Ambisome Side Effects Center
- Generic Name: amphotericin b
- Brand Name: Ambisome
Ambisome (Amphotericin B) side effects drug center
Ambisome Side Effects Center
What Is Ambisome?
AmBisome (amphotericin B) Liposome for Injection is an antifungal antibiotic used to treat serious, life-threatening fungal infections, including a certain form of meningitis in people infected with HIV (human immunodeficiency virus), and is usually given after other antifungal antibiotics have been tried without successful treatment of symptoms.
What Are Side Effects of AmBisome?
Common side effects of AmBisome include:
- fever,
- shaking,
- chills,
- flushing (warmth, redness, or tingly feeling),
- loss of appetite,
- dizziness,
- nausea,
- vomiting,
- stomach pain,
- diarrhea,
- headache,
- shortness of breath,
- fast breathing 1 to 2 hours after the infusion is started,
- sleep problems (insomnia), or
- skin rash.
Tell your doctor if you have serious side effects of AmBisome including:
- swelling or pain at injection site,
- muscle or joint pain,
- unusual tiredness,
- weakness,
- muscle cramping,
- changes in the amount of urine,
- painful urination,
- numbness or tingling of arms or legs,
- vision changes,
- hearing changes (e.g., ringing in the ears),
- dark urine,
- severe stomach or abdominal pain,
- yellowing eyes or skin,
- swelling ankles or feet,
- fast/slow/irregular heartbeat,
- cold sweats,
- blue lips,
- easy bruising or bleeding,
- other signs of infection (e.g., fever, persistent sore throat),
- mental/mood changes,
- seizures,
- black stools, or
- vomit that looks like coffee grounds.
Dosage for AmBisome
AmBisome is administered by intravenous infusion, using a controlled infusion device, over a period of approximately 2 hours. Dose is based on the patient's weight.
What Drugs, Substances, or Supplements Interact with AmBisome?
AmBisome may interact with flucytosine, digoxin, pentamidine, tacrolimus, muscle relaxers, steroids, antifungal antibiotics, antibiotics, antiviral medicines, or cancer medicines. Tell your doctor all medications you use.
AmBisome During Pregnancy and Breastfeeding
During pregnancy, AmBisome should be used only when prescribed. It is unknown if this drug passes into breast milk. Consult your doctor before breastfeeding.
Additional Information
Our AmBisome (amphotericin B) Side Effects Drug Center provides a comprehensive view of available drug information on the potential side effects when taking this medication.
Ambisome Consumer Information
Get emergency medical help if you have signs of an allergic reaction: hives; difficult breathing; swelling of your face, lips, tongue, or throat.
Some side effects may occur during the injection. Tell your caregiver right away if you feel dizzy, nauseated, light-headed, sweaty, hot or cold, or if you have a fast heartbeat, chest tightness, or trouble breathing.
Call your doctor at once if you have:
- kidney problems--little or no urination, swelling in your feet or ankles, feeling tired or short of breath;
- low calcium level--muscle spasms or contractions, numbness or tingly feeling (around your mouth, or in your fingers and toes);
- low magnesium--dizziness, irregular heartbeats, feeling jittery, muscle cramps, muscle spasms, cough or choking feeling; or
- low potassium level--leg cramps, constipation, irregular heartbeats, fluttering in your chest, increased thirst or urination, numbness or tingling, muscle weakness or limp feeling.
Common side effects may include:
- stomach pain, nausea, vomiting, diarrhea;
- trouble breathing;
- chills;
- weakness; or
- rash.
This is not a complete list of side effects and others may occur. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
Read the entire detailed patient monograph for Ambisome (Amphotericin B)
Ambisome Professional Information
SIDE EFFECTS
The following adverse events are based on the experience of 592 adult patients (295 treated with AmBisome and 297 treated with amphotericin B deoxycholate) and 95 pediatric patients (48 treated with AmBisome and 47 treated with amphotericin B deoxycholate) in Study 94-0-002, a randomized double-blind, multi-center study in febrile, neutropenic patients. AmBisome and amphotericin B were infused over two hours.
