Padcev Drug Description: Detailed Datasheet
- Generic Name: enfortumab vedotin-ejfv for injection
- Brand Name: Padcev
- Drug Class: Antineoplastics, Antimicrotubular
side effects drug center padcev (enfortumab vedotin-ejfv for injection) drug
Drug Description
What is PADCEV and how is it used?
PADCEV is a prescription medicine used to treat adults with bladder cancer and cancers of the urinary tract (renal pelvis, ureter or urethra) that has spread or cannot be removed by surgery. PADCEV may be used if you have:
- received an immunotherapy medicine and
- also received a chemotherapy-containing platinum medicine.
It is not known if PADCEV is safe and effective in children.
What are the possible side effects of PADCEV?
PADCEV may cause serious side effects, including:
- High blood sugar (hyperglycemia). You can develop high blood sugar during treatment with PADCEV. Tell your healthcare provider right away if you have any symptoms of high blood sugar, including:
- frequent urination
- increased thirst
- blurred vision
- confusion
- it becomes harder to control your blood sugar
- drowsiness
- loss of appetite
- fruity smell on your breath
- nausea, vomiting, or stomach pain
- Peripheral neuropathy. While receiving PADCEV you may experience nerve problems called peripheral neuropathy. Tell your healthcare provider right away if you get numbness or tingling in your hands or feet or muscle weakness.
- Eye problems. You can develop certain eye problems while receiving PADCEV. Tell your healthcare provider right away if you have dry eyes, or blurred vision.
- Skin Reactions. Rashes and severe skin reactions can happen while receiving PADCEV. Tell your healthcare provider right away if you get a rash or a skin reaction that continues to get worse.
Leakage of PADCEV out of your vein into the tissues around your infusion site (extravasation). If PADCEV leaks from the injection site or the vein into the nearby skin and tissues, it could cause an infusion site reaction. These reactions can happen right after you receive an infusion, but sometimes may happen days after your infusion. Tell your healthcare provider or get medical help right away if you notice any redness, swelling, itching, or discomfort at the infusion site.
The most common side effects of PADCEV include:
- numbness or tingling in your hands or feet, or muscle weakness
- fatigue
- decreased appetite
- rash
- hair loss
- nausea
- diarrhea
- change in sense of taste
- dry eyes
- dry skin
If you have certain side effects, your healthcare provider may decrease your dose or stop your treatment with PADCEV for a period of time (temporarily) or completely.
PADCEV may cause fertility problems in males, which may affect the ability to father children. Talk to your healthcare provider if you have concerns about fertility.
These are not all of the possible side effects of PADCEV.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
DESCRIPTION
Enfortumab vedotin-ejfv is a Nectin-4 directed antibody-drug conjugate (ADC) comprised of a fully human anti-Nectin-4 IgG1 kappa monoclonal antibody (AGS-22C3) conjugated to the small molecule microtubule disrupting agent, monomethyl auristatin E (MMAE) via a protease-cleavable maleimidocaproyl valine-citrulline (vc) linker (SGD-1006). Conjugation takes place on cysteine residues that comprise the interchain disulfide bonds of the antibody to yield a product with a drug-to-antibody ratio of approximately 3.8:1. The molecular weight is approximately 152 kDa.
Figure 1: Structural Formula
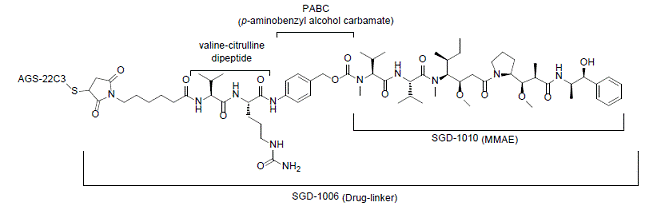 |
Approximately 4 molecules of MMAE are attached to each antibody molecule. Enfortumab vedotin-ejfv is produced by chemical conjugation of the antibody and small molecule components. The antibody is produced by mammalian (Chinese hamster ovary) cells and the small molecule components are produced by chemical synthesis.
PADCEV (enfortumab vedotin-ejfv) for injection is provided as a sterile, preservative-free, white to off-white lyophilized powder in single-dose vials for intravenous use. PADCEV is supplied as a 20 mg per vial and a 30 mg per vial and requires reconstitution with Sterile Water for Injection, USP, (2.3 mL and 3.3 mL, respectively) resulting in a clear to slightly opalescent, colorless to slightly yellow solution with a final concentration of 10 mg/mL [see DOSAGE AND ADMINISTRATION]. After reconstitution, each vial allows the withdrawal of 2 mL (20 mg) and 3 mL (30 mg). Each mL of reconstituted solution contains 10 mg of enfortumab vedotin-ejfv, histidine (1.4 mg), histidine hydrochloride monohydrate (2.31 mg), polysorbate 20 (0.2 mg) and trehalose dihydrate (55 mg) with a pH of 6.0.
Indications & Dosage
INDICATIONS
PADCEV® is indicated for the treatment of adult patients with locally advanced or metastatic urothelial cancer (mUC) who have previously received a programmed death receptor-1 (PD-1) or programmed death-ligand 1 (PD-L1) inhibitor, and a platinum-containing chemotherapy in the neoadjuvant/adjuvant, locally advanced or metastatic setting.
This indication is approved under accelerated approval based on tumor response rate [see Clinical Studies]. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials.
DOSAGE AND ADMINISTRATION
Recommended Dosage
The recommended dose of PADCEV is 1.25 mg/kg (up to a maximum of 125 mg for patients ≥100 kg) administered as an intravenous infusion over 30 minutes on Days 1, 8 and 15 of a 28-day cycle until disease progression or unacceptable toxicity.
