Esperoct Drug Description: Detailed Datasheet
- Generic Name: [antihemophilic factor (recombinant), glycopegylated-exei] injection
- Brand Name: Esperoct
side effects drug center esperoct ([antihemophilic factor (recombinant), glycopegylated-exei] injection) drug
Drug Description
ESPEROCT®
[antihemophilic factor (recombinant), glycopegylated-exei] for Injection
DESCRIPTION
ESPEROCT is a sterile, preservative-free, non-pyrogenic lyophilized powder for intravenous injection after reconstitution with the provided saline diluent. The active ingredient in ESPEROCT is a recombinant analogue of human coagulation Factor VIII (FVIII) conjugated with a 40-kDa polyethylene glycol (PEG) molecule. ESPEROCT is formulated with the following excipients: sodium chloride, L-histidine, sucrose, polysorbate 80, L-methionine, and calcium chloride.
FVIII activity in ESPEROCT is determined using the chromogenic assay described in the European Pharmacopoeia. The activity assignment employs a FVIII reference material that is traceable to the current World Health Organization (WHO) international standard for FVIII concentrate, and evaluated by appropriate methodologies to ensure the accuracy of the results. ESPEROCT is available in single-dose vials that contain nominally 500, 1000, 1500, 2000, or 3000 IU of FVIII. Each vial of ESPEROCT is labeled with the actual FVIII activity. After reconstitution with the supplied diluent (0.9% saline), each mL of the solution contains approximately 125, 250, 375, 500, or 750 IU of FVIII, respectively.
The FVIII protein in ESPEROCT is produced in Chinese Hamster Ovary (CHO) cells using recombinant DNA technology, and contains a truncated B domain, which is O-glycosylated. The polypeptide part of the molecule has a molecular mass of 166 kDa (calculated excluding post-translational modifications) and represents a heterodimer of a heavy chain and a light chain, which are held together by non-covalent interactions. The recombinant FVIII protein is purified using a series of chromatographic steps, one of which is affinity chromatography, with the use of a monoclonal antibody to selectively isolate the rFVIII from the cell culture medium. The 40-kDa PEG molecule is conjugated to the O-glycan moiety of the B domain using an enzymatic reaction to produce a glycopegylated FVIII (FVIII-PEG). The purification process includes two viral clearance steps, namely detergent (Triton X-100) treatment for inactivation of enveloped viruses, and 20-nm filtration for removal of enveloped and non-enveloped viruses. No additives of human or animal origin are used during the manufacturing process and formulation of ESPEROCT.
In the blood circulation, when FVIII-PEG is activated by thrombin, the B-domain portion with the attached PEG moiety is cleaved off, and the resulting activated FVIII (FVIIIa) is similar in structure and function to native FVIIIa.
Indications & Dosage
INDICATIONS
ESPEROCT [antihemophilic factor (recombinant), glycopegylated-exei] is a recombinant DNA-derived coagulation Factor VIII concentrate indicated for use in adults and children with hemophilia A for:
- On-demand treatment and control of bleeding episodes
- Perioperative management of bleeding
- Routine prophylaxis to reduce the frequency of bleeding episodes
Limitation Of Use
ESPEROCT is not indicated for the treatment of von Willebrand disease. (1)
DOSAGE AND ADMINISTRATION
For Intravenous Infusion After Reconstitution Only.
Dose
- Dosage and duration of treatment depend on the severity of the Factor VIII deficiency, on the location and extent of bleeding, and on the patient's clinical condition. Careful monitoring of replacement therapy is necessary in cases of major surgery or life-threatening bleeding episodes.
- Each vial of ESPEROCT contains the labeled amount of recombinant Factor VIII in international units (IU). One IU of Factor VIII activity corresponds to the quantity of Factor VIII in one milliliter of normal human plasma. The calculation of the required dosage of Factor VIII is based on the empirical finding that one IU of Factor VIII per kg body weight raises the plasma Factor VIII activity by two IU/dL.
On–Demand Treatment And Control Of Bleeding Episodes
Table 1 can be used to guide dosing of ESPEROCT for treatment of bleeding episodes.
Table 1:Dosing of ESPEROCT to Control Bleeding Episodes
| Type of bleeding | Adolescents/Adults ≥12 years Dose (IU/kg) |
Children <12 years Dose (IU/kg) |
Additional doses |
Minor |
40 | 65 | One dose should be sufficient |
Moderate |
40 | 65 | An additional dose may be administered after 24 hours |
Major |
50 | 65 | Additional dose(s) may be administered approximately every 24 hours |
Perioperative Management
The dose level and dosing intervals for surgery depend on the procedure and local practice. A guide for dosing with ESPEROCT during surgery (perioperative management) is provided in Table 2 below.
Table 2:Dosing for Perioperative Management with ESPEROCT
| Type of surgery | Adolescents/Adults ≥12 years Pre-operative Dose (IU/kg) |
Children <12 years Pre-operative Dose (IU/kg) |
Additional Doses |
| Minor Including tooth extraction |
50 | 65 | Additional dose(s) can be administered after 24 hours if necessary |
| Major Intracranial, intra-abdominal, intrathoracic, or joint replacement surgery |
50 | 65 | Additional doses can be administered approximately every 24 hours for the first week and then approximately every 48 hours until wound healing has occurred |
Routine Prophylaxis
Adults and adolescents (≥ 12 years): The recommended starting dose is 50 IU of ESPEROCT per kg body weight every 4 days. This regimen may be individually adjusted to less or more frequent dosing based on bleeding episodes.