The incidence of common adverse events (incidence of 10% or greater) occurring with AmBisome compared to amphotericin B deoxycholate, regardless of relationship to study drug, is shown in the following table:
Empirical Therapy Study 94-0-002 Common Adverse Events
| Adverse Event by Body System | AmBisome N = 343 % | Amphotericin BN = 344 % |
| Body as a Whole | ||
| Abdominal pain | 19.8 | 21.8 |
| Asthenia | 13.1 | 10.8 |
| Back pain | 12 | 7.3 |
| Blood product transfusion reaction | 18.4 | 18.6 |
| Chills | 47.5 | 75.9 |
| Infection | 11.1 | 9.3 |
| Pain | 14 | 12.8 |
| Sepsis | 14 | 11.3 |
| Cardiovascular System | ||
| Chest pain | 12 | 11.6 |
| Hypertension | 7.9 | 16.3 |
| Hypotension | 14.3 | 21.5 |
| Tachycardia | 13.4 | 20.9 |
| Digestive System | ||
| Diarrhea | 30.3 | 27.3 |
| Gastrointestinal hemorrhage | 9.9 | 11.3 |
| Nausea | 39.7 | 38.7 |
| Vomiting | 31.8 | 43.9 |
| Metabolic and Nutritional Disorders | ||
| Alkaline phosphatase increased | 22.2 | 19.2 |
| ALT (SGPT) increased | 14.6 | 14 |
| AST (SGOT) increased | 12.8 | 12.8 |
| Bilirubinemia | 18.1 | 19.2 |
| BUN increased | 21 | 31.1 |
| Creatinine increased | 22.4 | 42.2 |
| Edema | 14.3 | 14.8 |
| Hyperglycemia | 23 | 27.9 |
| Hypernatremia | 4.1 | 11 |
| Hypervolemia | 12.2 | 15.4 |
| Hypocalcemia | 18.4 | 20.9 |
| Hypokalemia | 42.9 | 50.6 |
| Hypomagnesemia | 20.4 | 25.6 |
| Peripheral edema | 14.6 | 17.2 |
| Nervous System | ||
| Anxiety | 13.7 | 11 |
| Confusion | 11.4 | 13.4 |
| Headache | 19.8 | 20.9 |
| Insomnia | 17.2 | 14.2 |
| Respiratory System | ||
| Cough increased | 17.8 | 21.8 |
| Dyspnea | 23 | 29.1 |
| Epistaxis | 14.9 | 20.1 |
| Hypoxia | 7.6 | 14.8 |
| Lung disorder | 17.8 | 17.4 |
| Pleural effusion | 12.5 | 9.6 |
| Rhinitis | 11.1 | 11 |
| Skin and Appendages | ||
| Pruritus | 10.8 | 10.2 |
| Rash | 24.8 | 24.4 |
| Sweating | 7 | 10.8 |
| Urogenital System | ||
| Hematuria | 14 | 14 |
AmBisome was well tolerated. AmBisome had a lower incidence of chills, hypertension, hypotension, tachycardia, hypoxia, hypokalemia, and various events related to decreased kidney function as compared to amphotericin B deoxycholate.
In pediatric patients (16 years of age or less) in this double-blind study, AmBisome compared to amphotericin B deoxycholate, had a lower incidence of hypokalemia (37% versus 55%), chills (29% versus 68%), vomiting (27% versus 55%), and hypertension (10% versus 21%). Similar trends, although with a somewhat lower incidence, were observed in open-label, randomized Study 104-14 involving 205 febrile neutropenic pediatric patients (141 treated with AmBisome and 64 treated with amphotericin B deoxycholate). Pediatric patients appear to have more tolerance than older individuals for the nephrotoxic effects of amphotericin B deoxycholate.
