Androderm Side Effects Center
- Generic Name: testosterone transdermal system
- Brand Name: Androderm
- Drug Class: ANDROGENS
Androderm (Testosterone Transdermal System) side effects drug center
Androderm Side Effects Center
What Is Androderm?
Androderm (testosterone transdermal system) topical (for the skin) is a naturally occurring male hormone used to treat conditions in men that result from a lack of natural testosterone.
What Are Side Effects of Androderm?
Common side effects of Androderm include:
- redness, itching, burning, irritation, or hardened skin where the skin patch is worn;
- breast swelling or tenderness,
- increased acne or hair growth,
- headache,
- depressed mood, or
- changes in your sex drive.
Tell your doctor if you have serious side effects of Androderm including:
- burn-like blistering of the skin where the transdermal patch is worn,
- skin irritation with patch-wearing that does not get better with time,
- problems with urination,
- swelling of your ankles,
- frequent/prolonged erections,
- nausea,
- stomach pain,
- low fever,
- loss of appetite,
- dark urine,
- clay-colored stools, or
- jaundice (yellowing of the skin or eyes).
Dosage for Androderm
The recommended starting dose is one Androderm 4 mg/day system (not two 2 mg/day systems) applied nightly for 24 hours.
What Drugs, Substances, or Supplements Interact with Androderm?
Androderm may interact with insulin, blood thinners, oxyphenbutazone, or corticosteroids. Other drugs may interact with Androderm topical. Tell your doctor all prescription and over-the-counter medications and supplements you use.
Androderm During Pregnancy and Breastfeeding
Women should not use this medication. Therefore, it is unlikely to be used during pregnancy or breastfeeding. Testosterone can cause birth defects in a fetus. A pregnant woman should avoid coming into contact with testosterone topical gel, or with a man's skin areas where a testosterone topical patch has been worn or the gel has been applied. If contact occurs, wash with soap and water right away.
Additional Information
Our Androderm (testosterone transdermal system) Side Effects Drug Center provides a comprehensive view of available drug information on the potential side effects when taking this medication.
Androderm Consumer Information
Get emergency medical help if you have signs of an allergic reaction: hives; difficulty breathing; swelling of your face, lips, tongue, or throat.
Stop using testosterone topical and call your doctor at once if you have:
- increased urination (many times per day), loss of bladder control;
- painful or difficult urination;
- breast pain or swelling;
- painful or bothersome erections;
- swelling, rapid weight gain, shortness of breath during sleep;
- chest pain or pressure, pain spreading to your jaw or shoulder;
- liver problems--nausea, upper stomach pain, itching, tired feeling, loss of appetite, dark urine, clay-colored stools, jaundice (yellowing of the skin or eyes);
- signs of a blood clot in the lung--chest pain, sudden cough, wheezing, rapid breathing, coughing up blood; or
- signs of a blood clot deep in the body--swelling, warmth, or redness in an arm or leg.
Topical testosterone is absorbed through the skin and can cause side effects or symptoms of male features in a child or woman who comes into contact with this medicine. Call your doctor if a person who has close contact with you develops enlarged genitals, premature pubic hair, increased libido, aggressive behavior, male-pattern baldness, excessive body hair growth, increased acne, irregular menstrual periods, or any signs of male characteristics.
Common side effects may include:
- redness, itching, burning, hardened skin or other irritation where the medicine was applied or where the skin patch was worn;
- increased red blood cells (may cause dizziness, itching, redness in your face, or muscle pain);
- increased prostate-specific antigen;
- increased blood pressure;
- headache;
- mood changes, strange dreams;
- frequent or prolonged erections;
- nausea, vomiting; or
- swelling in your lower legs.
This is not a complete list of side effects and others may occur. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
Read the entire detailed patient monograph for Androderm (Testosterone Transdermal System)
Androderm Professional Information
What is Olumiant?
- Olumiant is a prescription medicine used to treat adult patients with moderately to severely active rheumatoid arthritis after treatment with at least one other medicine called a Tumor Necrosis Factor (TNF) antagonist has been used, and did not work well enough or could not be tolerated.
- It is not known if Olumiant is safe and effective in children.
Before taking Olumiant, tell your healthcare provider about all your medical conditions, including if you:
- See “What is the most important information I should know about Olumiant?”
- have an infection.
- have kidney problems.
- have liver problems.
- have low red or white blood cell counts.
- have recently received or are scheduled to receive a vaccine. People who take Olumiant should not receive live vaccines.
