Xelpros Drug Description: Detailed Datasheet
- Generic Name: latanoprost ophthalmic emulsion
- Brand Name: Xelpros
side effects drug center xelpros (latanoprost ophthalmic emulsion) drug
Drug Description
What is Xelpros and how is it used?
Xelpros (latanoprost ophthalmic emulsion) is a prostaglandin F2a analog indicated for reduction of elevated intraocular pressure in patients with open-angle glaucoma, or ocular hypertension.
What are Side effects of Xelpros?
Common side effects of Xelpros include:
- eye pain/stinging,
- ocular hyperemia,
- eye redness,
- eye discharge,
- growth of eyelashes,
- eyelash thickening,
- itchy eyes,
- dry eye,
- vision problems,
- eyelid redness or swelling, or
- feeling as if something is in the eye
DESCRIPTION
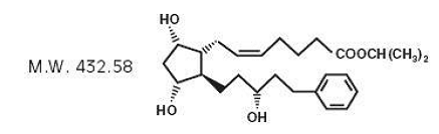
Latanoprost is a prostaglandin F2α analogue. Its chemical name is isopropyl-(Z)- 7[(1R,2R,3R,5S)3,5-dihydroxy-2-[(3R)-3-hydroxy-5-phenylpentyl]cyclopentyl]-5- heptenoate. Its molecular formula is C26H40O5 and its chemical structure is:
 |
Latanoprost is a pale yellow to yellow viscous oil that is very soluble in acetonitrile and freely soluble in acetone, ethanol, ethyl acetate, isopropanol, methanol, and octanol. It is practically insoluble in water.
XELPROS (latanoprost ophthalmic emulsion) 0.005% is a sterile, isotonic, buffered aqueous emulsion of latanoprost with a pH approximately 7.0 and an osmolality of approximately 375mOsmol/kg. Each mL of XELPROS contains 50 micrograms of latanoprost. Potassium sorbate 0.47% is added as a preservative. The inactive ingredients are: castor oil, sodium borate, boric acid, propylene glycol, edetate disodium, polyoxyl 15 hydroxystearate, sodium hydroxide, hydrochloric acid, and water for injection. One drop contains approximately 1.5 mcg of latanoprost.
Indications & Dosage
INDICATIONS
XELPROS® (latanoprost ophthalmic emulsion) 0.005% is indicated for the reduction of elevated intraocular pressure (IOP) in patients with open-angle glaucoma or ocular hypertension.
DOSAGE AND ADMINISTRATION
The recommended dosage is one drop in the affected eye(s) once daily in the evening. If one dose is missed, treatment should continue with the next dose as normal.
The dosage of XELPROS should not exceed once daily; the combined use of two or more prostaglandins, or prostaglandin analogs including XELPROS is not recommended. It has been shown that administration of these prostaglandin drug products more than once daily may decrease the intraocular pressure (IOP) lowering effect or cause paradoxical elevations in IOP.
Reduction of the intraocular pressure starts approximately 3 to 4 hours after administration and the maximum effect is reached after 8 to 12 hours.
XELPROS may be used concomitantly with other topical ophthalmic drug products to lower intraocular pressure. If more than one topical ophthalmic drug is being used, the drugs should be administered at least five (5) minutes apart. Contact lenses should be removed prior to the administration of XELPROS, and may be reinserted 15 minutes after administration.
HOW SUPPLIED
Dosage Forms And Strengths
Ophthalmic emulsion containing latanoprost 50 mcg/mL (0.005%).
Storage And Handling
XELPROS (latanoprost ophthalmic emulsion) is supplied as an off-white to pale yellow, translucent, isotonic, sterile, buffered emulsion of latanoprost 0.005% (50 mcg/mL). It is supplied as a 2.5 mL emulsion filled in a 5-mL clear low density polyethylene bottle with a clear low density polyethylene dropper tip, and a turquoise high density polyethylene pilfer-proof cap. Each mL contains 50 mcg of latanoprost.