Dose Modifications
Table 1. Dose Modifications
| Adverse Reaction | Severity1 | Dose Modification1 |
| Skin Reactions [see WARNINGS AND PRECAUTIONS] |
Suspected Stevens-Johnson syndrome (SJS) or toxic epidermal necrolysis (TEN), or Grade 3 (severe) skin reactions | Withhold until Grade ≤1, then resume treatment at the same dose level or consider dose reduction by one dose level. |
| Confirmed SJS or TEN; Grade 4 or recurrent Grade 3 skin reactions | Permanently discontinue. | |
| Hyperglycemia [see WARNINGS AND PRECAUTIONS] |
Blood glucose >250 mg/dL | Withhold until elevated blood glucose has improved to ≤ 250 mg/dL, then resume treatment at the same dose level. |
| Peripheral Neuropathy [see WARNINGS AND PRECAUTIONS] |
Grade 2 | Withhold until Grade ≤1, then resume treatment at the same dose level (if first occurrence). For a recurrence, withhold until Grade ≤1 then, resume treatment reduced by one dose level. |
| Grade ≥3 | Permanently discontinue. | |
| Other nonhematologic toxicity | Grade 3 | Withhold until Grade ≤ 1, then resume treatment at the same dose level or consider dose reduction by one dose level. |
| Grade 4 | Permanently discontinue. | |
| Hematologic toxicity | Grade 3, or Grade 2 thrombocytopenia | Withhold until Grade ≤ 1, then resume treatment at the same dose level or consider dose reduction by one dose level. |
| Grade 4 | Withhold until Grade ≤ 1, then reduce dose by one dose level or discontinue treatment. | |
| 1. Grade 1 is mild, Grade 2 is moderate, Grade 3 is severe, Grade 4 is life-threatening. | ||
Table 2. Recommended Dose Reduction Schedule
| Dose Level | |
| Starting dose | 1.25 mg/kg up to 125 mg |
| First dose reduction | 1.0 mg/kg up to 100 mg |
| Second dose reduction | 0.75 mg/kg up to 75 mg |
| Third dose reduction | 0.5 mg/kg up to 50 mg |
Instructions For Preparation And Administration
- Administer PADCEV as an intravenous infusion only.
- PADCEV is a cytotoxic drug. Follow applicable special handling and disposal procedures.1
Prior to administration, the PADCEV vial is reconstituted with Sterile Water for Injection (SWFI). The reconstituted solution is subsequently diluted in an intravenous infusion bag containing either 5% Dextrose Injection, USP, 0.9% Sodium Chloride Injection, USP, or Lactated Ringer’s Injection, USP.
Reconstitution In Single-Dose Vial
- Follow procedures for proper handling and disposal of anticancer drugs.
- Use appropriate aseptic technique for reconstitution and preparation of dosing solutions.
- Calculate the recommended dose based on the patient’s weight to determine the number and strength (20 mg or 30 mg) of vials needed.
- Reconstitute each vial as follows and, if possible, direct the stream of SWFI along the walls of the vial and not directly onto the lyophilized powder:
- 20 mg vial: Add 2.3 mL of SWFI, resulting in 10 mg/mL PADCEV.
- 30 mg vial: Add 3.3 mL of SWFI, resulting in 10 mg/mL PADCEV.
- Slowly swirl each vial until the contents are completely dissolved. Allow the reconstituted vial(s) to settle for at least 1 minute until the bubbles are gone. DO NOT SHAKE THE VIAL. Do not expose to direct sunlight.
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. The reconstituted solution should be clear to slightly opalescent, colorless to light yellow and free of visible particles. Discard any vial with visible particles or discoloration.
- Based upon the calculated dose amount, the reconstituted solution from the vial(s) should be added to the infusion bag immediately. This product does not contain a preservative. If not used immediately, reconstituted vials may be stored for up to 4 hours in refrigeration at 2°C to 8°C (36 °F to 46 °F). DO NOT FREEZE. Discard unused vials with reconstituted solution beyond the recommended storage time.
Dilution In Infusion Bag
- Withdraw the calculated dose amount of reconstituted solution from the vial(s) and transfer into an infusion bag.
- Dilute PADCEV with either 5% Dextrose Injection, 0.9% Sodium Chloride Injection, or Lactated Ringer's Injection. The infusion bag size should allow enough diluent to achieve a final concentration of 0.3 mg/mL to 4 mg/mL PADCEV.
- Mix diluted solution by gentle inversion. DO NOT SHAKE THE BAG. Do not expose to direct sunlight.
- Visually inspect the infusion bag for any particulate matter or discoloration prior to use. The reconstituted solution should be clear to slightly opalescent, colorless to light yellow and free of visible particles. DO NOT USE the infusion bag if particulate matter or discoloration is observed.
- Discard any unused portion left in the single-dose vials.
Administration
- Immediately administer the infusion over 30 minutes through an intravenous line.
- If the infusion is not administered immediately, the prepared infusion bag should not be stored longer than 8 hours at 2°C to 8°C (36 °F to 46 °F). DO NOT FREEZE.
DO NOT administer PADCEV as an intravenous push or bolus.
DO NOT mix PADCEV with, or administer as an infusion with, other medicinal products.
HOW SUPPLIED
Dosage Forms And Strengths
For Injection
20 mg and 30 mg of enfortumab vedotin-ejfv as a white to off-white lyophilized powder in a single-dose vial for reconstitution.