Children (< 12 years): A dose of 65 IU of ESPEROCT per kg body weight twice weekly. This regimen may be individually adjusted to less or more frequent dosing based on bleeding episodes.
- ESPEROCT also may be dosed to achieve a specific target Factor VIII activity level, depending on the severity of hemophilia, for on-demand treatment/control of bleeding episodes or perioperative management. To achieve a specific target Factor VIII activity level, use the following formula:
Dosage (IU) = Body Weight (kg) x Desired Factor VIII Increase (IU/dL or % normal) x 0.5
- Base the dose and frequency of ESPEROCT on the individual clinical response. Patients may vary in their pharmacokinetic and clinical responses.
- If monitoring of Factor VIII activity is performed, use a chromogenic or one-stage clotting assay appropriate for use with ESPEROCT [see WARNINGS AND PRECAUTIONS].
Preparation And Reconstitution
- Always wash hands and ensure that the area is clean before performing the reconstitution procedures.
- Use aseptic technique during the reconstitution procedures.
- If the dose requires more than one vial of ESPEROCT per infusion, reconstitute each vial according to the following instructions.
Overview Of Esperoct Package
 |
Reconstitution
- Bring the ESPEROCT vial and the pre-filled diluent syringe to room temperature.
- Remove the plastic cap from the ESPEROCT vial.
- Wipe the rubber stopper on the vial with a sterile alcohol swab and allow it to dry prior to use.
- Remove the protective paper from the vial adapter.Do not remove the vial adapter from the protective cap.
- Place the ESPEROCT vial on a flat and solid surface. While holding the protective cap, place the vial adapter over the ESPEROCT vial and press down firmly on the protective cap until the vial adapter spike penetrates the rubber stopper.
- Carefully remove the protective cap from the vial adapter.
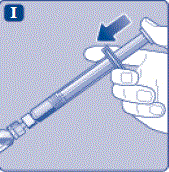
- Grasp the plunger rod as shown in the diagram. Attach the plunger rod to the syringe by holding the plunger rod by the wide top end. Turn the plunger rod clockwise into the rubber plunger inside the pre-filled diluent syringe until resistance is felt.
- Break off the syringe cap from the pre-filled diluent syringe by snapping the perforation of the cap.
- Connect the pre-filled diluent syringe to the vial adapter by turning it clockwise until it is secured.
- Push the plunger rod to slowly inject all the diluent into the vial.
- Without removing the syringe, gently swirl the ESPEROCT vial until all of the powder is dissolved. Avoid shaking the vial and foaming the solution.
 |
 |
 |
 |
 |
 |
 |
 |
 |
 |
Administration
For Intravenous Infusion Only
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. The solution should be clear and have no particles. Do not use if particulate matter or discoloration is observed.
- Do not administer ESPEROCT in the same tubing or container with other medicinal products.
- Administer the ESPEROCT solution immediately [see Administration]. If not used immediately after reconstitution, store the solution in the vial with the vial adapter and the syringe attached, at room temperature ≤ 86°F (30°C) for ≤ 4 hours, or stored in a refrigerator at 36°F to 46°F (2°C to 8°C) for ≤ 24 hours.
- Invert the ESPEROCT vial and slowly draw the solution into the syringe.
- Detach the syringe from the vial adapter by turning the syringe counterclockwise.
- Attach the syringe to the luer end of an infusion needle set.
- Infuse the reconstituted ESPEROCT intravenously slowly over approximately 2 minutes.
- After infusion, safely dispose of the syringe with the infusion set, the vial with the vial adapter, any unused ESPEROCT, and other waste materials.
 |
Caution
The pre-filled diluent syringe is made of glass with an internal tip diameter of 0.037 inches and is compatible with a standard Luer-lock connector.
Some needleless connectors for intravenous catheters are incompatible with the glass diluent syringes (for example, certain connectors with an internal spike, such as Clave®/MicroClave®, InVision-Plus®, InVision-Plus CS®, Invision-Plus Junior®, Bionector®), and their use can damage the connector and affect administration. To administer ESPEROCT through incompatible needleless connectors, withdraw the reconstituted product into a standard 10 mL sterile Luer-lock plastic syringe.
HOW SUPPLIED
Dosage Forms And Strengths
ESPEROCT is available as a sterile white to off-white lyophilized powder supplied in single-dose vials containing nominally 500, 1000, 1500, 2000 or 3000 IU. The actual FVIII activity is printed on each ESPEROCT vial and carton.
After reconstitution with 4 mL of saline diluent, the reconstituted solution contains approximately 125, 250, 375, 500 or 750 IU per mL of ESPEROCT, respectively.
How Supplied
- ESPEROCT is supplied in packages comprised of a single-dose vial containing nominally 500, 1000, 1500, 2000 or 3000 IU of Factor VIII activity; a MixPro® pre-filled diluent syringe containing 0.9% saline solution; and a sterile vial adapter with a 25-micrometer filter, which serves as a needleless reconstitution device.