The following adverse events are based on the experience of 244 patients (202 adult and 42 pediatric patients) of whom 85 patients were treated with AmBisome 3 mg/kg, 81 patients were treated with AmBisome 5 mg/kg and 78 patients were treated with amphotericin B lipid complex 5 mg/kg in Study 97-0-034, a randomized, double-blind, multi-center study in febrile, neutropenic patients. AmBisome and amphotericin B lipid complex were infused over two hours. The incidence of adverse events occurring in more than 10% of subjects in one or more arms, regardless of relationship to study drug, are summarized in the following table:
Empirical Therapy Study 97-0-034 Common Adverse Events
| Adverse Event byBody System | AmBisome 3 mg/kg/day N = 85 % | AmBisome 5 mg/kg/day N = 81 % | Amphotericin BLipid Complex 5 mg/kg/day N = 78 % |
| Body as a Whole | |||
| Abdominal pain | 12.9 | 9.9 | 11.5 |
| Asthenia | 8.2 | 6.2 | 11.5 |
| Chills/rigors | 40 | 48.1 | 89.7 |
| Sepsis | 12.9 | 7.4 | 11.5 |
| Transfusion reaction | 10.6 | 8.6 | 5.1 |
| Cardiovascular System | |||
| Chest pain | 8.2 | 11.1 | 6.4 |
| Hypertension | 10.6 | 19.8 | 23.1 |
| Hypotension | 10.6 | 7.4 | 19.2 |
| Tachycardia | 9.4 | 18.5 | 23.1 |
| Digestive System | |||
| Diarrhea | 15.3 | 17.3 | 14.1 |
| Nausea | 25.9 | 29.6 | 37.2 |
| Vomiting | 22.4 | 25.9 | 30.8 |
| Metabolic and Nutritional Disorders | |||
| Alkaline phosphatase increased | 7.1 | 8.6 | 12.8 |
| Bilirubinemia | 16.5 | 11.1 | 11.5 |
| BUN increased | 20 | 18.5 | 28.2 |
| Creatinine increased | 20 | 18.5 | 48.7 |
| Edema | 12.9 | 12.3 | 12.8 |
| Hyperglycemia | 8.2 | 8.6 | 14.1 |
| Hypervolemia | 8.2 | 11.1 | 14.1 |
| Hypocalcemia | 10.6 | 4.9 | 5.1 |
| Hypokalemia | 37.6 | 43.2 | 39.7 |
| Hypomagnesemia | 15.3 | 25.9 | 15.4 |
| Liver function tests abnormal | 10.6 | 7.4 | 11.5 |
| Nervous System | |||
| Anxiety | 10.6 | 7.4 | 9 |
| Confusion | 12.9 | 8.6 | 3.8 |
| Headache | 9.4 | 17.3 | 10.3 |
| Respiratory System | |||
| Dyspnea | 17.6 | 22.2 | 23.1 |
| Epistaxis | 10.6 | 8.6 | 14.1 |
| Hypoxia | 7.1 | 6.2 | 20.5 |
| Lung disorder | 14.1 | 13.6 | 15.4 |
| Skin and Appendages | |||
| Rash | 23.5 | 22.2 | 14.1 |
The following adverse events are based on the experience of 267 patients (266 adult patients and 1 pediatric patient) of whom 86 patients were treated with AmBisome 3 mg/kg, 94 patients were treated with AmBisome 6 mg/kg and 87 patients were treated with amphotericin B deoxycholate 0.7 mg/kg in Study 94-0-013 a randomized, double-blind, comparative multi-center trial, in the treatment of cryptococcal meningitis in HIV-positive patients. The incidence of adverse events occurring in more than 10% of subjects in one or more arms regardless of relationship to study drug are summarized in the following table:
Cryptococcal Meningitis Therapy Study 94-0-013 Common Adverse Events
| Adverse Event by BodySystem | AmBisome 3 mg/kg/day N = 86 % | AmBisome 6 mg/kg/day N = 94 % | Amphotericin B 0.7 mg/kg/day N = 87 % |
| Body as a Whole | |||
| Abdominal pain | 7 | 7.4 | 10.3 |
| Infection | 12.8 | 11.7 | 6.9 |
| Procedural Complication | 8.1 | 9.6 | 10.3 |
| Cardiovascular System | |||
| Phlebitis | 9.3 | 10.6 | 25.3 |
| Digestive System | |||
| Anorexia | 14 | 9.6 | 11.5 |
| Constipation | 15.1 | 14.9 | 20.7 |