Storage And Handling
PADCEV (enfortumab vedotin-ejfv) 20 mg and 30 mg are supplied as a sterile, preservative-free, white to off-white lyophilized powder in single-dose vials. PADCEV vials are available in the following packages:
- Carton of one 20 mg single-dose vial (NDC 51144-020-01)
- Carton of one 30 mg single-dose vial (NDC 51144-030-01)
Storage
Store PADCEV vials refrigerated at 2°C to 8°C (36°F to 46°F) in the original carton. Do not freeze. Do not shake.
Special Handling
PADCEV is a cytotoxic drug. Follow applicable special handling and disposal procedures.1
REFERENCES
1. "OSHA Hazardous Drugs." OSHA. http://www.osha.gov/SLTC/hazardousdrugs/index.html
Manufactured and Marketed by: Astellas Pharma US, Inc., Northbrook, Illinois 60062. Revised: Mar 2021
Side Effects & Drug Interactions
SIDE EFFECTS
The following serious adverse reactions are described elsewhere in the labeling:
- Skin Reactions [see WARNINGS AND PRECAUTIONS]
- Hyperglycemia [see WARNINGS AND PRECAUTIONS]
- Peripheral Neuropathy [see WARNINGS AND PRECAUTIONS]
- Ocular Disorders [see WARNINGS AND PRECAUTIONS]
- Infusion Site Extravasation [see WARNINGS AND PRECAUTIONS]
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The data in the WARNINGS AND PRECAUTIONS section reflect exposure to PADCEV as a single agent at 1.25 mg/kg in 310 patients in EV-201, EV-101 (NCT02091999), and EV-102 (NCT03219333). Among 310 patients receiving PADCEV, 30% were exposed for ≥ 6 months and 8% were exposed for ≥12 months.
The data described in this section reflect exposure to PADCEV from EV-201, a single arm study in patients (n=125) with locally advanced or metastatic urothelial cancer who had received prior treatment with a PD-1 or PD-L1 inhibitor and platinum-based chemotherapy. Patients received PADCEV 1.25 mg/kg on Days 1, 8 and 15 of a 28-day cycle until disease progression or unacceptable toxicity. The median duration of exposure to PADCEV was 4.6 months (range: 0.5-15.6).
Serious adverse reactions occurred in 46% of patients treated with PADCEV. The most common serious adverse reactions (≥3%) were urinary tract infection (6%), cellulitis (5%), febrile neutropenia (4%), diarrhea (4%), sepsis (3%), acute kidney injury (3%), dyspnea (3%), and rash (3%). Fatal adverse reactions occurred in 3.2% of patients, including acute respiratory failure, aspiration pneumonia, cardiac disorder, and sepsis (each 0.8%).
Adverse reactions leading to discontinuation occurred in 16% of patients; the most common adverse reaction leading to discontinuation was peripheral neuropathy (6%). Adverse reactions leading to dose interruption occurred in 64% of patients; the most common adverse reactions leading to dose interruption were peripheral neuropathy (18%), rash (9%) and fatigue (6%). Adverse reactions leading to dose reduction occurred in 34% of patients; the most common adverse reactions leading to dose reduction were peripheral neuropathy (12%), rash (6%) and fatigue (4%).
The most common adverse reactions (≥20%) were fatigue, peripheral neuropathy, decreased appetite, rash, alopecia, nausea, dysgeusia, diarrhea, dry eye, pruritus and dry skin. The most common Grade ≥3 adverse reaction (≥5%) were rash, diarrhea, and fatigue.
Table 3 summarizes the all grade and Grade ≥3 adverse reactions reported in patients in EV-201.
Table 3. Adverse Reactions Reported in ≥15% (Any Grade) or ≥5% (Grade ≥3) of Patients Treated with PADCEV in EV-201
| Adverse Reaction | PADCEV n=125 |
|
| All Grades % |
Grade ≥3 % |
|
| Any | 100 | 73 |
| General disorders and administration site conditions | ||
| Fatigue1 | 56 | 6 |
| Nervous system disorders | ||
| Peripheral neuropathy2 | 56 | 4 |
| Dysgeusia | 42 | 0 |
| Metabolism and nutrition disorders | ||
| Decreased appetite | 52 | 2 |
| Skin and subcutaneous tissue disorders | ||
| Rash3 | 52 | 13 |
| Alopecia | 50 | 0 |
| Dry skin | 26 | 0 |
| Pruritus4 | 26 | 2 |
| Eye disorders | ||
| Dry eye5 | 40 | 0 |
| Gastrointestinal disorders | ||
| Nausea | 45 | 3 |
| Diarrhea6 | 42 | 6 |
| Vomiting | 18 | 2 |
| 1 Includes: asthenia and fatigue. 2Includes: hypoesthesia, gait disturbance, muscular weakness, neuralgia, paresthesia, peripheral motor neuropathy, peripheral sensory neuropathy and peripheral sensorimotor neuropathy. 3 Includes: dermatitis acneiform, dermatitis bullous, dermatitis contact, dermatitis exfoliative, drug eruption, erythema, erythema multiforme, exfoliative rash, palmar-plantar erythrodysesthesia syndrome, photosensitivity reaction, rash, rash erythematous, rash generalized, rash macular, rash maculo-papular, rash papular, rash pustular, rash pruritic, rash vesicular, skin exfoliation, stasis dermatitis, and symmetrical drug-related intertriginous and flexural exanthema (SDRIFE) and urticaria. 4 Includes: pruritus and pruritus generalized. 5 Includes: blepharitis, conjunctivitis, dry eye, eye irritation, keratitis, keratopathy, lacrimation increased, limbal stem cell deficiency, Meibomian gland dysfunction, ocular discomfort, punctate keratitis, tear break up time decreased. 6 Includes: colitis, diarrhea and enterocolitis. |
||
Other clinically significant adverse reactions (≤15%) include: herpes zoster (3%) and infusion site extravasation (2%).