- The actual Factor VIII activity in IU is stated on each ESPEROCT carton and vial label.
Table 9: ESPEROCT Presentations
| Nominal Dosage Strength | Cap Color Indicator | Carton NDC Number | Components |
| 500 IU | Red | NDC 0169 8500 01 |
|
| 1000 IU | Green | NDC 0169 8100 01 |
|
| 1500 IU | Gray | NDC 0169 8150 01 |
|
| 2000 IU | Yellow | NDC 0169 8200 01 |
|
| 3000 IU | Black | NDC 0169 8300 01 |
|
| IU = International Units | |||
- The ESPEROCT vials are made of glass, closed with a chlorobutyl rubber stopper (not made with natural rubber latex), and sealed with an aluminum cap.
- The pre-filled diluent syringes are made of glass with a siliconized bromobutyl rubber plunger (not made with rubber latex).
- The closed vials and pre-filled diluent syringes are equipped with a tamper-evident snap-off cap which is made of polypropylene.
Storage And Handling
- Store ESPEROCT in the original package to protect the ESPEROCT vial from light.
- Store ESPEROCT in a powder form under refrigeration at 36°F to 46°F (2°C to 8°C) for up to 30 months from the date of manufacture until the expiration date stated on the label.
- ESPEROCT may be stored at room temperature [not to exceed 86°F(30°C)] for up to 12 months within the 30-month time period. Record the date on the carton when the product was removed from the refrigerator. The total time of storage at room temperature should not exceed 12 months. Do not return the product to the refrigerator after room temperature storage.
- Do not use ESPEROCT beyond the expiration date printed on the vial or 12 months after the date it was removed from refrigeration (whichever is earlier).
- Do not freeze ESPEROCT.
- Use ESPEROCT within 4 hours after reconstitution when stored at room temperature, or within 24 hours when stored in the refrigerator. Store the reconstituted product in the vial.
- Reconstituted product must be discarded after 4 hours at room temperature, or 24 hours in the refrigerator.
Manufactured by: Novo Nordisk A/S Novo Allé, DK-2880 Bagsvaerd. Revised: n/a
Side Effects & Drug Interactions
SIDE EFFECTS
The most frequently reported adverse reactions (incidence ≥1%) in clinical trials were rash, redness, itching (pruritus), and injection site reactions.
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in clinical trials of another drug and may not reflect the rates observed in clinical practice.
The safety of ESPEROCT has been evaluated in 270 subjects (202 adolescents/adults and 68 children) in five prospective, multi-center clinical studies in previously treated patients (PTPs) with severe hemophilia A (<1% endogenous Factor VIII activity) and no history of inhibitors. All subjects received at least one dose of ESPEROCT. A previously treated patient was defined as a subject with a history of at least 150 exposure days to other Factor VIII products (adolescent/adult subjects) or 50 exposure days to other Factor VIII products (pediatric subjects). Total exposure to ESPEROCT was 80,425 exposure days corresponding to 889 patient years of treatment.
During the clinical trials in PTPs, adverse reactions occurred at a rate of 0.10 events per patient year of exposure. The most frequently reported adverse reactions were rash (5.2%), injection site reaction (2.6%), redness (1.9%), and itching (pruritus) (1.5%).
Immunogenicity
Subjects were monitored for neutralizing and non-neutralizing antibodies to Factor VIII, polyethylene glycol (PEG), and CHO host cell protein. One previously treated subject developed confirmed neutralizing antibodies to Factor VIII (13.5 Bethesda Units). In addition, two subjects had transient low titer FVIII antibody (<5 Bethesda Units) test results at a single occasion. Anti-PEG antibodies of no clinical consequence were detected in 45 subjects, 32 of whom had pre-existing anti-PEG antibodies. Nine subjects developed anti-CHO host cell protein antibodies of no clinical consequence.
The detection of antibodies is highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of antibody (including neutralizing antibody) positivity in an assay may be influenced by several factors, including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease.
DRUG INTERACTIONS
No Information Provided
Warnings & Precautions
WARNINGS
Included as part of the "PRECAUTIONS" Section
PRECAUTIONS
Hypersensitivity Reactions
Allergic-type hypersensitivity reactions, including anaphylaxis, are possible with ESPEROCT. The product contains traces of hamster proteins, which in some patients may cause allergic reactions [see DESCRIPTION]. Early signs of allergic reactions, which can progress to anaphylaxis, may include angioedema, chest tightness, difficulty breathing, wheezing, rash, hives, and itching. Observe patients for signs and symptoms of acute hypersensitivity reactions, particularly during the early phases of exposure to the product. Discontinue use of ESPEROCT if allergic- or anaphylactic-type reactions occur, and initiate appropriate treatment.
Neutralizing Antibodies
The formation of neutralizing antibodies (inhibitors) to Factor VIII has occurred following administration of ESPEROCT. Monitor patients for the development of Factor VIII inhibitors by appropriate clinical observations and laboratory tests. If expected Factor VIII activity plasma levels are not attained, or if bleeding is not controlled after ESPEROCT administration, suspect the presence of an inhibitor (neutralizing antibody)[see Monitoring Laboratory Tests].
