Sirturo Drug Description: Detailed Datasheet
- Generic Name: bedaquiline tablets
- Brand Name: Sirturo
- Drug Class: Antitubercular Agents
side effects drug center sirturo (bedaquiline tablets) drug
Drug Description
What is Sirturo and how is it used?
Sirturo is a prescription medicine used to treat the symptoms of Multidrug Resistant Pulmonary Tuberculosis. Sirturo may be used alone or with other medications.
Sirturo belongs to a class of drugs called Antitubercular Agents.
It is not known if Sirturo is safe and effective in children younger than 5 years of age.
What are the possible side effects of Sirturo?
Sirturo may cause serious side effects including:
- hives,
- difficulty breathing,
- swelling of your face, lips, tongue, or throat,
- fast or pounding heartbeats,
- fluttering in your chest,
- shortness of breath,
- sudden dizziness,
- chest pain,
- coughing up blood,
- vomit that looks like coffee grounds,
- loss of appetite,
- stomach pain (upper right side),
- tiredness,
- dark urine,
- clay-colored stools, and
- yellowing of the skin or eyes (jaundice)
Get medical help right away, if you have any of the symptoms listed above.
The most common side effects of Sirturo include:
- nausea,
- stomach pain,
- coughing up blood,
- headache,
- skin rash, and
- joint pain
Tell the doctor if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of Sirturo. For more information, ask your doctor or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
WARNING
INCREASED MORTALITY; QT PROLONGATION
Increased Mortality
- An increased risk of death was seen in the SIRTURO treatment group (9/79, 11.4%) compared to the placebo treatment group (2/81, 2.5%) in one placebo-controlled trial. Only use SIRTURO when an effective treatment regimen cannot otherwise be provided [see INDICATIONS and WARNINGS AND PRECAUTIONS].
QT Prolongation
- QT prolongation can occur with SIRTURO. Use with drugs that prolong the QT interval may cause additive QT prolongation. Monitor ECGs. Discontinue SIRTURO if significant ventricular arrhythmia or if QTcF interval prolongation of greater than 500 ms develops [see WARNINGS AND PRECAUTIONS].
DESCRIPTION
SIRTURO (bedaquiline) for oral administration is available as 100 mg strength tablets. Each tablet contains 120.89 mg of bedaquiline fumarate drug substance, which is equivalent to 100 mg of bedaquiline. Bedaquiline is a diarylquinoline antimycobacterial drug.
Bedaquiline fumarate is a white to almost white powder and is practically insoluble in aqueous media. The chemical name of bedaquiline fumarate is (1R, 2S)-1-(6-bromo-2-methoxy-3-quinolinyl)-4- (dimethylamino)-2-(1-naphthalenyl)-1-phenyl-2-butanol compound with fumaric acid (1:1). It has a molecular formula of C32H31BrN2O2 · C4H4O4 and a molecular weight of 671.58 (555.50 + 116.07). The molecular structure of bedaquiline fumarate is the following:
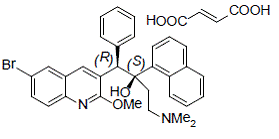 |
SIRTURO (bedaquiline) contains the following inactive ingredients: colloidal silicon dioxide, corn starch, croscarmellose sodium, hypromellose 2910 15 mPa.s, lactose monohydrate, magnesium stearate, microcrystalline cellulose, polysorbate 20, purified water (removed during processing).
Indications
SIRTURO is a diarylquinoline antimycobacterial drug indicated as part of combination therapy in the treatment of adult and pediatric patients (5 years and older and weighing at least 15 kg) with pulmonary multi-drug resistant tuberculosis (MDR-TB). Reserve SIRTURO for use when an effective treatment regimen cannot otherwise be provided.
This indication is approved under accelerated approval based on time to sputum culture conversion [see Clinical Studies]. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials.
Limitations Of Use
- Do not use SIRTURO for the treatment of:
- Latent infection due to Mycobacterium tuberculosis
- Drug-sensitive tuberculosis
- Extra-pulmonary tuberculosis
- Infections caused by non-tuberculous mycobacteria
- The safety and efficacy of SIRTURO in the treatment of HIV infected patients with MDR-TB have not been established as clinical data are limited [see Clinical Studies].
Dosage
DOSAGE AND ADMINISTRATION
Important Administration Instructions
- Administer SIRTURO by directly observed therapy (DOT).
- Only use SIRTURO in combination with at least 3 other drugs to which the patient’s MDR-TB isolate has been shown to be susceptible in vitro. If in vitro testing results are unavailable, SIRTURO treatment may be initiated in combination with at least 4 other drugs to which the patient’s MDR-TB isolate is likely to be susceptible. Refer to the prescribing information of the drugs used in combination with SIRTURO for further information.
- SIRTURO (20 mg and 100 mg) must be taken with food.
- SIRTURO 20 mg are functionally scored tablets which can be split at the scored lines into two equal halves of 10 mg each to provide doses less than 20 mg [see Method of Administration].
- As an alternative method of administration, SIRTURO 20 mg tablets can be dispersed in water and administered or dispersed in water and further mixed with a beverage or soft food, or crushed and mixed with soft food, or administered through a nasogastric tube [see Method of Administration].
- Emphasize the need for compliance with the full course of therapy.
Required Testing Prior To Administration
Prior to treatment with SIRTURO, obtain the following:
- Susceptibility information for the background regimen against M. tuberculosis isolate if possible [see Important Administration Instructions]
- ECG [see WARNINGS AND PRECAUTIONS]
- Serum potassium, calcium, and magnesium concentrations [see WARNINGS AND PRECAUTIONS]
- Liver enzymes [see WARNINGS AND PRECAUTIONS]
Recommended Dosage In Adult Patients
The recommended dosage of SIRTURO in adult patients is:
Table 1: Recommended Dosage of SIRTURO In Adult Patients
| Dosage Recommendation | |
| Weeks 1 and 2 | Weeks 3 to 24a |
| 400 mg (4 of the 100 mg tablets OR 20 of the 20 mg tablets) orally once daily | 200 mg (2 of the 100 mg tablets OR 10 of the 20 mg tablets) orally three times per week |
| a=At least 48 hours between doses | |
Recommended dosage in pediatric patients are described in Table 2 below [See Recommended Dosage in Pediatric Patients (5 years and older and weighing at least 15 kg)].