2.5 mL fill, 0.005% (50 mcg/mL)
Package of 1 bottle: NDC 47335-317-90
Multi-Pack of 3 bottles: NDC 47335-317-92
Storage
Protect from light. Store at 2°C to 25°C (36°F to 77°F). During shipment to the patient, the bottle may be maintained at temperatures up to 40°C (104°F) for a period not exceeding 8 days. After opening, XELPROS can be used until the expiration date stamped on bottle and then discarded.
Manufactured By: Sun Pharmaceutical Industries, Ltd. Halol-Baroda Highway Halol-389, Gujarat, India. Revised: Sep 2018
Side Effects & Drug Interactions
SIDE EFFECTS
The following adverse reactions were reported in postmarketing experience and are discussed in greater detail in other sections of the label:
- Iris pigmentation changes [see WARNINGS AND PRECAUTIONS]
- Eyelid skin darkening [see WARNINGS AND PRECAUTIONS]
- Eyelash changes (increased length, thickness, pigmentation, and number of lashes) [see WARNINGS AND PRECAUTIONS]
- Intraocular inflammation (iritis/uveitis) [see WARNINGS AND PRECAUTIONS]
- Macular edema, including cystoid macular edema [see WARNINGS AND PRECAUTIONS]
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly compared to rates in the clinical studies of another drug and may not reflect the rates observed in clinical practice.
Across multiple clinical trials conducted with XELPROS (latanoprost ophthalmic emulsion) 0.005%, the most frequently reported ocular adverse reactions were eye pain/stinging upon instillation and ocular hyperemia, reported in 55% and 41% of XELPROS treated patients, respectively (Table 1). Less than 1% of patients discontinued therapy because of intolerance to the eye pain/stinging or to the ocular hyperemia.
Table 1. Ocular Adverse Reactions Reported by ≥ 1% of Subjects Receiving XELPROS
| System Organ Class/ Preferred Term | XELPROS (N = 448) |
| Eye disorders | 325 (73%) |
| Eye pain / stinging | 246 (55%) |
| Ocular hyperemia | 185 (41%) |
| Conjunctival hyperemia | 65 (15%) |
| Eye discharge | 53 (12%) |
| Growth of eyelashes | 47 (11%) |
| Eyelash thickening | 35 (8%) |
| Ocular Itching | 20 (5%) |
| Visual acuity reduced | 16 (4%) |
| Dry eye | 13 (3%) |
| Erythema of eyelid | 14 (3%) |
| Foreign body sensation in eyes | 9 (2%) |
| Punctate keratitis | 6 (1%) |
| Eyelash discoloration | 5 (1%) |
| Eyelid edema | 7 (2%) |
| Conjunctival edema | 5 (1%) |
Postmarketing Experience
The following reactions have been identified during postmarketing use of topical latanoprost products in clinical practice. Because they are reported voluntarily from a population of unknown size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
The reactions, which have been chosen for inclusion due to either their seriousness, frequency of reporting, possible causal connection to latanoprost ophthalmic emulsion or a combination of these factors, include:
- Nervous System Disorders: Dizziness, headache, toxic epidermal necrolysis
- Eye Disorders: Corneal edema and erosions; intraocular inflammation (iritis/uveitis); macular edema, including cystoid macular edema; trichiasis; periorbital and lid changes resulting in deepening of the eyelid sulcus; iris cyst; eyelid skin darkening; localized skin reaction on the eyelids; conjunctivitis; pseudopemphigoid of the ocular conjunctiva
- Respiratory, Thoracic and Mediastinal Disorders: Asthma and exacerbation of asthma; dyspnea
- Skin and Subcutaneous Tissue Disorders: Pruritus
- Infections and Infestations: Herpes keratitis
- Cardiac Disorders: Angina; palpitations; angina unstable
- General Disorders and Administration Site Conditions: Chest pain
DRUG INTERACTIONS
In vitro studies have shown that precipitation occurs when eye drops containing thimerosal are mixed with XELPROS. If such drugs are used, they should be administered at least five (5) minutes apart.
The combined use of two or more prostaglandins, or prostaglandin analogs including XELPROS is not recommended. It has been shown that administration of these prostaglandin drug products more than once daily may decrease the IOP lowering effect or cause paradoxical elevations in IOP.