Monitoring Laboratory Tests
If monitoring of Factor VIII is performed, use a chromogenic or one-stage clotting assay appropriate for use with ESPEROCT [see DOSAGE AND ADMINISTRATION].
Factor VIII activity levels can be affected by the type of activated partial thromboplastin time (aPTT) reagent used in the assay. Some silica-based aPTT reagents can underestimate the activity of ESPEROCT by up to 60%; other reagents may overestimate the activity by 20%. If an appropriate one-stage clotting or chromogenic assay is not available locally, then use a reference laboratory.
If bleeding is not controlled with the recommended dose of ESPEROCT or if the expected Factor VIII activity levels in plasma are not attained, then perform a Bethesda assay to determine if Factor VIII inhibitors are present.
Patient Counseling Information
Advise patients:
- To read the FDA-approved patient labeling (PATIENT INFORMATION and Instructions for Use).
- That allergic-type hypersensitivity reactions or anaphylaxis are possible with use of ESPEROCT. Inform patients of the early signs of hypersensitivity reactions including rash, hives, itching, facial swelling, tightness of the chest, and wheezing. Advise patients to discontinue use of ESPEROCT immediately and contact their healthcare provider and/or seek emergency care promptly if these symptoms occur.
- To contact their healthcare provider or treatment facility for further treatment and/or assessment if they experience a lack of a clinical response to Factor VIII replacement therapy, as this may be a manifestation of an inhibitor.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment Of Fertility
Carcinogenesis, mutagenesis, and impairment of fertility studies in animals have not been performed.
Use In Specific Populations
Pregnancy
Risk Summary
There are no data with ESPEROCT use in pregnant women to determine whether there is a drug-associated risk. Animal reproduction studies have not been conducted with ESPEROCT. It is unknown whether ESPEROCT can cause fetal harm when administered to a pregnant woman or can affect fertility.
In the U.S. general population, the estimated background risk of major birth defect and miscarriage in clinically recognized pregnancies is 2–4% and 15–20%, respectively.
Lactation
Risk Summary
There is no information regarding the presence of ESPEROCT in human milk, the effect on the breastfed infant, and the effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for ESPEROCT and any potential adverse effects on the breastfed infant from ESPEROCT or from the underlying maternal condition.
Pediatric Use
Safety and efficacy were evaluated in 93 previously treated pediatric patients <18 years of age, who received at least one dose of ESPEROCT; all received routine prophylaxis [see Clinical Studies]. Thirty-four (34) of these subjects (36.6%) were 1 to <6 years of age; 34 subjects (36.6%) were 6 to <12 years of age; and 25 subjects (27%) were 12 to <18 years of age. Pharmacokinetic parameters were evaluated for 27 of these subjects who were treated with ESPEROCT [see CLINICAL PHARMACOLOGY].
No difference in the safety profile of ESPEROCT was observed between previously treated pediatric subjects and adult subjects. Pharmacokinetic studies in children <12 years of age demonstrated higher clearance, a shorter half-life, and lower incremental recovery of Factor VIII compared to adults, but the pharmacokinetic parameters are comparable between young children (1–<6 years) and older children (6–<12 years). Because clearance (per kg body weight) is higher in children (<12 years), a higher dose and more frequent dosing may be needed in this population [see CLINICAL PHARMACOLOGY].
Geriatric Use
Clinical studies of ESPEROCT did not include sufficient numbers of subjects age 65 years and over to determine whether or not they respond differently than younger subjects.Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease and other drug therapy.
Overdosage & Contraindications
OVERDOSE
No Information Provided
CONTRAINDICATIONS
ESPEROCT is contraindicated in patients who have known hypersensitivity to ESPEROCT or its components (including hamster proteins) [see WARNINGS AND PRECAUTIONS and DESCRIPTION].
Clinical Pharmacology
Mechanism Of Action
ESPEROCT, a glycopegylated form of recombinant anti-hemophilic factor, temporarily replaces the missing coagulation Factor VIII needed for effective hemostasis in congenital hemophilia A patients. The Factor VIII in ESPEROCT is conjugated to a 40-kDa polyethylene glycol molecule which increases the half-life and decreases the clearance compared to the non-pegylated molecule.
Pharmacodynamics
The administration of ESPEROCT increases plasma levels of Factor VIII and can temporarily correct the coagulation defect in hemophilia A patients, as reflected by a decrease in activated partial thromboplastin time (aPTT).
Pharmacokinetics
All pharmacokinetic studies with ESPEROCT were conducted in previously treated subjects with severe hemophilia A (Factor VIII<1%). In total, 129 single-dose pharmacokinetic profiles of ESPEROCT were evaluated in 86 subjects (including 24 pediatric subjects, 1-<12 years).
Table 3 shows data for subjects who each received a single dose of 50 IU/kg. The plasma samples were analyzed using the one-stage clotting assay. There was a trend of increasing incremental recovery and AUC, and decreasing clearance, with age.