The total duration of treatment with SIRTURO in adults is 24 weeks. Administer SIRTURO tablets with food.
Recommended Dosage In Pediatric Patients (5 Years And Older And Weighing At Least 15 kg)
The recommended dosage of SIRTURO in pediatric patients (5 years and older and weighing at least 15 kg) is based on body weight and shown in Table 2:
Table 2: Recommended Dosage of SIRTURO in Pediatric Patients (5 years and older and weighing at least 15 kg)
| Body Weight | Dosage Recommendation | |
| Weeks 1 and 2 | Weeks 3 to 24a | |
| 15 kg to less than 30 kg | 200 mg (2 of the 100 mg tablets OR 10 of the 20 mg tablets) orally once daily | 100 mg (1 of the 100 mg tablets OR 5 of the 20 mg tablets) orally three times per week |
| Greater than or equal to 30 kg | 400 mg (4 of the 100 mg tablets OR 20 of the 20 mg tablets) orally once daily | 200 mg (2 of the 100 mg tablets OR 10 of the 20 mg tablets) orally three times per week |
| a=At least 48 hours between doses | ||
The total duration of treatment with SIRTURO in pediatric patients is 24 weeks. Administer SIRTURO tablets with food.
Missed Dose
If a dose is missed during the first 2 weeks of treatment, do not administer the missed dose (skip the dose and then continue the daily dosing regimen). From Week 3 onwards, if a dose is missed, administer the missed dose as soon as possible, and then resume the 3 times a week dosing regimen. The total dose of SIRTURO during a 7-day period should not exceed the recommended weekly dose (with at least 24 hours between each intake).
Method Of Administration
There is one method of administration of SIRTURO 100 mg tablet and four different methods of administration of SIRTURO 20 mg tablet as follows:
- For SIRTURO 100 mg tablet, administer the tablet whole with water. Take with food.
- For SIRTURO 20 mg tablet, the four different methods of administration are outlined below. Each administration method requires SIRTURO to be taken with food.
Methods Of Administration Of SIRTURO 20 mg Tablet
Administration of 20 mg Tablets To Patients Who Can Swallow Intact Tablets
Administer SIRTURO 20 mg tablet whole or divided in half along the functional score line into two equal halves of 10 mg each. Administer SIRTURO 20 mg tablet with water. Take with food.
Administration Of 20 mg Tablets To Patients Who Cannot Swallow Intact Tablets
Dispersed In Water And Administered With Beverage Or Soft Food
For patients who have difficulty swallowing intact tablets, SIRTURO 20 mg tablet can be dispersed in water and administered. To aid with administration, the dispersed mixture in water can be further mixed with a beverage (e.g., water, milk products, apple juice, orange juice, cranberry juice or carbonated beverage) or soft food (e.g., yogurt, apple sauce, mashed banana or porridge) as follows:
- Disperse tablets in water (maximum of 5 tablets in 5 mL of water) in a drinking cup.
- Mix the contents of the cup well until the tablets are completely dispersed and then orally administer the contents of the cup immediately with food. To aid with administration, the dispersed mixture in water can be further mixed with at least 5 mL of beverage or 1 teaspoonful of soft food and then orally administer the contents of the cup immediately.
- If the total dose requires more than 5 tablets, repeat the above preparation steps with the appropriate number of additional tablets until the desired dose is reached.
- Ensure no tablet residue is left in the cup, rinse with beverage or add more soft food and orally administer the contents of the cup immediately.
Crushed And Mixed With Soft Food
SIRTURO 20 mg tablet can be crushed and mixed with soft food (e.g., yogurt, apple sauce, mashed banana or porridge) immediately prior to use and administered orally. Ensure no tablet residue is left in container, add more soft food and administer the contents immediately.
Administration Through A Nasogastric Tube
SIRTURO 20 mg tablet can be administered through a nasogastric tube (8 French or greater) as follows:
- Disperse 5 tablets or less in 50 mL of non-carbonated water and mix well. Mixture should be white to almost white with visible particles expected.
- Administer through the nasogastric tube immediately.
- Repeat with additional tablets until desired dose is reached.
- Rinse and flush with 25 mL of additional water to ensure no tablet residue is left in materials used for preparation or the nasogastric tube.
HOW SUPPLIED
Dosage Forms And Strengths
- SIRTURO 20 mg tablet: uncoated, white to almost white oblong functionally scored tablet, with a score line on both sides, debossed with “2” and “0” on one side and plain on the other side.
- SIRTURO 100 mg tablet: uncoated, white to almost white round biconvex tablet with debossing of “T” over “207” on one side and “100” on the other side.
SIRTURO® 20 mg tablets are supplied as uncoated white to almost white oblong functionally scored tablets with a score line on both sides, debossed with “2” and “0” on one side and plain on the other side.
SIRTURO 100 mg tablets are supplied as uncoated white to almost white round biconvex 100 mg tablets with debossing of “T” over “207” on one side and “100” on the other side.
SIRTURO tablets are packaged in white high density polyethylene (HDPE) bottles with child-resistant polypropylene (PP) closure with induction seal liner in the following configurations:
20 mg tablets - bottles of 60 tablets. Each bottle contains silica gel desiccant (NDC 59676Â702-60)
100 mg tablets - bottles of 188 tablets (NDC 59676-701-01).
Storage And Handling
SIRTURO 20 mg Tablet
Store in original container. Bottle contains desiccant. Do not discard desiccant. Protect from light and moisture. Keep the container tightly closed.
Store at 25°C (77°F); excursions permitted between 15°C to 30°C (59°F to 86°F). [See USP Controlled Room Temperature]
SIRTURO 100 mg Tablet
Dispense in original container. Store tablets dispensed outside the original container in a tight light-resistant container with an expiration date not to exceed 3 months. Protect from light. Keep the container tightly closed.
Store at 25°C (77°F); excursions permitted from 15°C to 30°C (59°F to 86°F) [see USP Controlled Room Temperature].