- have any stomach area (abdominal) pain or been diagnosed with diverticulitis or ulcers in your stomach or intestines.
- are pregnant or plan to become pregnant. It is not known if Olumiant will harm an unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if Olumiant passes into your breast milk. You and your healthcare provider should decide if you will take Olumiant or breastfeed. You should not do both.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Olumiant and other medicines may affect each other causing side effects.
Especially tell your healthcare provider if you take:
- a medicine called probenecid.
- any other medicines to treat your rheumatoid arthritis. For example, you should not take tocilizumab (Actemra®), etanercept (Enbrel®), adalimumab (Humira®), infliximab (Remicade®), rituximab (Rituxan®), abatacept (Orencia®), anakinra (Kineret®), certolizumab pegol (Cimzia®), golimumab (Simponi®), tofacitinib (Xeljanz®, Xeljanz® XR), sarilumab (Kevzara®), azathioprine or cyclosporine while you are taking Olumiant. Taking Olumiant with these medicines may increase your risk of infection.
Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine.
How should I take Olumiant?
- Take Olumiant exactly as your healthcare provider tells you to take it.
- Take Olumiant 1 time a day with or without food.
What are the possible side effects of Olumiant?
Olumiant can cause serious side effects including:
- See “What is the most important information I should know about Olumiant?”
Common side effects of Olumiant include (these are not all of the possible side effects of Olumiant):
- upper respiratory tract infections (common cold, sinus infections)
- nausea
- cold sores
- shingles
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
WARNING
SERIOUS INFECTION, MALIGNANCY, AND THROMBOSIS
Serious Infections
Patients treated with Olumiant are at risk for developing serious infections that may lead to hospitalization or death [see WARNINGS AND PRECAUTIONS and ADVERSE REACTIONS]. Most patient who developed these infections were taking concomitant immunosuppressants such as methotrexate or corticosteroids.
If a serious infection develops, interrupt Olumiant until the infection is controled.
Reported infections include:
- Active tuberculosis, which may present with pulmonary or extrapulmonary disease. Patients should be tested for latent tuberculosis before initiating Olumiant and during therapy. Treatment for latent infection should be considered prior to Olumiant use.
- Invasive fungal infections, including candidiasis and pneumocystosis. Patients with invasive fungal infections may present with disseminated, rather then locatized, disease.
- Bacterial, viral, and other infections due to opportunistic pathogens.
The risks and benefits of treatment with Olumiant should be carefully considered prior to initiating therapy in patients with chronic or recurrent infections.
Patients should be closely monitored for the developement of signs and symptoms of infection during and after treatment with Olumiant including the possible development of tuberculosis in patients who tested negative for latent tuberculosis infection prior to initiating therapy [see WARNINGS AND PRECAUTIONS].
Malignancies
Lymphoma and other malignancies have been observed in patients treated with Olumiant [see WARNINGS AND PRECAUTIONS].
Thrombosis
Thrombosis, including deep venous thrombosis and pulmonary embolism, has been observed at an increased incidence in patients treated with Olumiant compared to placebo. In addition, there were cases of arterial thrombosis. Many of these adverse events were serious and some resulted in death. Patients with symptoms of thrombosis should be promptly evaluated. [See WARNINGS AND PRECAUTIONS].
DESCRIPTION
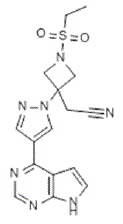
Olumiant (baricitinib) is a Janus kinase (JAK) inhibitor with the chemical name {1-(ethylsulfonyl)-3-[4-(7Hpyrrolo[ 2,3-d]pyrimidin-4-yl)-1H-pyrazol-1-yl]azetidin-3-yl}acetonitrile. Baricitinib has an empirical formula of C16H17N7O2S and a molecular weight of 371.42. Baricitinib has the following structural formula:
 |
Olumiant tablets contain a recessed area on each face of the tablet surface and are available for oral administration as debossed, film-coated, immediate-release tablets. The 2 mg tablet is light pink, oblong, debossed with “Lilly” on one side and “2” on the other.
Each tablet contains 2 mg of baricitinib and the following inactive ingredients: croscarmellose sodium, magnesium stearate, mannitol, microcrystalline cellulose, ferric oxide, lecithin (soya), polyethylene glycol, polyvinyl alcohol, talc and titanium dioxide.
Read the entire FDA prescribing information for Androderm (Testosterone Transdermal System)
© Androderm Patient Information is supplied by Cerner Multum, Inc. and Androderm Consumer information is supplied by First Databank, Inc., used under license and subject to their respective copyrights.