Table 4. Selected Laboratory Abnormalities Reported in ≥ 10% (Grades 2-4) or ≥ 5% (Grade 3-4) of Patients Treated with PADCEV in EV-201
| Adverse Reaction | PADCEV | |
| Grades 2-41 % |
Grade 3-41 % |
|
| Hematology | ||
| Hemoglobin decreased | 34 | 10 |
| Lymphocytes decreased | 32 | 10 |
| Neutrophils decreased | 14 | 5 |
| Leukocytes decreased | 14 | 4 |
| Chemistry | ||
| Phosphate decreased | 34 | 10 |
| Creatinine increased | 20 | 2 |
| Potassium decreased | 192 | 1 |
| Lipase increased | 14 | 9 |
| Glucose increased | -3 | 8 |
| Sodium decreased | 8 | 8 |
| Urate increased | 7 | 7 |
| 1 Denominator for each laboratory parameter is based on the number of patients with a baseline and post-treatment laboratory value available for 121 or 122 patients. 2Includes Grade 1 (potassium 3.0-3.5 mmol/L) – Grade 4. 3CTCAE Grade 2 is defined as fasting glucose >160-250 mg/dL. Fasting glucose levels were not measured in EV-201. However, 23 (19%) patients had non-fasting glucose >160-250 mg/dL. |
||
Post Marketing Experience
The following adverse reactions have been identified during post-approval use of PADCEV. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Skin and subcutaneous tissue disorders: Epidermal necrosis, Stevens-Johnson syndrome, toxic epidermal necrolysis [see WARNINGS AND PRECAUTIONS].
Immunogenicity
As with all therapeutic proteins, there is a potential for immunogenicity. The detection of antibody formation is highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of antibody (including neutralizing antibody) positivity in an assay may be influenced by several factors including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of the incidence of antibodies in the studies described below with the incidence of antibodies in other studies or other enfortumab vedotin products may be misleading.
A total of 365 patients were tested for immunogenicity to PADCEV; 4 patients (1%) were confirmed to be transiently positive for anti-therapeutic antibody (ATA), and 1 patient (0.3%) was confirmed to be persistently positive for ATA at any post-baseline time point. No impact of ATA on efficacy, safety and pharmacokinetics was observed.
DRUG INTERACTIONS
Effects Of Other Drugs On PADCEV
Strong CYP3A4 Inhibitors
Concomitant use with a strong CYP3A4 inhibitor may increase free MMAE exposure [see CLINICAL PHARMACOLOGY], which may increase the incidence or severity of PADCEV toxicities. Closely monitor patients for signs of toxicity when PADCEV is given concomitantly with strong CYP3A4 inhibitors.
Warnings & Precautions
WARNINGS
Included as part of the "PRECAUTIONS" Section
PRECAUTIONS
Skin Reactions
Severe cutaneous adverse reactions, including fatal cases of Stevens-Johnson syndrome (SJS) or toxic epidermal necrolysis (TEN) occurred in patients treated with PADCEV. SJS and TEN occurred predominantly during the first cycle of treatment but may occur later.
Skin reactions occurred in 54% of the 310 patients treated with PADCEV in clinical trials. Twenty-six percent (26%) of patients had maculopapular rash and 30% had pruritus. Grade 3-4 skin reactions occurred in 10% of patients and included symmetrical drug-related intertriginous and flexural exanthema (SDRIFE), dermatitis bullous, dermatitis exfoliative, and palmar-plantar erythrodysesthesia. In study EV-201, the median time to onset of severe skin reactions was 0.8 months (range: 0.2 to 5.3). Of the patients who experienced rash, 65% had complete resolution and 22% had partial improvement. [see ADVERSE REACTIONS].
Monitor patients closely throughout treatment for skin reactions. Consider topical corticosteroids and antihistamines, as clinically indicated. Withhold PADCEV and consider referral for specialized care for severe (Grade 3) skin reactions, suspected SJS or TEN. Permanently discontinue PADCEV in patients with confirmed SJS or TEN; or Grade 4 or recurrent Grade 3 skin reactions [see DOSAGE AND ADMINISTRATION].
Hyperglycemia
Hyperglycemia occurred in patients treated with PADCEV, including death, and diabetic ketoacidosis (DKA) in those with and without pre-existing diabetes mellitus. The incidence of Grade 3-4 hyperglycemia increased consistently in patients with higher body mass index and in patients with higher baseline A1C. In EV-201, 8% of patients developed Grade 3-4 hyperglycemia. In this trial, patients with baseline hemoglobin A1C ≥8% were excluded. Closely monitor blood glucose levels in patients with, or at risk for, diabetes mellitus or hyperglycemia. If blood glucose is elevated (>250 mg/dL), withhold PADCEV [see DOSAGE AND ADMINISTRATION].
Peripheral Neuropathy
Peripheral neuropathy, predominantly sensory, occurred in 49% of the 310 patients treated with PADCEV in clinical trials; 2% experienced Grade 3 reactions.
In study EV-201, peripheral neuropathy occurred in patients treated with PADCEV with or without preexisting peripheral neuropathy. The median time to onset of Grade ≥2 was 3.8 months (range: 0.6 to 9.2). Neuropathy led to treatment discontinuation in 6% of patients. At the time of their last evaluation, 19% had complete resolution, and 26% had partial improvement.
Monitor patients for symptoms of new or worsening peripheral neuropathy and consider dose interruption or dose reduction of PADCEV when peripheral neuropathy occurs. Permanently discontinue PADCEV in patients that develop Grade ≥3 peripheral neuropathy [see DOSAGE AND ADMINISTRATION].