Manufactured by: Recipharm Pharmaservices Pvt. Ltd., Bangalore, India Manufactured for: Janssen Therapeutics, Division of Janssen Products, LP Titusville, NJ 08560. Revised: May 2020
Side Effects
The following serious adverse reactions are discussed elsewhere in the labeling:
- Increased mortality [see WARNINGS AND PRECAUTIONS]
- QT Prolongation [see WARNINGS AND PRECAUTIONS and CLINICAL PHARMACOLOGY]
- Hepatotoxicity [see WARNINGS AND PRECAUTIONS]
- Drug Interactions [see WARNINGS AND PRECAUTIONS]
Clinical Studies Experience
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly compared to the rates in the clinical studies of another drug and may not reflect the rates observed in clinical practice.
Clinical Studies Experience In Adults
Adverse reactions for SIRTURO were identified from the pooled safety data from 335 SIRTURO-exposed patients who received 8 weeks (Study 2) and 24 weeks (Studies 1 and 3) at the proposed dose. Studies 1 and 2 were randomized, double-blind, placebo-controlled trials in newly diagnosed patients with pulmonary MDR-TB. In both treatment arms, patients received SIRTURO or placebo in combination with other drugs used to treat MDR-TB. Study 3 was an open-label, noncomparative study with SIRTURO administered as part of an individualized pulmonary MDR-TB treatment regimen in previously treated patients.
In Study 1, 35% were Black, 17.5% were Hispanic, 12.5% were White, 9.4% were Asian, and 25.6% were of another race. Eight of 79 (10.1%) patients in the SIRTURO group and 16 of 81 (19.8%) patients in the placebo treatment group were HIV-infected. Seven (8.9%) SIRTURO-treated patients and six (7.4%) placebo-treated patients discontinued Study 1 because of an adverse reaction.
Table 3: Select Adverse Reactions from Study 1 That Occurred More Frequently Than Placebo During Treatment with SIRTURO
| Adverse Reactions | SIRTURO Treatment Group N=79 n (%) | Placebo Treatment Group N=81 n (%) |
| Nausea | 30 (38) | 26 (32) |
| Arthralgia | 26 (33) | 18 (22) |
| Headache | 22 (28) | 10 (12) |
| Hemoptysis | 14 (18) | 9 (11) |
| Chest Pain | 9 (11) | 6 (7) |
| Anorexia | 7 (9) | 3 (4) |
| Transaminases Increased* | 7 (9) | 1 (1) |
| Rash | 6 (8) | 3 (4) |
| Blood Amylase Increased | 2 (3) | 1 (1) |
| * Terms represented by 'transaminases increased' included transaminases increased, AST increased, ALT increased, hepatic enzyme increased, and hepatic function abnormal. | ||
No additional unique adverse reactions were identified from the uncontrolled Study 3.
In both Studies 1 and 2, aminotransferase elevations of at least 3 times the upper limit of normal developed more frequently in the SIRTURO treatment group (11/102 [10.8%] vs 6/105 [5.7%]) than in the placebo treatment group. In Study 3, 22/230 (9.6%) patients had alanine aminotransferase (ALT) or aspartate aminotransferase (AST) greater than or equal to 3 times the upper limit of normal during the overall treatment period.
Increased Mortality
In Study 1, there was a statistically significant increased mortality risk by Week 120 in the SIRTURO treatment group compared to the placebo treatment group (9/79 (11.4%) versus 2/81 (2.5%), p-value=0.03, an exact 95% confidence interval of the difference [1.1%, 18.2%]). Five of the 9 SIRTURO deaths and the 2 placebo deaths were tuberculosis-related. One death occurred during the 24-week SIRTURO treatment period. The median time to death for the remaining eight patients in the SIRTURO treatment group was 329 days after last intake of SIRTURO. The imbalance in deaths is unexplained; no discernible pattern between death and sputum conversion, relapse, sensitivity to other drugs used to treat tuberculosis, HIV status, and severity of disease was observed.
In the open-label Study 3, 6.9% (16/233) of patients died. The most common cause of death as reported by the investigator was TB (9 patients). All but one patient who died of TB had not converted or had relapsed. The causes of death in the remaining patients varied.
Clinical Studies Experience In Pediatric Patients
The safety assessment of bedaquiline is based on the Week 24 analysis from 30 pediatric patients in an ongoing, single-arm, open-label, multi-cohort trial, (Study 4).
Pediatric Patients (12 Years To Less Than 18 Years Of Age)
The first cohort was designed to enroll patients 12 years to less than 18 years of age (fifteen patients 14 years to less than 18 years of age were enrolled) with confirmed or probable pulmonary MDR-TB infection who received SIRTURO (400 mg once daily for the first 2 weeks and 200 mg 3 times/week for the following 22 weeks) in combination with a background regimen [see Clinical Studies].
The most common adverse reactions were arthralgia in 6/15 (40%) patients, nausea in 2/15 (13%) patients, and abdominal pain in 2/15 (13%) patients. Among the 15 patients, no deaths occurred during treatment with SIRTURO. Observed laboratory abnormalities were comparable to those in adults.
Pediatric Patients (5 Years To Less Than 12 Years Of Age)
The second cohort was designed to enroll patients 5 years to less than 12 years of age (fifteen patients aged 5 years to less than 11 years of age were enrolled) with confirmed or probable pulmonary MDR-TB infection who received SIRTURO (200 mg once daily for the first 2 weeks and 100 mg 3 times/week for the following 22 weeks) in combination with a background regimen [see Clinical Studies].
The most common adverse reactions were related to elevations in liver enzymes (5/15, 33%), and led to discontinuation of SIRTURO in three patients. Elevations in liver enzymes were reversible upon discontinuation of SIRTURO and some of the background regimen drugs. Among these 15 pediatric patients, no deaths occurred during treatment with SIRTURO.
Drug Interactions
CYP3A4 Inducers/Inhibitors
Bedaquiline exposure may be reduced during co-administration with inducers of CYP3A4 and increased during co-administration with inhibitors of CYP3A4.
CYP3A4 Inducers
Due to the possibility of a reduction of the therapeutic effect of bedaquiline because of the decrease in systemic exposure, co-administration of strong CYP3A4 inducers, such as rifamycins (i.e., rifampin, rifapentine and rifabutin), or moderate CYP3A4 inducers should be avoided during treatment with SIRTURO [see CLINICAL PHARMACOLOGY].