| Diarrhea | 10.5 | 16 | 10.3 |
| Nausea | 16.3 | 21.3 | 25.3 |
| Vomiting | 10.5 | 21.3 | 20.7 |
| Hemic and Lymphatic System | |||
| Anemia | 26.7 | 47.9 | 43.7 |
| Leukopenia | 15.1 | 17 | 17.2 |
| Thrombocytopenia | 5.8 | 12.8 | 6.9 |
| Metabolic and Nutritional Disorders | |||
| Bilirubinemia | 0 | 8.5 | 12.6 |
| BUN increased | 9.3 | 7.4 | 10.3 |
| Creatinine increased | 18.6 | 39.4 | 43.7 |
| Hyperglycemia | 9.3 | 12.8 | 17.2 |
| Hypocalcemia | 12.8 | 17 | 13.8 |
| Hypokalemia | 31.4 | 51.1 | 48.3 |
| Hypomagnesemia | 29.1 | 48.9 | 40.2 |
| Hyponatremia | 11.6 | 8.5 | 9.2 |
| Liver Function Tests Abnormal | 12.8 | 4.3 | 9.2 |
| Nervous System | |||
| Dizziness | 7 | 8.5 | 10.3 |
| Insomnia | 22.1 | 17 | 20.7 |
| Respiratory System | |||
| Cough Increased | 8.1 | 2.1 | 10.3 |
| Skin and Appendages | |||
| Rash | 4.7 | 11.7 | 4.6 |
Infusion-Related Reactions
In Study 94-0-002, the large, double-blind study of pediatric and adult febrile neutropenic patients, no premedication to prevent infusion-related reaction was administered prior to the first dose of study drug (Day 1). AmBisome-treated patients had a lower incidence of infusion-related fever (17% versus 44%), chills/rigors (18% versus 54%) and vomiting (6% versus 8%) on Day 1 as compared to amphotericin B deoxycholate-treated patients.
The incidence of infusion-related reactions on Day 1 in pediatric and adult patients is summarized in the following table:
Incidence of Day 1 Infusion-Related Reactions (IRR) By Patient Age
| Pediatric Patients (≤ 16 years of age) | Adult Patients (> 16 years of age) | |||
| AmBisome 3 mg/kg/day | Amphotericin B 0.6 mg/kg/day | AmBisome 3 mg/kg/day | Amphotericin B 0.6 mg/kg/day | |
| Total number of patients receiving at least one dose of study drug | 48 | 47 | 295 | 297 |
| Patients with fever* increase ≥ 1.0°C | 6 (13%) | 22 (47%) | 52 (18%) | 128 (43%) |
| Patients with chills/rigors | 4 (8%) | 22 (47%) | 59 (20%) | 165 (56%) |
| Patients with nausea | 4 (8%) | 4 (9%) | 38 (13%) | 31 (10%) |
| Patients with vomiting | 2 (4%) | 7 (15%) | 19 (6%) | 21 (7%) |
| Patients with other reactions | 10 (21%) | 13 (28%) | 47 (16%) | 69 (23%) |
| * Day 1 body temperature increased above the temperature taken within 1 hour prior to infusion (preinfusion temperature) or above the lowest infusion value (no preinfusion temperature recorded). | ||||
Cardiorespiratory events, except for vasodilatation (flushing), during all study drug infusions were more frequent in amphotericin B-treated patients as summarized in the following table:
Incidence of Infusion-Related Cardiorespiratory Events
| Event | AmBisome 3 mg/kg/day N = 343 | Amphotericin B 0.6 mg/kg/day N = 344 |
| Hypotension | 12 (3.5%) | 28 (8.1%) |
| Tachycardia | 8 (2.3%) | 43 (12.5%) |
| Hypertension | 8 (2.3%) | 39 (11.3%) |
| Vasodilatation | 18 (5.2%) | 2 (0.6%) |
| Dyspnea | 16 (4.7%) | 25 (7.3%) |
| Hyperventilation | 4 (1.2%) | 17 (4.9%) |
| Hypoxia | 1 (0.3%) | 22 (6.4%) |
The percentage of patients who received drugs either for the treatment or prevention of infusion related reactions (e.g., acetaminophen, diphenhydramine, meperidine and hydrocortisone) was lower in AmBisome-treated patients compared with amphotericin B deoxycholate-treated patients.