Ocular Disorders
Ocular disorders occurred in 46% of the 310 patients treated with PADCEV. The majority of these events involved the cornea and included keratitis, blurred vision, limbal stem cell deficiency and other events associated with dry eyes.
Dry eye symptoms occurred in 36% of patients, and blurred vision occurred in 14% of patients, during treatment with PADCEV. The median time to onset to symptomatic ocular disorder was 1.9 months (range: 0.3 to 6.2).
Monitor patients for ocular disorders. Consider artificial tears for prophylaxis of dry eyes and ophthalmologic evaluation if ocular symptoms occur or do not resolve. Consider treatment with ophthalmic topical steroids, if indicated after an ophthalmic exam. Consider dose interruption or dose reduction of PADCEV for symptomatic ocular disorders.
Infusion Site Extravasation
Skin and soft tissue reactions secondary to extravasation have been observed after administration of PADCEV. Of the 310 patients, 1.3% of patients experienced skin and soft tissue reactions. Reactions may be delayed. Erythema, swelling, increased temperature, and pain worsened until 2-7 days after extravasation and resolved within 1-4 weeks of peak. One percent of patients developed extravasation reactions with secondary cellulitis, bullae, or exfoliation. Ensure adequate venous access prior to starting PADCEV and monitor for possible extravasation during administration. If extravasation occurs, stop the infusion and monitor for adverse reactions.
Embryo-Fetal Toxicity
Based on the mechanism of action and findings in animals, PADCEV can cause fetal harm when administered to a pregnant woman. In animal reproduction studies, administration of enfortumab vedotin to pregnant rats during the period of organogenesis caused maternal toxicity, embryo-fetal lethality, structural malformations and skeletal anomalies at maternal exposures approximately similar to the clinical exposures at the recommended human dose of 1.25 mg/kg.
Advise patients of the potential risk to the fetus. Advise female patients of reproductive potential to use effective contraception during treatment with PADCEV and for 2 months after the last dose. Advise male patients with female partners of reproductive potential to use effective contraception during treatment with PADCEV and for 4 months after the last dose [see Use In Specific Populations and CLINICAL PHARMACOLOGY].
Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (PATIENT INFORMATION).
Skin Reactions
Inform patients that severe skin reactions including SJS and TEN with fatal outcomes have occurred after administration of PADCEV, predominantly during the first cycle of treatment but may occur later. Advise patients to contact their healthcare provider immediately if they develop new target lesions, progressively worsening skin reactions, severe blistering or peeling of the skin [see WARNINGS AND PRECAUTIONS].
Hyperglycemia
Inform patients about the risk of hyperglycemia and how to recognize associated symptoms [see WARNINGS AND PRECAUTIONS].
Peripheral Neuropathy
Inform patients to report to their healthcare provider any numbness and tingling of the hands or feet or muscle weakness [see WARNINGS AND PRECAUTIONS].
Ocular Disorders
Advise patients to contact their healthcare provider if they experience any visual changes [see WARNINGS AND PRECAUTIONS]. In order to prevent or treat dry eyes, advise patients to use artificial tear substitutes.
Infusion Site Extravasation
Inform patients that infusion site reactions have occurred after administration of PADCEV. These reactions generally occurred immediately after administration but, in some instances, had a delayed onset (e.g., 24 hours). Instruct patients to contact their healthcare provider immediately if they experience an infusion site reaction [see WARNINGS AND PRECAUTIONS].
Embryo-Fetal Toxicity
Advise pregnant women and females of reproductive potential of the potential risk to the fetus. Advise females to inform their healthcare providers of a known or suspected pregnancy [see WARNINGS AND PRECAUTIONS and Use In Specific Populations].
Females And Males Of Reproductive Potential
Advise female patients of reproductive potential to use effective contraception during treatment with PADCEV and for 2 months after the last dose. Advise male patients with female partners of reproductive potential to use effective contraception during treatment with PADCEV and for 4 months after the last dose [see Use In Specific Populations].
Lactation
Advise women not to breastfeed during treatment with PADCEV and for 3 weeks after the last dose [see Use In Specific Populations].
Infertility
Advise males of reproductive potential that PADCEV may impair fertility [see Use In Specific Populations].
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment Of Fertility
Carcinogenicity studies with enfortumab vedotin-ejfv or the small molecule cytotoxic agent (MMAE) have not been conducted.
MMAE was genotoxic in the rat bone marrow micronucleus study through an aneugenic mechanism. This effect is consistent with the pharmacological effect of MMAE as a microtubule-disrupting agent. MMAE was not mutagenic in the bacterial reverse mutation assay (Ames test) or the L5178Y mouse lymphoma forward mutation assay.
Fertility studies with enfortumab vedotin-ejfv or MMAE have not been conducted. However, results of repeat-dose toxicity studies in rats indicate the potential for enfortumab vedotin-ejfv to impair male reproductive function and fertility.
In repeat-dose toxicology studies conducted in rats for up to 13 weeks, doses ≥2 mg/kg enfortumab vedotin-ejfv (at exposures similar to the exposures at the recommended human dose) resulted in decreases in testes and epididymis weights, seminiferous tubule degeneration, spermatid/spermatocyte depletion in the testes and cell debris, sperm granuloma and hypospermia/abnormal spermatids in the epididymis. Findings in the testes and epididymis did not reverse by the end of the recovery period.
Use In Specific Populations
Pregnancy
Risk Summary
Based on the mechanism of action and findings in animals, PADCEV can cause fetal harm when administered to a pregnant woman [see CLINICAL PHARMACOLOGY]. There are no available human data on PADCEV use in pregnant women to inform a drug-associated risk. In an animal reproduction study, administration of enfortumab vedotin-ejfv to pregnant rats during organogenesis caused maternal toxicity, embryo-fetal lethality, structural malformations and skeletal anomalies at maternal exposures approximately similar to the exposures at the recommended human dose of 1.25 mg/kg (see Data). Advise patients of the potential risk to the fetus.