Table 3: Single-dose PK parameters of ESPEROCT 50 IU/kg, by age, using one-stage clotting assay (geometric mean (CV%))
| PK Parameter No. of subjects |
1 to <6 years N=12 |
6 to <12 years N=10 |
12 to <18 years N=3 |
>18 years N=42 |
| No. of profiles | 12 | 10 | 5 | 78 |
| IR (IU/dL) per IU/kg)a | 1.82 (32) | 1.67 (22) | 2.45 (16) | 2.53 (24) |
| FVIII recovery (IU/dL)a | 103.2 (27) | 98.7 (18) | 117.7 (14) | 130.4 (26) |
| t1/2 (hours) | 14.7 (27) | 13.8 (32) | 17.4 (39) | 21.7 (33) |
| AUCinf (IU*hour/dL) | 2305 (42) | 2197 (38) | 3063 (40) | 4110 (38) |
| CL (mL/hour/kg) | 2.4 (42) | 2.7 (42) | 1.6 (39) | 1.2 (34) |
| Vss (mL/kg) | 44.2 (25) | 47.3 (28) | 36.4 (12) | 37.3 (26) |
| MRT (hours) | 18.1 (27) | 17.8 (35) | 23.4 (43) | 27.4 (28)b |
|
PK parameters are presented in geometric mean. Abbreviations: IR = Incremental recovery; t1/2 = terminal half-life; AUC = area under the FVIII activity time profile; CL = clearance; Vss = volume of distribution at steady-state; MRT = mean residence time; CV% = coefficient of variation a IR and FVIII recovery were assessed 30 minutes post-dosing 50 IU/kg for patients ≥12 years and 60 minutes post-dosing 50 IU/kg (first sample) for children <12 years. b Calculation based on 64 profiles |
||||
In the single-dose PK assessment in adult subjects, whose body mass index (BMI) ranged from 17-35 kg/m2, differences were noted for individuals who were overweight (BMI 25 - <30 kg/m2) and obese (BMI 30 - <35 kg/m2). Incremental recovery was increased by approximately 17% and 41%, AUC was increased by approximately 10% and 27%, and clearance was decreased by approximately 8% and 23% respectively, all in comparison to those subjects with BMI <25 kg/m2. There is insufficient data to recommend specific dose adjustments for overweight and obese patients. The dose may be adjusted as necessary per prescriber’s discretion.
Observed pre-dose (trough) and post-dose (peak) plasma Factor VIII activity levels at steady-state during prophylactic treatment with ESPEROCT are presented in Table 4 by dose regimen and age range.
Table 4: Steady-state trough and peak plasma FVIII activity by age and dose regimen, chromogenic assay (geometric mean [95% CI])
| Dose Regimen | 60 IU/kg twice weekly** (50-75 IU/kg) |
50 IU/kg Q4D* | 75 IU/kg Q7D* | |||
| Age range No. patients |
<6 years N=31 |
6-<12 years N=34 |
12-<18 years N=23 |
≥18 years N=143 |
12-<18 years N=6 |
≥18 years N=29 |
| Trough, IU/dL | 1.2 (0.8; 1.6) |
2.0 (1.5; 2.7) |
2.7 (1.8; 4.0) |
3.0 (2.6; 3.5) |
0.6 (0.2; 1.6) |
1.3 (0.9; 2.0) |
| Peak, IU/dL |
125.0 (118.7; 131.6) |
143.3 (136.8; 150.2) |
125.1 (116.0; 135.0) |
137.9 (133.9; 142.2) |
198.0 (166.8; 235.2) |
197.9 (184.9; 212.7) |
| *Data included in analysis: adolescents/adults Main Phase until Visit 8 (end of the Main Phase) 50 IU/kg Q4D, and extension 1 for 75 IU/kg Q7D. Only measurements collected at steady-state for the given prophylaxis treatment are included in the analyses. **Data included in analysis: pediatric Main Phase 60 IU/kg (50-75 IU/kg) twice weekly. Only measurements collected at steady-state for the given prophylaxis treatment are included in the analyses. |
||||||
Time Of Factor VIII Activity Above 5%
Steady-state Factor VIII activity profiles were estimated using a one-compartment model with first-order elimination with PK parameters of clearance (CL) and volume of distribution (Table 5). Pharmacokinetic predictions showed that in all age groups, patients dosed twice weekly (dosing interval alternating between 3 and 4 days) or Q4D will be above 5% Factor VIII activity (i.e., in the range of mild hemophilia) for the majority of time (72-95% of time). Patients dosed with 50 IU/kg every 4 days will be above 1% Factor VIII activity 100% of the dosing interval. Patients dosed with 75 IU/kg every 7 days are predicted to be above 5% for 57% of time and above 1% for 83% of time.