In the empirical therapy study 97-0-034, on Day 1, where no premedication was administered, the overall incidence of infusion-related events of chills/rigors was significantly lower for patients administered AmBisome compared with amphotericin B lipid complex. Fever, chills/rigors and hypoxia were significantly lower for each AmBisome group compared with the amphotericin B lipid complex group. The infusion-related event hypoxia was reported for 11.5% of amphotericin B lipid complex-treated patients compared with 0% of patients administered 3 mg/kg per day AmBisome and 1.2% of patients treated with 5 mg/kg per day AmBisome.
Incidence of Day 1 Infusion-Related Reactions (IRR) Chills/Rigors Empirical Therapy Study 97-0-034
| AmBisome | Amphotericin Blipid complex5 mg/kg/day | |||
| 3 mg/kg/day | 5 mg/kg/day | BOTH | ||
| Total number of patients | 85 | 81 | 166 | 78 |
| Patients with Chills/Rigors (Day 1) | 16 (18.8%) | 19 (23.5%) | 35 (21.1%) | 62 (79.5%) |
| Patients with other notable reactions: | ||||
| Fever(≥1.0°C increase in temperature) | 20 (23.5%) | 16 (19.8%) | 36 (21.7%) | 45 (57.7%) |
| Nausea | 9 (10.6%) | 7 (8.6%) | 16 (9.6%) | 9 (11.5%) |
| Vomiting | 5 (5.9%) | 5 (6.2%) | 10 (6%) | 11 (14.1%) |
| Hypertension | 4 (4.7%) | 7 (8.6%) | 11 (6.6%) | 12 (15.4%) |
| Tachycardia | 2 (2.4%) | 8 (9.9%) | 10 (6%) | 14 (17.9%) |
| Dyspnea | 4 (4.7%) | 8 (9.9%) | 12 (7.2%) | 8 (10.3%) |
| Hypoxia | 0 | 1 (1.2%) | 1 (< 1%) | 9 (11.5%) |
| Day 1 body temperature increased above the temperature taken within 1 hour prior to infusion (preinfusion temperature) or above the lowest infusion value (no preinfusion temperature recorded). Patients were not administered premedications to prevent infusion-related reactions prior to the Day 1 study drug infusion. | ||||
In Study 94-0-013, a randomized, double-blind, multicenter trial comparing AmBisome and amphotericin B deoxycholate as initial therapy for cryptococcal meningitis, premedications to prevent infusion-related reactions were permitted. AmBisome-treated patients had a lower incidence of fever, chills/rigors and respiratory adverse events as summarized in the following table:
Incidence of Infusion-Related Reactions Study 94-0-013
| AmBisome 3 mg/kg/day | AmBisome 6 mg/kg/day | Amphotericin B 0.7 mg/kg/day | |
| Total number of patients receiving at least one dose of study drug | 86 | 94 | 87 |
| Patients with fever increase of >1°C | 6 (7%) | 8 (9%) | 24 (28%) |
| Patients with chills/rigors | 5 (6%) | 8 (9%) | 42 (48%) |
| Patients with nausea | 11 (13%) | 13 (14%) | 18 (20%) |
| Patients with vomiting | 14 (16%) | 13 (14%) | 16 (18%) |
| Respiratory adverse events | 0 | 1 (1%) | 8 (9%) |
There have been a few reports of flushing, back pain with or without chest tightness, and chest pain associated with AmBisome administration; on occasion this has been severe. Where these symptoms were noted, the reaction developed within a few minutes after the start of infusion and disappeared rapidly when the infusion was stopped. The symptoms do not occur with every dose and usually do not recur on subsequent administrations when the infusion rate is slowed.