Warnings & Precautions
WARNINGS
Included as part of the "PRECAUTIONS" Section
PRECAUTIONS
Pigmentation
Topical latanoprost ophthalmic products, including XELPROS, have been reported to cause changes to pigmented tissues. The most frequently reported changes have been increased pigmentation of the iris, periorbital tissue (eyelid) and eyelashes. Pigmentation is expected to increase as long as latanoprost is administered.
The pigmentation change is due to increased melanin content in the melanocytes rather than to an increase in the number of melanocytes. After discontinuation of latanoprost, pigmentation of the iris is likely to be permanent, while pigmentation of the periorbital tissue and eyelash changes have been reported to be reversible in some patients. Patients who receive treatment should be informed of the possibility of increased pigmentation. The long-term effects of increased pigmentation are not known.
Iris color change may not be noticeable for several months to years. Typically, the brown pigmentation around the pupil spreads concentrically towards the periphery of the iris and the entire iris or parts of the iris become more brownish. Neither nevi nor freckles of the iris appear to be affected by treatment. While treatment with XELPROS can be continued in patients who develop noticeably increased iris pigmentation, these patients should be examined regularly. [see PATIENT INFORMATION].
Eyelash Changes
Latanoprost ophthalmic products, including XELPROS, may gradually change eyelashes and vellus hair in the treated eye; these changes include increased length, thickness, pigmentation, the number of lashes or hairs, and misdirected growth of eyelashes. Eyelash changes are usually reversible upon discontinuation of treatment. [see PATIENT INFORMATION].
Intraocular Inflammation
XELPROS should be used with caution in patients with a history of intraocular inflammation (iritis/uveitis) and should generally not be used in patients with active intraocular inflammation because inflammation may be exacerbated.
Macular Edema
Macular edema, including cystoid macular edema, has been reported during treatment with latanoprost ophthalmic products, including XELPROS. XELPROS should be used with caution in aphakic patients, in pseudophakic patients with a torn posterior lens capsule, or in patients with known risk factors for macular edema.
Herpetic Keratitis
Reactivation of herpes simplex keratitis has been reported during treatment with latanoprost. XELPROS should be used with caution in patients with a history of herpetic keratitis. XELPROS should be avoided in cases of active herpes simplex keratitis because inflammation may be exacerbated.
Bacterial Keratitis
There have been reports of bacterial keratitis associated with the use of multiple-dose containers of topical ophthalmic products. These containers had been inadvertently contaminated by patients who, in most cases, had a concurrent corneal disease or a disruption of the ocular epithelial surface [see PATIENT INFORMATION].
Use With Contact Lens
Contact lenses should be removed prior to the administration of XELPROS and may be reinserted 15 minutes after administration [see PATIENT INFORMATION].
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment Of Fertility
Latanoprost was not carcinogenic in either mice or rats when administered by oral gavage at doses of up to 170 mg/kg/day (approximately 2,800 times the recommended maximum human dose) for up to 20 and 24 months, respectively.
Latanoprost was not mutagenic in bacteria, in mouse lymphoma, or in mouse micronucleus tests. Chromosome aberrations were observed in vitro with human lymphocytes. Additional in vitro and in vivo studies on unscheduled DNA synthesis in rats were negative.
Latanoprost has not been found to have any effect on male or female fertility in animal studies.
Use In Specific Populations
Pregnancy
Pregnancy Category C
Reproduction studies have been performed in rats and rabbits. In rabbits, an incidence of 4 of 16 dams had no viable fetuses at a dose that was approximately 80 times the maximum human dose, and the highest nonembryocidal dose in rabbits was approximately 15 times the maximum human dose. There are no adequate and well-controlled studies in pregnant women. XELPROS should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Nursing Mothers
It is not known whether latanoprost or its metabolites are excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when XELPROS is administered to a nursing woman.
Pediatric Use
Safety and effectiveness in pediatric patients have not been established.
Geriatric Use
No overall differences in safety or effectiveness have been observed between elderly and younger patients.
Overdosage & Contraindications
OVERDOSE
Intravenous infusion of up to 3 mcg/kg in healthy volunteers produced mean plasma concentrations 200 times higher than during clinical treatment and no adverse reactions were observed. Intravenous dosages of 5.5 to 10 mcg/kg caused abdominal pain, dizziness, fatigue, hot flashes, nausea, and sweating.