Table 5:Estimation of steady-state peak and trough FVIII activity and time to 5% FVIII activity for ESPEROCT
| Dose regimen |
60 IU/kg (50-75 IU/kg) twice weekly |
50 IU/kg twice weekly | 50 IU/kg Q4D | 75 IU/kg Q7D |
| Age range | <12 years | ≥12 years | ≥12 years | ≥12 years |
| Peak FVIII activity (%) | 110/112* | 133/138* | 132 | 194 |
| Trough FVIII activity (%) | 2.8/0.8* | 8.6/3.6* | 3.5 | 0.3 |
| Time to 5% FVIII activity (days) | 2.5/2.5* | 3.6/3.6* | 3.6 | 4.0 |
| % of time in dosing interval above 5% FVIII activity | 72 | 95 | 90 | 57 |
| *Twice weekly values are shown as 3 day/4 day. Only 50 IU/kg data are used for the analysis. | ||||
Animal Toxicology And/Or Pharmacology
No adverse effects were observed in immune-deficient rats intravenously injected with ESPEROCT (50-1200 IU/kg/ injection), once every 4th day for 52 weeks. No evidence of polyethylene glycol accumulation was detected by immunohistochemical staining of brain tissue, including the choroid plexus.
Clinical Studies
The safety and efficacy of ESPEROCT have been evaluated in five multinational, open-label trials in male subjects with severe hemophilia A (<1% endogenous Factor VIII activity). One trial was subsequently partially randomized to evaluate two different prophylaxis regimens. All subjects were previously treated, which was defined as having received other Factor VIII products for ≥150 exposure days for adolescents and adults, and ≥50 exposure days for pediatric subjects. The key exclusion criteria across trials included known or suspected hypersensitivity to trial or related products and known history of Factor VIII inhibitors or current inhibitor ≥0.6 Bethesda units (BU).
The efficacy evaluation included 254 subjects, who received at least one dose of ESPEROCT, in the following trials:
- Adolescent/adult trial: This trial included 186 subjects, 161 adults (18 to 65 years old) and 25 adolescents (12 to <18 years old); it consisted of a Main Phase and optional Extension Phase. During the Main Phase, 175 subjects received the prophylaxis regimen which consisted of 50 IU/kg every 4 days (Q4D), while 12 adults chose to be treated on-demand. (One subject changed from on-demand to prophylaxis and is counted in both groups.) Thirteen (7%) of 175 adults in the prophylaxis arm modified their dosing regimen to Q3-4D dosing for ease of use. All subjects received at least one dose of ESPEROCT and are evaluable for safety and efficacy. A total of 165 subjects (91%) completed the Main Phase of this trial.
- Extension: This extension compared two dose regimens: 75 IU/kg every 7 days (Q7D) and 50 IU/kg Q4D. The randomization was open to subjects who experienced 2 or fewer bleeds during the last 6 months in the Main Phase.
- Pediatric trial: This trial included 68 subjects who were evenly divided with 34 in each age group, 0-<6 and 6-<12 years of age. All subjects received the same prophylaxis regimen of approximately 65 IU/kg (50-75 IU/kg) twice weekly. A total of 63 subjects (93%) completed the Main Phase.
- Surgery trial: In the surgery trial, 33 previously treated adolescents/adults underwent 45 major surgeries. The dose level of ESPEROCT was chosen so that FVIII activity at least as recommended by World Federation of Hemophilia (WFH) guidelines was targeted. All subjects returned to the adolescent/adult trial after the surgery trial assessments were completed.
On-Demand Treatment And Control Of Bleeding Episodes
There were 1506 bleeds reported in 171 of 254 subjects across the completed clinical trials, and the most common bleed types were joint (65.2%), muscle (14.5%), and subcutaneous (8.9%). Table 6 summarizes the efficacy in control of bleeding episodes by age.
Doses used for treatment of bleeding episodes depended on age, treatment regimen and the severity of the bleed.
Of the 1407 mild and moderate bleeding episodes in all subjects in the adolescent/adult study, the median dose used was 42 IU/kg. For subjects who were on the on-demand arm the median initial dose was 28 IU/kg and 88.4% of the bleeds were treated successfully with a single dose. In subjects receiving routine prophylaxis, the median initial dose was 52 IU/kg, and 76.4% of the bleeds were successfully treated with a single dose. Of the 15 severe bleeds, 12 (80%) required more than one dose with a total median dose of 111 IU/kg.
In the pediatric study, 70 mild/moderate bleeds in children < 12 years old receiving routine prophylaxis were treated with a median initial dose of 64 IU/kg per injection, with 63% treated with a single injection. When needed, additional median doses of 62 IU/kg were used at approximately 24 hour intervals. The median total dose was 70 IU/kg per bleed.
Table 6: Summary of efficacy in control of bleeding episodes by age
| Age range # of subjects |
<6 years N=34 |
6 - <12 years N=34 |
12 - <18years N=25 |
≥ 18 years N=161 |
Total N= 254 |
|
| # of bleeds | 30 | 40 | 112 | 1324 | 1506 | |
| # of injections | 1-2 | 76.7% | 82.5% | 88.4% | 95.5% | 94.3% |
| > 2 | 23.3% | 17.5% | 11.6% | 4.5% | 5.7% | |
| Response to first treatment | Excellent/ Good | 80.0% | 77.5% | 75.0% | 88.7% | 87.3% |
| Moderate | 13.3% | 17.5% | 17.9% | 10.3% | 11.1 % | |
|
Definition of Hemostatic Response: Excellent: Abrupt pain relief and/or unequivocal improvement in objective signs of bleeding within approximately 8 hours after a single injection. Good: Definite pain relief and/or improvement in signs of bleeding within approximately 8 hours after one injection, but possibly requiring more than one injection for complete resolution. Moderate: Probable or slight beneficial effect within approximately 8 hours after the first injection; usually requiring more than one injection. |
||||||
Perioperative Management
The efficacy analysis of ESPEROCT in perioperative management included 45 major surgical procedures performed in 33 adolescent and adult subjects. The procedures included 15 joint replacements, 9 arthroscopic orthopedic interventions, 17 other orthopedic interventions, and 4 non-orthopedic surgeries.