The background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2%-4% and 15%-20%, respectively.
Data
Animal Data
In a rat pilot embryo-fetal development study, administration of enfortumab vedotin-ejfv on gestation day 6 and 13 during the period of organogenesis resulted in a complete litter loss in all pregnant rats at the maternally toxic dose of 5 mg/kg (approximately 3 times the exposure at the recommended human dose). A dose of 2 mg/kg (approximately similar to the exposure at the recommended human dose) resulted in maternal toxicity, embryo-fetal lethality and structural malformations that included gastroschisis, malrotated hindlimb, absent forepaw, malpositioned internal organs and fused cervical arch. Additionally, skeletal anomalies (asymmetric, fused, incompletely ossified, and misshapen sternebrae, misshapen cervical arch, and unilateral ossification of the thoracic centra) and decreased fetal weight were observed.
Lactation
Risk Summary
There are no data on the presence of enfortumab vedotin-ejfv in human milk, the effects on the breastfed child, or the effects on milk production. Because of the potential for serious adverse reactions in a breastfed child, advise lactating women not to breastfeed during treatment with PADCEV and for at least 3 weeks after the last dose.
Females And Males Of Reproductive Potential
Pregnancy Testing
Verify pregnancy status in females of reproductive potential prior to initiating PADCEV treatment [see Pregnancy].
Contraception
Females
PADCEV can cause fetal harm when administered to a pregnant woman [see Pregnancy]. Advise females of reproductive potential to use effective contraception during treatment with PADCEV and for 2 months after the last dose.
Males
Advise male patients with female partners of reproductive potential to use effective contraception during treatment with PADCEV and for 4 months after the last dose.
Infertility
Males
Based on findings from animal studies, PADCEV may impair male fertility [see Nonclinical Toxicology].
Pediatric Use
Safety and effectiveness of PADCEV in pediatric patients have not been established.
Geriatric Use
Of the 310 patients treated with PADCEV in clinical studies, 187 (60%) were 65 years or older and 80 (26%) were 75 years or older. No overall differences in safety or effectiveness were observed between these patients and younger patients [see CLINICAL PHARMACOLOGY].
Hepatic Impairment
Avoid the use of PADCEV in patients with moderate or severe hepatic impairment. PADCEV has not been studied in patients with moderate or severe hepatic impairment [see CLINICAL PHARMACOLOGY]. In another ADC that contains MMAE, the frequency of ≥ Grade 3 adverse reactions and deaths was greater in patients with moderate (Child-Pugh B) or severe (Child-Pugh C) hepatic impairment compared to patients with normal hepatic function. No adjustment in the starting dose is required when administering PADCEV to patients with mild hepatic impairment.
Renal Impairment
No dose adjustment is required in patients with mild (CrCL >60-90 mL/min), moderate (CrCL 30-60 mL/min) or severe (CrCL <30 mL/min) renal impairment [see CLINICAL PHARMACOLOGY].
Overdosage & Contraindications
OVERDOSE
No Information Provided
CONTRAINDICATIONS
None.
Clinical Pharmacology
Mechanism Of Action
Enfortumab vedotin-ejfv is an ADC. The antibody is a human IgG1 directed against Nectin-4, an adhesion protein located on the surface of cells. The small molecule, MMAE, is a microtubule-disrupting agent, attached to the antibody via a protease-cleavable linker. Nonclinical data suggest that the anticancer activity of enfortumab vedotin-ejfv is due to the binding of the ADC to Nectin-4-expressing cells, followed by internalization of the ADC-Nectin-4 complex, and the release of MMAE via proteolytic cleavage. Release of MMAE disrupts the microtubule network within the cell, subsequently inducing cell cycle arrest and apoptotic cell death.
Pharmacodynamics
In an exposure-response analysis, higher enfortumab vedotin exposure was associated with higher incidence of some adverse reactions (e.g., Grade ≥2 peripheral neuropathy, Grade ≥3 hyperglycemia) and a lower exposure was associated with lower efficacy.
Cardiac Electrophysiology
At the recommended dose, PADCEV had no large QTc prolongation (>20 msec).
Pharmacokinetics
Population pharmacokinetic analysis included data from 369 patients based on three Phase 1 studies and one Phase 2 study. Enfortumab vedotin-ejfv pharmacokinetics were characterized after single and multiple doses in patients with locally advanced or metastatic urothelial carcinoma and other solid tumors.
The exposure parameters of ADC and unconjugated MMAE (the cytotoxic component of enfortumab vedotin-ejfv) are summarized in Table 5 below. Peak ADC concentrations were observed near the end of intravenous infusion while peak MMAE concentrations were observed approximately 2 days after enfortumab vedotin-ejfv dosing. Minimal accumulation of the ADC and MMAE was observed following repeat administration of enfortumab vedotin-ejfv in patients. Steadystate concentrations of ADC and MMAE were reached after 1 treatment cycle.
Table 5. Exposure parameters of ADC and unconjugated MMAE after first treatment cycle of 1.25 mg/kg of enfortumab vedotin-ejfv dose of Days 1, 8 and 15
| ADC Mean (± SD) |
Unconjugated MMAE Mean (± SD) |
|
| Cmax | 28 (6.8) μg/mL | 4.8 (2.7) ng/mL |
| AUC0-28d | 111 (38) μg·d/mL | 69 (42) ng·d/mL |
| Ctrough,0-28d | 0.27 (0.22) μg/mL | 0.57 (0.58) ng/mL |
| Cmax = maximum concentration, AUC0-28d = area under the concentration-time curve from time zero to 28 days, Ctrough,0-28d = predose concentration on day 28 | ||
Distribution
The estimated mean steady-state volume of distribution of ADC was 11 liters following administration of enfortumab vedotin-ejfv. Plasma protein binding of MMAE ranged from 68% to 82%, in vitro.