CYP3A4 Inhibitors
Due to the potential risk of adverse reactions to bedaquiline because of the increase in systemic exposure, prolonged co-administration of bedaquiline and strong CYP3A4 inhibitors, such as ketoconazole or itraconazole, for more than 14 consecutive days should be avoided unless the benefit outweighs the risk [see CLINICAL PHARMACOLOGY]. Appropriate clinical monitoring for SIRTURO-related adverse reactions is recommended.
Other Antimicrobial Medications
No dose-adjustment of isoniazid or pyrazinamide is required during co-administration with SIRTURO.
In a placebo-controlled clinical trial in adult patients with MDR-TB, no major impact of co-administration of SIRTURO on the pharmacokinetics of ethambutol, kanamycin, pyrazinamide, ofloxacin or cycloserine was observed.
Antiretroviral Medications
Lopinavir/Ritonavir
Although clinical data in HIV/MDR-TB co-infected patients on the combined use of lopinavir (400 mg)/ritonavir (100 mg) with SIRTURO are not available, use SIRTURO with caution when co-administered with lopinavir/ritonavir and only if the benefit outweighs the risk [see CLINICAL PHARMACOLOGY].
Nevirapine
No dosage adjustment of bedaquiline is required when co-administered with nevirapine [see CLINICAL PHARMACOLOGY].
Efavirenz
Concomitant administration of bedaquiline and efavirenz, or other moderate CYP3A inducers, should be avoided [see WARNINGS AND PRECAUTIONS].
QT Interval Prolonging Drugs
In a drug interaction study of bedaquiline and ketoconazole in adults, a greater effect on QTc was observed after repeated dosing with bedaquiline and ketoconazole in combination than after repeated dosing with the individual drugs. Additive or synergistic QT prolongation was observed when bedaquiline was co-administered with other drugs that prolong the QT interval.
In Study 3, mean increases in QTc were larger in the 17 adult patients who were taking clofazimine with bedaquiline at Week 24 (mean change from reference of 31.9 ms) than in patients who were not taking clofazimine with bedaquiline at Week 24 (mean change from baseline of 12.3 ms). Monitor ECGs if SIRTURO is co-administered to patients receiving other drugs that prolong the QTc interval, and discontinue SIRTURO if evidence of serious ventricular arrhythmia or QTcF interval greater than 500 ms. [see WARNINGS AND PRECAUTIONS and CLINICAL PHARMACOLOGY].
Warnings & Precautions
WARNINGS
Included as part of the "PRECAUTIONS" Section
PRECAUTIONS
Reduced Efficacy In Nonvalvular Atrial Fibrillation Patients With CrCL > 95 mL/min
SAVAYSA should not be used in patients with CrCL > 95 mL/min. In the randomized ENGAGE AF-TIMI 48 study, NVAF patients with CrCL > 95 mL/min had an increased rate of ischemic stroke with SAVAYSA 60 mg daily compared to patients treated with warfarin. In these patients another anticoagulant should be used [see DOSAGE AND ADMINISTRATION and Clinical Studies].
Increased Risk Of Stroke With Discontinuation Of SAVAYSA In Patients With Nonvalvular Atrial Fibrillation
Premature discontinuation of any oral anticoagulant in the absence of adequate alternative anticoagulation increases the risk of ischemic events. If SAVAYSA is discontinued for a reason other than pathological bleeding or completion of a course of therapy, consider coverage with another anticoagulant as described in the transition guidance [see DOSAGE AND ADMINISTRATION and Clinical Studies].
Risk Of Bleeding
SAVAYSA increases the risk of bleeding and can cause serious and potentially fatal bleeding. Promptly evaluate any signs or symptoms of blood loss.
Discontinue SAVAYSA in patients with active pathological bleeding.
Concomitant use of drugs affecting hemostasis may increase the risk of bleeding. These include aspirin and other antiplatelet agents, other antithrombotic agents, fibrinolytic therapy, chronic use of nonsteroidal anti-inflammatory drugs (NSAIDs), selective serotonin reuptake inhibitors (SSRIs) and serotonin norepinephrine reuptake inhibitors (SNRIs) [see DRUG INTERACTIONS].
Reversal Of Anticoagulant Effect
There is no established way to reverse the anticoagulant effects of SAVAYSA, which can be expected to persist for approximately 24 hours after the last dose. The anticoagulant effect of SAVAYSA cannot be reliably monitored with standard laboratory testing. A specific reversal agent for edoxaban is not available. Hemodialysis does not significantly contribute to edoxaban clearance [see CLINICAL PHARMACOLOGY]. Protamine sulfate, vitamin K, and tranexamic acid are not expected to reverse the anticoagulant activity of SAVAYSA. The use of prothrombin complex concentrates (PCC), or other procoagulant reversal agents such as activated prothrombin complex concentrate (APCC) or recombinant factor VIIa (rFVIIa) may be considered but has not been evaluated in clinical outcome studies [see CLINICAL PHARMACOLOGY]. When PCCs are used, monitoring for anticoagulation effect of edoxaban using clotting test (PT, INR, or aPTT) or anti-FXa activity is not useful and is not recommended.
Spinal/Epidural Anesthesia Or Puncture
When neuraxial anesthesia (spinal/epidural anesthesia) or spinal/epidural puncture is employed, patients treated with antithrombotic agents for prevention of thromboembolic complications are at risk of developing an epidural or spinal hematoma, which can result in long-term or permanent paralysis.
The risk of these events may be increased by the postoperative use of indwelling epidural catheters or the concomitant use of medicinal products affecting hemostasis. Indwelling epidural or intrathecal catheters should not be removed earlier than 12 hours after the last administration of SAVAYSA. The next dose of SAVAYSA should not be administered earlier than 2 hours after the removal of the catheter. The risk may also be increased by traumatic or repeated epidural or spinal puncture.
Monitor patients frequently for signs and symptoms of neurological impairment (e.g., numbness or weakness of the legs, bowel, or bladder dysfunction). If neurological compromise is noted, urgent diagnosis and treatment is necessary. Prior to neuraxial intervention the physician should consider the potential benefit versus the risk in anticoagulated patients or in patients to be anticoagulated for thromboprophylaxis.