The clinical evaluation of hemostatic response during major surgery was assessed using a 4-point scale of excellent, good, moderate, or none. The hemostatic effect of ESPEROCT was rated as “excellent” or “good” in 43 of 45 surgeries (95.6%), while the effect was rated as “moderate” in 2 surgeries (4.4%). No surgery had an outcome rated as “none” or “missing.”
The median pre-operative dose for adults and adolescents undergoing major surgeries was 52 IU/kg, and the median total dose was 702 IU/kg. During post-operative days 1-6, the median dose was 32 IU/kg at approximately 24 hour intervals. During post-operative days 7-14, the median dose was 36 IU/kg at approximately 28 hour intervals. The number of doses and duration of treatment varied by procedure.
Routine Prophylaxis In Adolescents/Adults
The efficacy of ESPEROCT in routine prophylaxis with Q4D dosing was demonstrated for the adult/adolescent population (see Table 7). In the extension part of the study, treatment success of the Q7D arm was not established. During the Main Phase of the adolescent/adult trial, 186 subjects had a total of 159 exposure years. The median annualized bleeding rate (ABR) for treated bleeds in adults and adolescents treated every 4 days was 1.2 (IQR: 0.0:4.3), and mean ABR was 3.0 (SD: 4.7). When including all bleeds (treated and non-treated), the median ABR was 1.2 (IQR: 0.0; 4.7) and the mean ABR was 3.3 (SD: 4.9).
Table 7: Efficacy in adolescent/adult prophylaxis, median and mean ABRs by age, treatment regimen, and bleed type
| Prophylaxis | On-demand | |||
| Age Range | 12-17 years | 18-70 years | 12-70 years | 18-70 years |
| # of subjects | 25 | 150 | 175 | 12 |
| Mean treatment duration (years) | 0.85 | 0.81 | 0.82 | 1.33 |
| Treated bleeds | ||||
| # of subjects with bleeds (%) | 19 (76) | 86 (57) | 105 (60) | 12 (100) |
| # of subjects without bleeds (%) | 6 (24) | 64 (43) | 70 (40) | 0 |
| # of bleeds | 67 | 369 | 436 | 532 |
| Median ABR (IQR) | 2.2 (0.9;4.7) | 1.2 (0.0;3.7) | 1.2 (0.0;4.3) | 30.9 (18.6;38.5) |
| Mean ABR (SD) | 3.5 (3.9) | 2.9 (4.8) | 3.0 (4.7) | 31.9 (19.1) |
| All bleeds (treated and untreated) | ||||
| # of subjects with bleeds (%) | 19 (76) | 88 (59) | 107 (61) | 12 (100) |
| # of subjects without bleeds (%) | 6 (24) | 62 (41) | 68 (39) | 0 |
| # of bleeds* | 72 | 386 | 458 | 536 |
| Median ABR (IQR) | 2.2 (0.9;6.0) | 1.2 (0.0;4.3) | 1.2 (0.0;4.7) | 31.3 (18.6;38.9) |
| Mean ABR (SD) | 3.7 (4.1) | 3.2 (5.1) | 3.3 (4.9) | 32.2 (19.1) |
| Treated spontaneous bleeds | ||||
| # of subjects with bleeds (%) | 11 (44) | 65 (43) | 76 (43) | 12 (100) |
| # of subjects without bleeds (%) | 14 (56) | 85 (57) | 99 (57) | 0 |
| # of bleeds | 30 | 221 | 251 | 415 |
| Median AsBR (IQR) | 0.0 (0.0;1.5) | 0.0 (0.0;1.9) | 0.0 (0.0;1.8) | 19.4 (12.1;31.0) |
| Mean AsBR (SD) | 1.4 (2.4) | 1.8 (3.7) | 1.7 (3.5) | 24.5 (17.3) |
| Treated traumatic bleeds | ||||
| # of subjects with bleeds (%) | 16 (64) | 57 (38) | 73 (42) | 10 (83) |
| # of subjects without bleeds (%) | 9 (36) | 93 (62) | 102 (58) | 2 (17) |
| # of bleeds | 37 | 146 | 183 | 110 |
| Median AtBR (IQR) | 1.3 (0.0;2.6) | 0.0 (0.0;1.4) | 0.0 (0.0;1.7) | 4.3 (0.8;9.9) |
| Mean AtBR (SD) | 2.1 (2.9) | 1.1 (2.2) | 1.2 (2.3) | 6.1 (6.2) |
| Treated joint bleeds | ||||
| # of subjects with bleeds (%) | 16 (64) | 74 (49) | 90 (51) | 12 (100) |
| # of subjects without bleeds (%) | 9 (36) | 76 (51) | 85 (49) | 0 |
| # of bleeds | 37 | 288 | 325 | 309 |
| Median AjBR (IQR) | 1.2 (0.0;2.8) | 0.0(0.0;2.8) | 0.9 (0.0;2.8) | 19.4 (4.5;28.8) |
| Mean AjBR (SD) | 1.8 (2.2) | 2.3 (4.3) | 2.2 (4.1) | 19.7 (15.1) |
|
ABR = annualized bleed rate; IQR = interquartile range, 25th percentile to 75th percentile; SD = standard deviation; AsBR = annualized spontaneous bleed rate; AtBR = annualized traumatic bleed rate; AjBR = annualized joint bleed rate. *Reflects all bleeds reported by patients including those where no ESPEROCT was administered |
||||
Routine Prophylaxis In Children <12 Years Of Age
Overall, 68 children below 12 years received prophylactic treatment with ESPEROCT at an average dose of approximately 65 IU/kg twice weekly. The prophylactic effect of ESPEROCT was demonstrated with a median ABR rate of 2.0 (IQR: 0.0; 2.8) and 2.0 (IQR: 0.0; 4.2) for treated bleeds and all bleeds respectively (see Table 8). The mean ABR (SD) for treated bleeds and all bleeds were 3.1 (7.1) and 4.4 (8.7), respectively. Of the 68 children, 22 (32%) did not experience any bleeding episodes and 29 (43%) did not experience any bleeding episodes that required treatment during the Main Phase of the trial. Of the 13 subjects with 17 documented target joints at baseline, 10 subjects (77%) and 14 target joints (82%) did not have any bleeds during the Main Phase of the trial.