Elimination
ADC and MMAE exhibited multi-exponential declines with an elimination half-life of 3.4 days and 2.4 days, respectively. The mean clearance (CL) of enfortumab vedotin-ejfv and free MMAE in patients was 0.10 L/h and 2.7 L/h, respectively, in patients. Elimination of MMAE appeared to be limited by its rate of release from enfortumab vedotin-ejfv.
Metabolism
Enfortumab vedotin-ejfv catabolism has not been studied in humans; however, it is expected to undergo catabolism to small peptides, amino acids, unconjugated MMAE, and unconjugated MMAE-related catabolites. Enfortumab vedotinejfv releases MMAE via proteolytic cleavage, and MMAE is primarily metabolized by CYP3A4 in vitro.
Excretion
The excretion of enfortumab vedotin-ejfv is not fully characterized. Following a single-dose of another ADC that contains MMAE, 17% of the total MMAE administered was recovered in feces and 6% in urine over a 1-week period, primarily as unchanged drug. A similar excretion profile of MMAE is expected after enfortumab vedotin-ejfv administration.
Specific Populations
Based on population pharmacokinetic analysis, no clinically significant differences in the pharmacokinetics of enfortumab vedotin-ejfv were observed based on age (24 to 87 years), sex, or race/ethnicity (Caucasian, Asian, Black, or others).
Hepatic Impairment
Based on population pharmacokinetics analysis, there was a 48% AUC increase in unconjugated MMAE exposure observed in patients with mild hepatic impairment (bilirubin of 1 to 1.5 × ULN and AST <ULN, or bilirubin ≤ULN and AST >ULN, n=31) compared to normal hepatic function. The effect of moderate or severe hepatic impairment (AST or ALT >2.5 x ULN or total bilirubin >1.5 x ULN) or liver transplantation on the pharmacokinetics of ADC or unconjugated MMAE is unknown.
Renal Impairment
The pharmacokinetics of enfortumab vedotin-ejfv and MMAE were evaluated after the administration of 1.25 mg/kg of enfortumab vedotin-ejfv to patients with mild (creatinine clearance; CrCL >60–90 mL/min; n=135), moderate (CrCL 30– 60 mL/min; n=147) and severe (CrCL <30 mL/min; n=8) renal impairment. No significant differences in exposure (AUC) of ADC and MMAE were observed in patients with mild, moderate or severe renal impairment compared to patients with normal renal function. The effect of end stage renal disease with or without dialysis on the pharmacokinetics of ADC or unconjugated MMAE is unknown.
Drug Interaction Studies
Clinical Studies
No clinical studies evaluating the drug-drug interaction potential of enfortumab vedotin-ejfv have been conducted. To characterize the drug-drug interaction potential of free MMAE, clinical studies with another ADC that contains MMAE are described below.
Strong CYP3A4 Inhibitors
Another ADC that contains MMAE co-administered with ketoconazole (a strong CYP3A4 inhibitor) increased MMAE Cmax by 25% and AUC by 34%, with no change in ADC exposure. The concomitant use of strong inhibitors of CYP3A4 with PADCEV would likely result in similar effects on free MMAE and ADC.
Strong CYP3A4 Inducers
Another ADC that contains MMAE co-administered with rifampin (a strong CYP3A4 inducer) decreased MMAE Cmax by 44% and AUC by 46%, with no change in ADC exposure. The concomitant use of strong inducers of CYP3A4 with PADCEV would likely result in similar effects on free MMAE and ADC.
Sensitive CYP3A4 Substrates
Another ADC that contains MMAE co-administered with midazolam (a sensitive CYP3A4 substrate) did not affect the exposure of midazolam. Similarly, PADCEV is not expected to alter the exposure of drugs that are metabolized by CYP3A4 enzymes.
In Vitro Studies
Transporter Systems
MMAE is a substrate of P-glycoprotein (P-gp), but not an inhibitor of P-gp.
Clinical Studies
Metastatic Urothelial Cancer
The efficacy of PADCEV was evaluated in EV-201 (NCT03219333), single-arm, multicenter trial that enrolled 125 patients with locally advanced or metastatic urothelial cancer who received prior treatment with a PD-1 or PD-L1 inhibitor and platinum-based chemotherapy. Patients were excluded if they had active CNS metastases, ongoing sensory or motor neuropathy ≥ Grade 2, or uncontrolled diabetes defined as hemoglobin A1C (HbA1c) ≥8% or HbA1c ≥7% with associated diabetes symptoms.
The median age was 69 years (range: 40 to 84 years), 70% were male, and 85% were Caucasian. All patients had a baseline Eastern Cooperative Oncology Group (ECOG) performance status of 0 (32%) or 1 (68%). Ninety percent of patients had visceral metastases including 40% with liver metastases. Two-thirds of patients had pure transitional cell carcinoma (TCC) histology; 33% had TCC with other histologic variants. An immunohistochemistry clinical trial assay was used to assess patients with tumor tissue available, and detected Nectin-4 expression in all patients tested (n=120). The median number of prior systemic therapies was 3 (range: 1 to 6). Forty-six percent of patients received prior PD-1 inhibitor, 42% received prior PD-L1 inhibitor, and an additional 13% received both PD-1 and PD-L1 inhibitors. Sixty-six percent of patients received prior cisplatin-based regimens, 26% received prior carboplatin-based regimens, and an additional 8% received both cisplatin and carboplatin-based regimens.