Patients With Mechanical Heart Valves Or Moderate To Severe Mitral Stenosis
The safety and efficacy of SAVAYSA has not been studied in patients with mechanical heart valves or moderate to severe mitral stenosis. The use of SAVAYSA is not recommended in these patients [see Clinical Studies].
Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Advise patients of the following:
Instructions For Patient Use
- Advise patients to take SAVAYSA exactly as prescribed.
- Remind patients to not discontinue SAVAYSA without talking to the healthcare provider who prescribed it.
- Instruct patients to keep an adequate supply of tablets to ensure continuous dosing of SAVAYSA
- Instruct patients who cannot swallow the tablet whole to crush SAVAYSA, combine with 2 to 3 ounces of water or applesauce and ingest immediately.
- Instruct patients who require a gastric tube to crush the SAVAYSA tablet and mix it with 2 to 3 ounces of water before administering immediately via the gastric feeding tube.
- Inform patients that if a dose is missed, they should take SAVAYSA as soon as possible the same day, and resume the normal dosing schedule the following day. The dose should not be doubled to make up for a missing dose.
Bleeding Risk
- Advise patients that they may bleed more easily, may bleed longer, or bruise more easily when treated with SAVAYSA.
- Instruct patients to report any unusual bleeding immediately to their healthcare provider.
- For patients that are having neuraxial anesthesia or spinal puncture, advise patients to watch for signs and symptoms of spinal or epidural hematoma, such as back pain, tingling, numbness (especially in the lower limbs), muscle weakness, and stool or urine incontinence. If any of these symptoms occur, advise the patient to contact his or her physician immediately [see BOX WARNING].
Invasive Or Surgical Procedures
- Remind patients to inform their healthcare providers that they are taking SAVAYSA before any surgery, medical, or dental procedure is scheduled.
Concomitant Medication And Herbals
- Remind patients to inform their healthcare providers and dentists if they plan to take, or are taking any prescription medications, over-the-counter drugs or herbal products.
Pregnancy
- Remind patients to inform their healthcare provider immediately if they become pregnant or intend to become pregnant during treatment with SAVAYSA
- Inform patients to not breastfeed if they are taking SAVAYSA [see Use In Specific Populations].
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment Of Fertility
Edoxaban was not carcinogenic when administered daily to mice and rats by oral gavage for up to 104 weeks. The highest dose tested (500 mg/kg/day) in male and female mice was 3 and 6 times, respectively, the human exposure (AUC) at the human dose of 60 mg/day, and the highest doses tested in male (600/400 mg/kg/day) and female (200 mg/kg/day) rats were 8 and 14 times, respectively, the human exposure at the human dose of 60 mg/day.
Edoxaban and its human-specific metabolite, M-4, were genotoxic in in vitro chromosomal aberration tests but were not genotoxic in the in vitro bacterial reverse mutation (Ames test), in in vitro human lymphocytes micronucleus test, in in vivo rat bone marrow micronucleus test, in in vivo rat liver micronucleus test, and in in vivo unscheduled DNA synthesis tests.
Edoxaban showed no effects on fertility and early embryonic development in rats at doses of up to 1000 mg/kg/day (162 times the human dose of 60 mg/day normalized to body surface area).
Use In Specific Populations
Pregnancy
Risk Summary
Available data about SAVAYSA use in pregnant women are insufficient to determine whether there are drug-associated risks for adverse developmental outcomes. In animal developmental studies, no adverse developmental effects were seen when edoxaban was administered orally to pregnant rats and rabbits during organogenesis at up to 16-times and 8-times, respectively, the human exposure, when based on body surface area and AUC, respectively (see Data).
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Clinical Considerations
Disease-associated maternal and/or embryo/fetal risk
Pregnancy confers an increased risk of thromboembolism that is higher for women with underlying thromboembolic disease and certain high-risk pregnancy conditions. Published data describe that women with a previous history of venous thrombosis are at high risk for recurrence during pregnancy.
Fetal/Neonatal adverse reactions
Use of anticoagulants, including edoxaban, may increase the risk of bleeding in the fetus and neonate. Monitor neonates for bleeding [see WARNINGS AND PRECAUTIONS].
Labor or delivery
All patients receiving anticoagulants, including pregnant women, are at risk for bleeding. SAVAYSA use during labor or delivery in women who are receiving neuraxial anesthesia may result in epidural or spinal hematomas. Consider use of a shorter acting anticoagulant as delivery approaches [see WARNINGS AND PRECAUTIONS].
Data
Animal Data
Embryo-fetal development studies were conducted in pregnant rats and rabbits during the period of organogenesis. In rats, no malformation was seen when edoxaban was administered orally at doses up to 300 mg/kg/day, or 49 times the human dose of 60 mg/day normalized to body surface area. Increased post-implantation loss occurred at 300 mg/kg/day, but this effect may be secondary to the maternal vaginal hemorrhage seen at this dose. In rabbits, no malformation was seen at doses up to 600 mg/kg/day (49 times the human exposure at a dose of 60 mg/day when based on AUC). Embryo-fetal toxicities occurred at maternally toxic doses, and included absent or small fetal gallbladder at 600 mg/kg/day, and increased post-implantation loss, increased spontaneous abortion, and decreased live fetuses and fetal weight at doses equal to or greater than 200 mg/kg/day, which is equal to or greater than 20 times the human exposure.
In a rat pre- and post-natal developmental study, edoxaban was administered orally during the period of organogenesis and through lactation day 20 at doses up to 30 mg/kg/day, which is up to 3 times the human exposure when based on AUC. Vaginal bleeding in pregnant rats and delayed avoidance response (a learning test) in female offspring were seen at 30 mg/kg/day.
Lactation
Risk Summary
There are no data on the presence of edoxaban in human milk, or its effects on the breastfeeding infant or on milk production. Edoxaban was present in rat milk. Because of the potential for serious adverse reactions in nursing infants, including hemorrhage, advise patients that breastfeeding is not recommended during treatment with SAVAYSA.
Pediatric Use
Safety and effectiveness in pediatric patients have not been established.
