconstitution or after removal of the grey rubber tip cap from the prefilled dual-chamber syringe. You can keep the reconstituted solution at room temperature before infusion for up to 3 hours. If it is not used in 3 hours, throw it away.
Do not use reconstituted XYNTHA if it is not clear to slightly opalescent and colorless.
Dispose of all materials, whether reconstituted or not, in an appropriate medical waste container.
What else should I know about XYNTHA?
Medicines are sometimes prescribed for purposes other than those listed here. Talk to your healthcare provider if you have any concerns. You can ask your healthcare provider for information about XYNTHA SOLOFUSE that was written for healthcare professionals.
Do not share XYNTHA SOLOFUSE with other people, even if they have the same symptoms that you have.
Instructions for Use
XYNTHA® SOLOFUSE®/ZIN-tha/
[Antihemophilic Factor (Recombinant)]
XYNTHA SOLOFUSE is supplied as a pre-filled dual-chamber syringe with lyophilized XYNTHA powder in one chamber and 0.9% sodium chloride solution in the other chamber. Before you can infuse it (intravenous injection), you must reconstitute the powder by mixing it with the sodium chloride solution.
Reconstitute and infuse XYNTHA SOLOFUSE using the infusion set provided in this kit. Please follow the directions below for the proper use of this product.
PREPARATION AND RECONSTITUTION OF XYNTHA SOLOFUSE
Preparation
1. Always wash your hands before doing the following steps.
2. Keep everything clean and germ-free while you are reconstituting XYNTHA SOLOFUSE.
3. Once the syringes are open, finish reconstituting XYNTHA SOLOFUSE as soon as possible. This will help to keep them germ-free.
4. For additional instructions on the use of a XYNTHA SOLOFUSE and a XYNTHA vial or multiple XYNTHA SOLOFUSE, see the detailed information provided after INFUSION OF XYNTHA section.
Reconstitution
1. Allow the XYNTHA SOLOFUSE to reach room temperature.
2. Remove the contents of the XYNTHA SOLOFUSE Kit and place on a clean surface, making sure you have all the supplies you will need.
3. Grasp the plunger rod as shown in the following diagram. Do not touch the shaft of the plunger rod. Screw the plunger rod firmly into the opening in the finger rest of the XYNTHA SOLOFUSE by pushing and turning firmly until resistance is felt (approximately 2 turns).
Throughout the reconstitution process, it is important to keep the XYNTHA SOLOFUSE upright to prevent possible leakage.
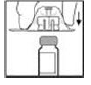 |
4. Holding the XYNTHA SOLOFUSE upright, remove the white tamper-evident seal by bending the seal right to left (or a gentle rocking motion) to break the perforation of the cap and expose the grey rubber tip cap of the XYNTHA SOLOFUSE.
 |
5. Remove the protective blue vented sterile cap from its package. While holding the XYNTHA SOLOFUSE upright, remove the grey rubber tip cap and replace it with the protective blue vented cap (prevents pressure build-up). Avoid touching the open end of both the syringe and the protective blue vented cap.
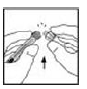 |
6. Gently and slowly push the plunger rod until the two stoppers inside the XYNTHA SOLOFUSE meet, and all of the diluent is transferred to the chamber containing the XYNTHA powder.
Note: To prevent the escape of fluid from the tip of the syringe, do not push the plunger rod with excessive force.
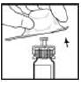 |
7. With the XYNTHA SOLOFUSE remaining upright, swirl gently several times until the powder is dissolved.
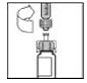 |
Look carefully at the solution in the XYNTHA SOLOFUSE. The solution should be clear to slightly opalescent and colorless. If it is not, throw away the solution and use a new kit.
8. Holding the XYNTHA SOLOFUSE in an upright position, slowly advance the plunger rod until most, but not all, of the air is removed from the drug product chamber.