Toxicity And Discontinuation Of Dosing
In Study 94-0-002, a significantly lower incidence of grade 3 or 4 toxicity was observed in the AmBisome group compared with the amphotericin B group. In addition, nearly three times as many patients administered amphotericin B required a reduction in dose due to toxicity or discontinuation of study drug due to an infusion-related reaction compared with those administered AmBisome.
In empirical therapy study 97-0-034, a greater proportion of patients in the amphotericin B lipid complex group discontinued the study drug due to an adverse event than in the AmBisome groups.
Less Common Adverse Events
The following adverse events also have been reported in 2% to 10% of AmBisome-treated patients receiving chemotherapy or bone marrow transplantation, or who had HIV disease in six comparative, clinical trials:
Body As A Whole
Abdomen enlarged, allergic reaction, cellulitis, cell-mediated immunological reaction, face edema, graft-versus-host disease, malaise, neck pain, and procedural complication.
Cardiovascular System
Arrhythmia, atrial fibrillation, bradycardia, cardiac arrest, cardiomegaly, hemorrhage, postural hypotension, valvular heart disease, vascular disorder, and vasodilatation (flushing).
Digestive System
Anorexia, constipation, dry mouth/nose, dyspepsia, dysphagia, eructation, fecal incontinence, flatulence, hemorrhoids, gum/oral hemorrhage, hematemesis, hepatocellular damage, hepatomegaly, liver function test abnormal, ileus, mucositis, rectal disorder, stomatitis, ulcerative stomatitis, and veno-occlusive liver disease.
Hemic & Lymphatic System
Anemia, coagulation disorder, ecchymosis, fluid overload, petechia, prothrombin decreased, prothrombin increased, and thrombocytopenia.
Metabolic & Nutritional Disorders
Acidosis, amylase increased, hyperchloremia, hyperkalemia, hypermagnesemia, hyperphosphatemia, hyponatremia, hypophosphatemia,hypoproteinemia, lactate dehydrogenase increased, nonprotein nitrogen (NPN) increased, and respiratory alkalosis.
Musculoskeletal System
Arthralgia, bone pain, dystonia, myalgia, and rigors.
Nervous System
Agitation, coma, convulsion, cough, depression, dysesthesia, dizziness, hallucinations, nervousness, paresthesia, somnolence, thinking abnormality, and tremor.
Respiratory System
Asthma, atelectasis, hemoptysis, hiccup, hyperventilation, influenza-like symptoms, lung edema, pharyngitis, pneumonia, respiratory insufficiency, respiratory failure, and sinusitis.
Skin & Appendages
Alopecia, dry skin, herpes simplex, injection site inflammation, maculopapular rash, purpura, skin discoloration, skin disorder, skin ulcer, urticaria, and vesiculobullous rash.
Special Senses
Conjunctivitis, dry eyes, and eye hemorrhage.
Urogenital System
Abnormal renal function, acute kidney failure, acute renal failure, dysuria, kidney failure, toxic nephropathy, urinary incontinence, and vaginal hemorrhage.
Post-Marketing Experience
The following infrequent adverse experiences have been reported in post-marketing surveillance, in addition to those mentioned above: angioedema, erythema, urticaria, bronchospasm, cyanosis/hypoventilation, pulmonary edema, agranulocytosis, hemorrhagic cystitis, and rhabdomyolysis.
Clinical Laboratory Values
The effect of AmBisome on renal and hepatic function and on serum electrolytes was assessed from laboratory values measured repeatedly in Study 94-0-002. The frequency and magnitude of hepatic test abnormalities were similar in the AmBisome and amphotericin B groups. Nephrotoxicity was defined as creatinine values increasing 100% or more over pretreatment levels in pediatric patients, and creatinine values increasing 100% or more over pretreatment levels in adult patients, provided the peak creatinine concentration was > 1.2 mg/dL. Hypokalemia was defined as potassium levels ≤ 2.5 mmol/L any time during treatment.