Geriatric Use
Of the total patients in the ENGAGE AF-TIMI 48 study, 5182 (74%) were 65 years and older, while 2838 (41%) were 75 years and older. In Hokusai VTE, 1334 (32%) patients were 65 years and older, while 560 (14%) patients were 75 years and older. In the Hokusai VTE Cancer Study, 539 (52%) patients were 65 years and older and 176 (17%) were 75 years and older. In clinical trials the efficacy and safety of SAVAYSA in elderly (65 years or older) and younger patients were similar [see ADVERSE REACTIONS, CLINICAL PHARMACOLOGY, and Clinical Studies].
Renal Impairment
Renal clearance accounts for approximately 50% of the total clearance of edoxaban. Consequently, edoxaban blood levels are increased in patients with poor renal function compared to those with higher renal function. Reduce SAVAYSA dose to 30 mg once daily in patients with CrCL 15-50 mL/min. There are limited clinical data with SAVAYSA in patients with CrCL < 15 mL/min; SAVAYSA is therefore not recommended in these patients. Hemodialysis does not significantly contribute to SAVAYSA clearance [see DOSAGE AND ADMINISTRATION and CLINICAL PHARMACOLOGY].
As renal function improves and edoxaban blood levels decrease, the risk for ischemic stroke increases in patients with NVAF [see INDICATIONS, DOSAGE AND ADMINISTRATION, and Clinical Studies].
Hepatic Impairment
The use of SAVAYSA in patients with moderate or severe hepatic impairment (Child-Pugh B and C) is not recommended as these patients may have intrinsic coagulation abnormalities. No dose reduction is required in patients with mild hepatic impairment (Child-Pugh A) [see CLINICAL PHARMACOLOGY].
Low Body Weight Consideration For Patients Treated For DVT And/Or PE
Based on the clinical experience from the Hokusai VTE study, reduce SAVAYSA dose to 30 mg in patients with body weight less than or equal to 60 kg [see DOSAGE AND ADMINISTRATION and Clinical Studies].
Overdosage & Contraindications
OVERDOSE
There is no experience with the treatment of acute overdose with SIRTURO. Take general measures to support basic vital functions including monitoring of vital signs and ECG (QT interval) in case of deliberate or accidental overdose. It is advisable to contact a poison control center to obtain the latest recommendations for the management of an overdose. Since bedaquiline is highly protein-bound, dialysis is not likely to significantly remove bedaquiline from plasma.
CONTRAINDICATIONS
None.
Clinical Pharmacology
Mechanism Of Action
Bedaquiline is a diarylquinoline antimycobacterial drug [see Microbiology].
Pharmacodynamics
Bedaquiline is primarily subjected to oxidative metabolism leading to the formation of N-monodesmethyl metabolite (M2). M2 is not thought to contribute significantly to clinical efficacy given its lower average exposure (23% to 31%) in humans and lower antimycobacterial activity (4-fold to 6-fold lower) compared to the parent compound. However, M2 plasma concentrations appeared to correlate with QT prolongation.
Cardiac Electrophysiology
In Study 1, in adults, the mean increases in QTcF, corrected using the Fridericia method, were greater in the SIRTURO treatment group compared to the placebo treatment group from the first week of treatment (9.9 ms at Week 1 for SIRTURO and 3.5 ms for placebo). The largest mean increase in QTcF during the 24 weeks of SIRTURO treatment was 15.7 ms compared to 6.2 ms with placebo treatment (at Week 18). After bedaquiline treatment ended, the QTcF gradually decreased, and the mean value was similar to that in the placebo group by study week 60.
In Study 3, where adult patients with no treatment options received other QT-prolonging drugs used to treat tuberculosis, including clofazimine, concurrent use with SIRTURO resulted in additive QTcF prolongation, proportional to the number of QT prolonging drugs in the treatment regimen. Patients taking SIRTURO alone with no other QT prolonging drug developed a mean QTcF increase over baseline of 23.7 ms with no QTcF segment duration in excess of 480 ms, whereas patients taking at least 2 other QT prolonging drugs developed a mean QTcF prolongation of 30.7 ms over baseline, and resulted in QTcF segment duration in excess of 500 ms in one patient [see WARNINGS AND PRECAUTIONS].
Pharmacokinetics
The pharmacokinetic parameters of bedaquiline in adult MDR-TB patients at the recommended dosing regimen of SIRTURO (400 mg for 2 weeks followed by 200 mg three times per week for 22 weeks) in combination with a background regimen are provided in Table 4.
Table 4: Pharmacokinetic Parameters of Bedaquiline Following Repeat Dose Administration of SIRTURO at the Recommended Dosing Regimen to Adult MDR-TB Patients at Week 8 Administered with Food (N = 18)
| Pharmacokinetic Parameter | Bedaquiline Mean (SD) |
| AUC24h (ng•h/mL) | 25,863 (13,259) |
| Cmax (ng/mL) | 1,659 (722) |
| Tmax (h)* | 5 (3-8) |
| Cmin (ng/mL) | 654 (498) |
| SD=Standard deviation * Median (range) | |
Absorption
After single oral dose administration of SIRTURO, maximum plasma concentrations (Cmax) are typically achieved at approximately 5 hours post-dose. Cmax and the area under the plasma concentration-time curve (AUC) increased proportionally up 700 mg (1.75 times the 400 mg loading dose).
Administration of SIRTURO with a standard meal containing approximately 22 grams of fat (558 total Kcal) increased the relative bioavailability by approximately 2-fold compared to administration under fasted conditions. SIRTURO should be taken with food to enhance its oral bioavailability.
Distribution
The plasma protein binding of bedaquiline is greater than 99.9%. The volume of distribution in the central compartment is estimated to be approximately 164 Liters.
Elimination
After reaching Cmax, bedaquiline concentrations decline tri-exponentially. The mean terminal elimination half-life of bedaquiline and the N-monodesmethyl metabolite (M2) is approximately 5.5 months. This long terminal elimination phase likely reflects slow release of bedaquiline and M2 from peripheral tissues.
Metabolism
CYP3A4 was the major CYP isoenzyme involved in the in vitro metabolism of bedaquiline and the formation of the N-monodesmethyl metabolite (M2).