The major efficacy outcome measures were confirmed objective response rate (ORR) and duration of response (DOR) assessed by blinded independent central review (BICR) using RECIST v1.1.
Efficacy results are presented in Table 6.
Table 6. Efficacy Results in EV201 (BICR Assessment)
| Endpoint | PADCEV n=1251 |
| Confirmed ORR (95% CI) |
44% (35.1, 53.2) |
| Complete Response Rate (CR) | 12% |
| Partial Response Rate (PR) | 32% |
| Median2 Duration of Response, months (95% CI) |
7.63 (6.3, NE) |
| NE = not estimable 1 Median follow-up duration of 10.2 months. 2 Kaplan-Meier estimate. 3 Based on patients (n=55) with a response by BICR. |
|
Medication Guide
PATIENT INFORMATION
PADCEV®
(PAD-sev)
(enfortumab vedotin-ejfv) for injection
What is PADCEV?
PADCEV is a prescription medicine used to treat adults with bladder cancer and cancers of the urinary tract (renal pelvis, ureter or urethra) that has spread or cannot be removed by surgery. PADCEV may be used if you have:
- received an immunotherapy medicine and
- also received a chemotherapy-containing platinum medicine.
It is not known if PADCEV is safe and effective in children.
Before receiving PADCEV, tell your healthcare provider about all of your medical conditions, including if you:
Females who are able to become pregnant:
Males with a female sexual partner who is able to become pregnant:
- are currently experiencing numbness or tingling in your hands or feet
- have a history of high blood sugar or diabetes
- are pregnant or plan to become pregnant. PADCEV can harm your unborn baby. Tell your healthcare provider right away if you become pregnant or think you may be pregnant during treatment with PADCEV.
- Your healthcare provider should do a pregnancy test before you start treatment with PADCEV.
- You should use an effective method of birth control during your treatment and for at least 2 months after the last dose of PADCEV.
- If your female partner is pregnant, PADCEV can harm the unborn baby.
- You should use an effective method of birth control during your treatment and for at least 4 months after the last dose of PADCEV.
- are breastfeeding or plan to breastfeed. It is not known if PADCEV passes into your breast milk. Do not breastfeed during treatment and for at least 3 weeks after the last dose of PADCEV.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
How will I receive PADCEV?
- PADCEV will be given to you by intravenous (IV) infusion into your vein over 30 minutes.
- You will receive your PADCEV over periods of time called cycles.
- Each PADCEV cycle is 28 days.
- You will receive PADCEV on days 1, 8 and 15 of every cycle.
- Your healthcare provider will decide how many treatment cycles you need.
- Your healthcare provider may do blood tests regularly during treatment with PADCEV.
What are the possible side effects of PADCEV?
PADCEV may cause serious side effects, including:
- Skin reactions. Severe skin reactions have happened after treatment with PADCEV, in some cases severe skin reactions have caused death. Most severe skin reactions occurred during the first cycle (28 days) of treatment but may happen later. Tell your healthcare provider right away if you develop any of these signs of a new or worsening skin reaction:
- target lesions (skin reactions that look like rings)
- rash or itching that continues to get worse
- blistering or peeling of the skin
- painful sores or ulcers in mouth or nose, throat, or genital area
- fever or flu-like symptoms
- swollen lymph nodes
- High blood sugar (hyperglycemia). You can develop high blood sugar during treatment with PADCEV. Tell your healthcare provider right away if you have any symptoms of high blood sugar, including:
- frequent urination
- increased thirst
- blurred vision
- confusion
- it becomes harder to control your blood sugar
- drowsiness
- loss of appetite
- fruity smell on your breath
- nausea, vomiting, or stomach pain
- Peripheral neuropathy. You may develop nerve problems called peripheral neuropathy during treatment with PADCEV. Tell your healthcare provider right away if you get new or worsening numbness or tingling in your hands or feet or muscle weakness.
- Eye problems. You can develop certain eye problems during treatment with PADCEV. Tell your healthcare provider right away if you have dry eyes, or blurred vision. You may use artificial tear substitutes to help prevent or treat dry eyes.
- Leakage of PADCEV out of your vein into the tissues around your infusion site (extravasation). If PADCEV leaks from the injection site or the vein into the nearby skin and tissues, it could cause an infusion site reaction. These reactions can happen right after you receive an infusion, but sometimes may happen days after your infusion. Tell your healthcare provider or get medical help right away if you notice any redness, swelling, itching, or discomfort at the infusion site.
The most common side effects of PADCEV include:
- tiredness (fatigue)
- numbness or tingling in your hands or feet, or muscle weakness
- decreased appetite
- rash
- hair loss
- nausea
- diarrhea
- change in sense of taste
- dry eyes
- dry skin
If you have certain side effects, your healthcare provider may decrease your dose or stop your treatment with PADCEV for a period of time (temporarily) or completely.
PADCEV may cause fertility problems in males, which may affect the ability to father children. Talk to your healthcare provider if you have concerns about fertility.
These are not all of the possible side effects of PADCEV.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
General information about the safe and effective use of PADCEV.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. If you would like more information about PADCEV, talk with your healthcare provider. You can ask your pharmacist or healthcare provider for information about PADCEV that is written for healthcare professionals.
What are the ingredients in PADCEV?
Active ingredient: enfortumab vedotin-ejfv
Inactive ingredients: histidine, histidine hydrochloride monohydrate, polysorbate 20, and trehalose dehydrate.
This Patient Information has been approved by the U.S. Food and Drug Administration.