Incidence of nephrotoxicity, mean peak serum creatinine concentration, mean change from baseline in serum creatinine, and incidence of hypokalemia in the double-blind, randomized study were lower in the AmBisome group as summarized in the following table:
Study 94-0-002 Laboratory Evidence of Nephrotoxicity
| AmBisome 3 mg/kg/day | Amphotericin B 0.6 mg/kg/day | |
| Total number of patients receiving at least one dose of study drug | 343 | 344 |
| Nephrotoxicity | 64 (18.7%) | 116 (33.7%) |
| Mean peak creatinine | 1.24 mg/dL | 1.52 mg/dL |
| Mean change from baseline in creatinine | 0.48 mg/dL | 0.77 mg/dL |
| Hypokalemia | 23 (6.7%) | 40 (11.6%) |
The effect of AmBisome (3 mg/kg/day) vs. amphotericin B (0.6 mg/kg/day) on renal function in adult patients enrolled in this study is illustrated in the following figure:
Mean Change in Creatinine Over Time in Study 94-0-002
 |
In empirical therapy study 97-0-034, the incidence of nephrotoxicity as measured by increases of serum creatinine from baseline was significantly lower for patients administered AmBisome (individual dose groups and combined) compared with amphotericin B lipid complex.
Incidence of Nephrotoxicity Empirical Therapy Study 97-0-034
| AmBisome | Amphotericin Blipid complex 5 mg/kg/day | |||
| 3 mg/kg/day | 5 mg/kg/day | BOTH | ||
| Total number of patients | 85 | 81 | 166 | 78 |
| Number with nephrotoxicity | ||||
| 1.5X baseline serum creatinine value | 25 (29.4%) | 21 (25.9%) | 46 (27.7%) | 49 (62.8%) |
| 2X baseline serum creatinine value | 12 (14.1%) | 12 (14.8%) | 24 (14.5%) | 33 (42.3%) |
The following graph shows the average serum creatinine concentrations in the compassionate use study and shows that there is a drop from pretreatment concentrations for all patients, especially those with elevated (greater than 1.7 mg/dL) pretreatment creatinine concentrations.
Mean Creatinine Concentrations Over Time
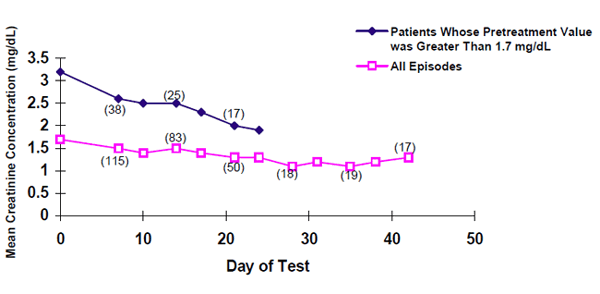 |
The incidence of nephrotoxicity in Study 94-0-013 comparative trial in cryptococcal meningitis was lower in the AmBisome groups as shown in the following table:
Laboratory Evidence of Nephrotoxicity Study 94-0-013
| AmBisome 3 mg/kg/day | AmBisome 6 mg/kg/day | Amphotericin B 0.7 mg/kg/day | |
| Total number of patients receiving at least one dose of study drug | 86 | 94 | 87 |
| Number with Nephrotoxicity (%) | |||
| 1.5X baseline serum creatinine | 30 (35%) | 44 (47%) | 52 (60%) |
| 2X baseline serum creatinine | 12 (14%) | 20 (21%) | 29 (33%) |
Read the entire FDA prescribing information for Ambisome (Amphotericin B)
© Ambisome Patient Information is supplied by Cerner Multum, Inc. and Ambisome Consumer information is supplied by First Databank, Inc., used under license and subject to their respective copyrights.