Excretion
Based on preclinical studies, bedaquiline is mainly excreted in feces. The urinary excretion of unchanged bedaquiline was less than or equal to 0.001% of the dose in clinical studies, indicating that renal clearance of unchanged drug is insignificant.
Specific Populations
Hepatic Impairment
After single-dose administration of 400 mg SIRTURO to 8 adult patients with moderate hepatic impairment (Child-Pugh B), mean exposure to bedaquiline and M2 (AUC672h) was approximately 20% lower compared to healthy subjects. SIRTURO has not been studied in patients with severe hepatic impairment. [See WARNINGS AND PRECAUTIONS and Use In Specific Populations].
Renal Impairment
SIRTURO has mainly been studied in adult patients with normal renal function. Renal excretion of unchanged bedaquiline is not substantial (less than or equal to 0.001%).
In a population pharmacokinetic analysis of MDR-TB adult patients treated with SIRTURO 200 mg three times per week, creatinine clearance was not found to influence the pharmacokinetic parameters of bedaquiline. It is therefore not expected that mild or moderate renal impairment will have a clinically relevant effect on the exposure to bedaquiline. However, in patients with severe renal impairment or end-stage renal disease requiring hemodialysis or peritoneal dialysis bedaquiline concentrations may be increased due to alteration of drug absorption, distribution, and metabolism secondary to renal dysfunction. As bedaquiline is highly bound to plasma proteins, it is unlikely that it will be significantly removed from plasma by hemodialysis or peritoneal dialysis [see Use In Specific Populations].
Sex
In a population pharmacokinetic analysis of MDR-TB adult patients treated with SIRTURO no clinically relevant difference in exposure between men and women were observed.
Race/Ethnicity
In a population pharmacokinetic analysis of MDR-TB adult patients treated with SIRTURO, systemic exposure (AUC) to bedaquiline was found to be 34% lower in Black patients than in patients from other race categories. This lower exposure was not considered to be clinically relevant as no clear relationship between exposure to bedaquiline and response has been observed in clinical trials of MDR-TB. Furthermore, response rates were comparable in patients of different race categories that completed 24 weeks of bedaquiline treatment.
HIV Co-Infection
There are limited data on the use of SIRTURO in HIV co-infected patients [see DRUG INTERACTIONS].
Geriatric Population
There are limited data on the use of SIRTURO in tuberculosis patients 65 years of age and older.
In a population pharmacokinetic analysis of MDR-TB adult patients treated with SIRTURO, age was not found to influence the pharmacokinetics of bedaquiline.
Pediatric Population
Pediatric Patients 12 Years To Less Than 18 Years Of Age With MDR-TB
The pharmacokinetic parameters of bedaquiline in 15 pediatric patients (body weight at baseline: 38 to 75 kg) who received the same adult dosage regimen of SIRTURO (400 mg once daily for the first 2 weeks and 200 mg 3 times/week for the following 22 weeks) in combination with a background regimen were comparable to those in adults. There was no impact of body weight on bedaquiline pharmacokinetics in this cohort.
Pediatric Patients 5 Years To Less Than 12 Years Of Age With MDR-TB
Fifteen MDR-TB pediatric patients (body weight at baseline: 14 to 36 kg) received SIRTURO (200 mg once daily for the first 2 weeks and 100 mg 3 times/week for the following 22 weeks) in combination with a background regimen. Of these 15 pediatric patients, complete pharmacokinetic data were obtained for 10 patients at the aforementioned dosage regimen of SIRTURO. In 9 of these 10 pediatric patients who weighed at least 15 kg at baseline, the mean bedaquiline Cmax and AUC24h were similar to that of adult MDR-TB patients receiving the recommended adult dosage regimen. In 1 of these 10 pediatric patients who weighed 14 kg at baseline, the bedaquiline mean Cmax and AUC24h were 3.8-fold and 2.6-fold, respectively, higher than the mean Cmax and AUC24h in adult MDR-TB patients administered the recommended adult dosage regimen. The clinical significance of this higher pharmacokinetic plasma exposure in this one pediatric patient is not known [see Use In Specific Populations].
See Table 5 for a summary of the pharmacokinetic parameters in pediatric patients 5 years to less than 18 years of age.
Table 5: Pharmacokinetic Parameters of Bedaquiline Following Repeat Dose Administration of SIRTURO to Pediatric MDR-TB Patients 5 to less than 18 years of age at Week 12 Administered with Food (N=25)
| Pharmacokinetic Parameter | Bedaquiline Mean (SD) | |
| 14 years to less than 18 years (N=15) | 5 years to less than 12 years (N=10) | |
| AUC24h (ng•h/mL) | 26,300 (10,300) | 32,200 (16,300) |
| Cmax (ng/mL) | 1,800 (736) | 2,430 (1,670) |
| Tmax (h)* | 4 (2-8) | 4 (2-8) |
| Cmin (ng/mL) | 544 (263) | 461 (173) |
| SD=Standard Deviation * Median (range) | ||
Drug-Drug Interactions
In vitro, bedaquiline does not significantly inhibit the activity of the following CYP450 enzymes that were tested: CYP1A2, CYP2A6, CYP2C8/9/10, CYP2C19, CYP2D6, CYP2E1, CYP3A4, CYP3A4/5 and CYP4A, and it does not induce CYP1A2, CYP2C9, CYP2C19, or CYP3A4 activities.
Bedaquiline is an in vitro substrate of CYP3A4, and because of this, the following clinical drug interaction studies were performed.
Ketoconazole
Co-administration of multiple-dose bedaquiline (400 mg once daily for 14 days) and multiple-dose ketoconazole (once daily 400 mg for 4 days) in healthy adult subjects increased the AUC24h, Cmax and Cmin of bedaquiline by 22% [90% CI (12; 32)], 9% [90% CI (-2, 21)] and 33% [90% CI (24, 43)] respectively [see DRUG INTERACTIONS].
Rifampin
In a drug interaction study of single-dose 300 mg bedaquiline and multiple-dose rifampin (once daily 600 mg for 21 days) in healthy adult subjects, the exposure (AUC) to bedaquiline was reduced by 52% [90% CI (-57; -46)] [see DRUG INTERACTIONS].