If overdosage with XELPROS occurs, treatment should be symptomatic.
CONTRAINDICATIONS
Known hypersensitivity to latanoprost, or any other ingredients in this product.
Clinical Pharmacology
Mechanism Of Action
Latanoprost is a prostaglandin F2α analogue that is believed to reduce the intraocular pressure (IOP) by increasing the outflow of aqueous humor. Studies in animals and man suggest that the main mechanism of action is increased uveoscleral outflow. Elevated IOP represents a major risk factor for glaucomatous field loss. The higher the level of IOP, the greater the likelihood of optic nerve damage and visual field loss.
Pharmacodynamics
Reduction of the IOP in man starts about 3-4 hours after administration and maximum effect is reached after 8-12 hours. IOP reduction is present for at least 24 hours.
Pharmacokinetics
Absorption
Latanoprost is absorbed through the cornea where the isopropyl ester prodrug is hydrolyzed to the acid form to become biologically active.
Distribution
The distribution volume in humans is 0.16 ± 0.02 L/kg. The acid of latanoprost can be measured in aqueous humor during the first 4 hours and in plasma only during the first hour after local administration. Studies in man indicate that the peak concentration in the aqueous humor is reached about 2 hours after topical administration.
Metabolism
Latanoprost, an isopropyl ester prodrug, is hydrolyzed by esterases in the cornea to the biologically active acid. The active acid of latanoprost reaching the systemic circulation is primarily metabolized by the liver to the 1,2-dinor and 1,2,3,4- tetranor metabolites via fatty acid β-oxidation.
Excretion
The elimination of the acid of latanoprost from human plasma is rapid (t1/2 =17 min) after both intravenous and topical administration. Systemic clearance is approximately 7 mL/min/kg. Following hepatic β-oxidation, the metabolites are mainly eliminated via the kidneys. Approximately 88% and 98% of the administered dose are recovered in the urine after topical and intravenous dosing, respectively.
Clinical Studies
Elevated Baseline IOP
In randomized, controlled clinical trials of patients with open angle glaucoma or ocular hypertension with mean baseline IOP of 23 - 26 mmHg, the mean IOP-lowering effect of XELPROS administered once daily in the evening was up to 6 - 8 mmHg.
Medication Guide
PATIENT INFORMATION
Potential For Pigmentation
Advise patients about the potential for increased brown pigmentation of the iris, which may be permanent. Patients should also be informed about the possibility of eyelid skin darkening, which may be reversible after discontinuation of XELPROS [see WARNINGS AND PRECAUTIONS].
Potential For Eyelash Changes
Inform patients of the possibility of eyelash and vellus hair changes in the treated eye during treatment with latanoprost ophthalmic emulsion. These changes may result in a disparity between eyes in length, thickness, pigmentation, number of eyelashes or vellus hairs, and/or direction of eyelash growth. Eyelash changes are usually reversible upon discontinuation of treatment [see WARNINGS AND PRECAUTIONS].
Handling The Container
Instruct patients to avoid allowing the tip of the dispensing container to contact the eye or surrounding structures because this could cause the tip to become contaminated by common bacteria known to cause ocular infections. Serious damage to the eye and subsequent loss of vision may result from using contaminated emulsions [see WARNINGS AND PRECAUTIONS].
When To Seek Physician Advice
Advise patients that if they develop an intercurrent ocular condition (e.g., trauma or infection) or have ocular surgery, or develop any ocular reactions, particularly conjunctivitis and eyelid reactions, they should immediately seek their physician’s advice concerning the continued use of the multiple-dose container.
Use With Contact Lenses
Advise patients that contact lenses should be removed prior to administration of the emulsion. Lenses may be reinserted 15 minutes following administration of XELPROS [see WARNINGS AND PRECAUTIONS].
Use With Other Ophthalmic Drugs
Advise patients that if more than one topical ophthalmic drug is being used, the drugs should be administered at least five (5) minutes apart [see DOSAGE AND ADMINISTRATION].