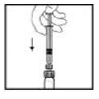 |
Note:
- If you are not using the solution immediately, store the syringe upright and keep the protective blue vent cap on the XYNTHA SOLOFUSE until ready to infuse.
- Infuse XYNTHA solution within 3 hours after reconstitution or removal of the grey tip cap from the XYNTHA SOLOFUSE. The reconstituted solution may be kept at room temperature for up to 3 hours prior to infusion. If you have not used it in 3 hours, throw it away.
- If more than one XYNTHA SOLOFUSE is needed for each infusion, a luer-to-luer syringe connector can be used (not included in this kit). Please contact your doctor or healthcare provider, or call the Wyeth Medical Information Department at 1-800-438-1985, for additional information.
INFUSION OF XYNTHA
Your healthcare provider will teach you how to infuse XYNTHA yourself. Once you learn how to do this, you can follow the instructions in this insert.
Before XYNTHA can be infused, you must reconstitute it as instructed above in the PREPARATION AND RECONSTITUTION OF XYNTHA SOLOFUSE section.
After reconstitution, be sure to look carefully at the XYNTHA solution. The solution should be clear to slightly opalescent and colorless. If it is not, throw away the solution and use a new kit.
Use the infusion set included in the kit to infuse XYNTHA. Do not infuse XYNTHA in the same tubing or container with other medicines.
1. After removing the protective blue vented cap, firmly attach the intravenous infusion set provided in the kit onto the XYNTHA SOLOFUSE.
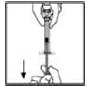 |
2. Apply a tourniquet and prepare the injection site by wiping the skin well with an alcohol swab provided in the kit.
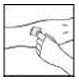 |
3. Remove the protective needle cover and insert the butterfly needle of the infusion set tubing into your vein as instructed by your healthcare provider. Remove the tourniquet. Verify proper needle placement.
4. Infuse the reconstituted XYNTHA product over several minutes. Your comfort level should determine the rate of infusion.
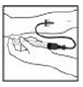 |
5. After infusing XYNTHA, remove the infusion set and throw it away. The amount of liquid left in the infusion set will not affect your treatment.
- Note: Dispose of all unused solution, the empty XYNTHA SOLOFUSE, and other used medical supplies in an appropriate container.
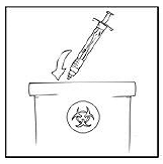 |
- It is a good idea to record the lot number from the XYNTHA SOLOFUSE label every time you use XYNTHA. You can use the peel-off label found on the XYNTHA SOLOFUSE to record the lot number.
ADDITIONAL INSTRUCTIONS
XYNTHA is also supplied in kits that include single-use vials with lyophilized powder and prefilled diluent syringes.
If you use one XYNTHA vial and one XYNTHA SOLOFUSE for the infusion, reconstitute the XYNTHA vial and the XYNTHA SOLOFUSE according to the specific directions for that respective product kit. Use a separate, 10 milliliter or larger luer lock syringe (not included in this kit) to draw back the reconstituted contents of the XYNTHA vial and the XYNTHA SOLOFUSE.
If you use multiple XYNTHA SOLOFUSE kits for the infusion, reconstitute each XYNTHA SOLOFUSE according to the directions above. Use a separate, 10 milliliter or larger luer lock syringe (not included in this kit) to draw back the reconstituted contents of any additional XYNTHA SOLOFUSE.
Use of a XYNTHA Vial Kit with a XYNTHA SOLOFUSE Kit
These instructions are for the use of only one XYNTHA vial kit with one XYNTHA SOLOFUSE Kit. For further information, please contact your healthcare provider or call the Medical Information Department at Wyeth Pharmaceuticals, 1-800-438-1985.
1. Reconstitute the XYNTHA vial using the instructions included with the kit. Detach the empty diluent syringe from the vial adapter by gently turning and pulling the syringe counterclockwise, leaving the contents in the XYNTHA vial with the vial adapter in place.