Table 8: Efficacy in pediatric prophylaxis, medianand mean ABR by age and bleed type
| Prophylaxis Regimen | |||
| Age range | < 6 years** | 6 to < 12 years | 0 to < 12 years |
| # of subjects | N=34 | N=34 | N=68 |
| Mean treatment duration (years) | 0.46 | 0.51 | 0.48 |
| Treated bleeds | |||
| # of subjects with bleeds (%) | 19 (56) | 20 (59) | 39 (57) |
| # of subjects without bleeds (%) | 15 (44) | 14 (41) | 29 (43) |
| # of bleeds | 30 | 40 | 70 |
| Median ABR (IQR) | 1.9 (0.0;2.1) | 2.0 (0.0;3.9) | 2.0 (0.0;2.8) |
| Mean ABR (SD) | 3.9 (9.7) | 2.3 (2.9) | 3.1 (7.1) |
| All Bleeds (treated and untreated) | |||
| # of subjects with bleeds (%) | 20 (59) | 26 (77) | 46 (68) |
| # of subjects without bleeds (%) | 14 (41) | 8 (24) | 22 (32) |
| # of bleeds* | 41 | 65 | 106 |
| Median ABR (IQR) | 2.0 (0.0;4.0) | 2.0 (1.9;6.0) | 2.0 (0.0;4.2) |
| Mean ABR (SD) | 5.0 (11.9) | 3.8 (3.6) | 4.4 (8.7) |
| Treated spontaneous bleeds | |||
| # of subjects with bleeds (%) | 6 (18) | 7 (21) | 13 (19) |
| # of subjects without bleeds (%) | 28 (82) | 27 (79) | 55 (81) |
| # of bleeds | 9 | 10 | 19 |
| Median AsBR (IQR) | 0.0 (0.0;0.0) | 0.0 (0.0; 0.0) | 0.0 (0.0; 0.0) |
| Mean AsBR (SD) | 2.1 (7.3) | 0.6 (1.5) | 1.3 (5.3) |
| Treated traumatic bleeds | |||
| # of subjects with bleeds (%) | 15 (44) | 17 (50) | 32 (47) |
| # of subjects without bleeds (%) | 19 (56) | 17 (50) | 36 (53) |
| # of bleeds | 20 | 30 | 50 |
| Median AtBR (IQR) | 0.0 (0.0; 2.0) | 0.9 (0.0;2.0) | 0.0 (0.0;2.0) |
| Mean AtBR (SD) | 1.7 (4.0) | 1.7 (2.5) | 1.7 (3.3) |
| Treated joint bleeds | |||
| # of subjects with bleeds (%) | 7 (21) | 12 (35) | 19 (28) |
| # of subjects without bleeds (%) | 27 (79) | 22 (65) | 49 (72) |
| # of bleeds | 10 | 24 | 34 |
| Median AjBR (IQR) | 0.0 (0.0;0.0) | 0.0 (0.0;2.0) | 0.0 (0.0;2.0) |
| Mean AjBR (SD) | 1.5 (6.3) | 1.4 (2.4) | 1.5 (4.7) |
| ABR = annualized bleed rate; IQR = interquartile range, 25th percentile to 75th percentile; SD = standard deviation; AsBR = annualized spontaneous bleed rate; AtBR = annualized traumatic bleed rate; AjBR = annualized joint bleed rate *Reflects all bleeds reported by patients including those where no ESPEROCT was administered **Elevated mean ABRs are due to subjects who withdrew from the study, whose bleeding rates were extrapolated to one year |
|||
Medication Guide
PATIENT INFORMATION
No information provided. Please refer to the WARNINGS AND PRECAUTIONS sections.