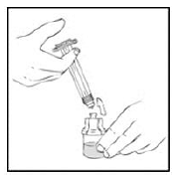 |
2. Reconstitute the XYNTHA SOLOFUSE using the instructions included with the product kit, remembering to remove most, but not all, of the air from the syringe.
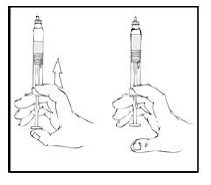 |
3. After removing the protective blue vented cap, connect the XYNTHA SOLOFUSE to the vial adapter by inserting the tip into the adapter opening while firmly pushing and turning the syringe clockwise until secured.
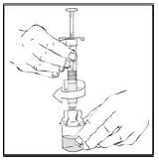 |
4. Slowly push the plunger rod of the XYNTHA SOLOFUSE to empty the contents into the XYNTHA vial. The plunger rod may move back slightly after release.
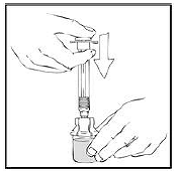 |
5. Detach the empty XYNTHA SOLOFUSE from the vial adapter and throw it away.
If the syringe turns without detaching from the vial adapter, grasp the white collar and turn.
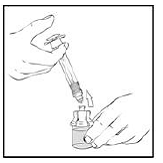 |
6. Connect a sterile 10 milliliter or larger luer lock syringe to the vial adapter. You may want to inject some air into the vial to make withdrawing the vial contents easier.
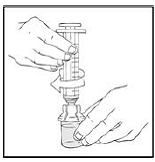 |
7. Invert the XYNTHA vial and slowly draw the solution into the large luer lock syringe.
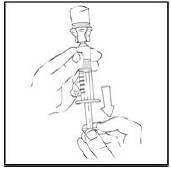 |
8. Detach the large luer lock syringe from the vial adapter by gently turning and pulling the syringe counterclockwise. Throw away the empty XYNTHA vial with the adapter attached.
9. Attach the infusion set to the large luer lock syringe as directed in the INFUSION OF XYNTHA section.
Note: Dispose of all unused solution, the empty XYNTHA SOLOFUSE, and other used medical supplies in an appropriate container.
 |
Use of Multiple XYNTHA SOLOFUSE Kits
The instructions below are for the use of multiple XYNTHA SOLOFUSE kits with a 10 milliliter or larger luer lock syringe. For further information, please contact your healthcare provider or call the Medical Information Department at Wyeth Pharmaceuticals, 1-800-438-1985.
Note: Luer-to-luer syringe connectors are not provided in the kits. Contact your XYNTHA supplier to order.
1. Reconstitute all XYNTHA SOLOFUSE according to instructions described in PREPARATION AND RECONSTITUTION OF XYNTHA SOLOFUSE section. Holding the XYNTHA SOLOFUSE in an upright position, slowly push the plunger rod until most, but not all, of the air is removed from the syringe.
 |
2. Remove the luer-to-luer syringe connector from its package.
3. After removing the protective blue vented cap, connect a sterile 10 milliliter or larger luer lock syringe to one opening (port) in the syringe connector and the XYNTHA SOLOFUSE to the remaining open port on the opposite end.
 |
4. With the XYNTHA SOLOFUSE on top, slowly push the plunger rod to empty all the XYNTHA SOLOFUSE content into the large luer lock syringe.
 |
5. Remove the empty XYNTHA SOLOFUSE and repeat procedures 3 and 4 above for any additional XYNTHA SOLOFUSE.
6. Remove the luer-to-luer syringe connector from the large luer lock syringe and attach the infusion set as directed in the INFUSION OF XYNTHA section.
Note: Dispose of all unused solution, the empty XYNTHA SOLOFUSE, and other used medical supplies in an appropriate container.
 |
This product's labeling may have been updated. For the most recent prescribing information, please visit www.pfizer.com.