Antimicrobial Agents
The combination of multiple-dose bedaquiline 400 mg once daily with multiple-dose isoniazid/pyrazinamide (300 mg/2000 mg once daily) in healthy adult subjects did not result in clinically relevant changes in the exposure (AUC) to bedaquiline, isoniazid or pyrazinamide [see DRUG INTERACTIONS].
In a placebo-controlled study in adult patients with MDR-TB, no major impact of co-administration of bedaquiline on the pharmacokinetics of ethambutol, kanamycin, pyrazinamide, ofloxacin or cycloserine was observed.
Lopinavir/Ritonavir
In a drug interaction study in healthy adult volunteers of single-dose bedaquiline (400 mg) and multiple-dose lopinavir (400 mg)/ritonavir (100 mg) given twice daily for 24 days, the mean AUC of bedaquiline was increased by 22% [90% CI (11; 34)] while the mean Cmax was not substantially affected [see DRUG INTERACTIONS].
Nevirapine
Co-administration of multiple-dose nevirapine 200 mg twice daily for 4 weeks in HIV-infected adult patients with a single 400 mg dose of bedaquiline did not result in clinically relevant changes in the exposure to bedaquiline [see DRUG INTERACTIONS].
Efavirenz
Co-administration of a single dose of bedaquiline 400 mg and efavirenz 600 mg daily for 27 days to healthy adult volunteers resulted in approximately a 20% decrease in the AUCinf of bedaquiline; the Cmax of bedaquiline was not altered. The AUC and Cmax of the primary metabolite of bedaquiline (M2) were increased by 70% and 80%, respectively. The effect of efavirenz on the pharmacokinetics of bedaquiline and M2 following steady-state administration of bedaquiline has not been evaluated [see DRUG INTERACTIONS].
Microbiology
Mechanism Of Action
SIRTURO is a diarylquinoline antimycobacterial drug that inhibits mycobacterial ATP (adenosine 5'-triphosphate) synthase, by binding to subunit c of the enzyme that is essential for the generation of energy in M. tuberculosis.
Resistance
A potential for development of resistance to bedaquiline in M. tuberculosis exists. Modification of the atpE target gene, and/or upregulation of the MmpS5-MmpL5 efflux pump (Rv0678 mutations) have been associated with increased bedaquiline MIC values in isolates of M. tuberculosis. Target-based mutations generated in preclinical studies lead to 8-to 133-fold increases in bedaquiline MIC, resulting in MICs ranging from 0.25 to 4 micrograms per mL. Efflux-based mutations have been seen in preclinical and clinical isolates. These lead to 2-to 8-fold increases in bedaquiline MICs, resulting in bedaquiline MICs ranging from 0.25 to 0.5 micrograms per mL.
M. tuberculosis isolates from a clinical study in adult patients with MDR-TB that developed at least 4-fold increase in bedaquiline MIC were associated with mutations in Rv0678 gene that lead to upregulation of the MmpS5-MmpL5 efflux pump. Isolates with these efflux-based mutations are less susceptible to clofazimine. Isolates that are phenotypically resistant to bedaquiline should be tested for cross-resistance to clofazimine, if clofazimine is being considered as part of the treatment regimen. In Study 2 and 3 there was no clear relationship between the presence of Rv0678 mutations at baseline and treatment outcome.
Activity In Vitro And In Clinical Infections
SIRTURO has been shown to be active in vitro and in clinical infections against most isolates of M. tuberculosis [see INDICATIONS AND USAGE and Clinical Studies].
Susceptibility Testing
The bedaquiline agar (left) and resazurin microtiter assay1 (REMA; a 7H9 broth microdilution to which resazurin, a bacterial growth indicator, was added) (right) MIC distributions against clinical isolates resistant to isoniazid and rifampin from Studies 1, 2, and 3 are provided below.
Figure 1: Bedaquiline MIC Distribution against Baseline MDRH&R-TB Isolates from Studies 1, 2, and 3 mITT Adult Patients: Agar Method (left) and Broth (REMA) Method (right)
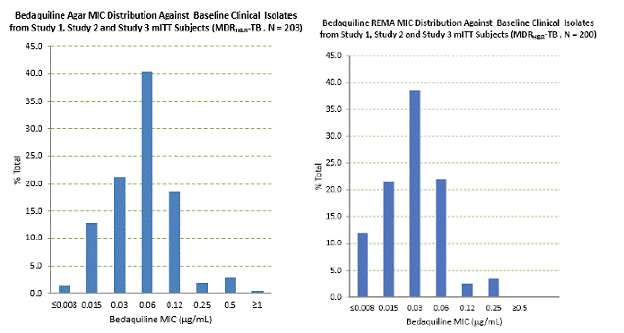 |
MICs for baseline M. tuberculosis isolates from patients in Studies 1 and 3 and their sputum culture conversion rates at Week 24 are shown in Table 6 below. Based on the available data, there was no trend for poor microbiologic outcomes related to baseline bedaquiline MIC.
Table 6: Culture Conversion Rates (Week 24 Data Selection, No Overruling for Discontinuation) at Week 24 By Baseline Bedaquiline MIC for mITT Patients from Study 1 and Study 3
| Baseline Bedaquiline MIC (micrograms/mL) | SIRTURO (Bedaquiline) Treatment Group 24-Week Culture Conversion Rate n/N (%) | |
| 7H11 Agar | 7H9 Broth (REMA) | |
| ≤ 0.008 | 2/2 (100) | 21/25 (84.0) |
| 0.015 | 13/15 (86.7) | 33/39 (84.6) |
| 0.03 | 36/46 (78.3) | 70/92 (76.1) |
| 0.06 | 82/107 (76.6) | 45/56 (80.4) |
| 0.12 | 36/42 (85.7) | 6/7 (85.7) |
| 0.25 | 3/4 (75.0) | 3/4 (75.0) |
| 0.5 | 5/6 (83.3) | 0/1 (0) |
| ≥ 1 | 0/1 (0) | |
| N=number of patients with data; n=number of patients with that result; MIC=minimum inhibitory concentration; BR=background regimen | ||
Nineteen patients in the efficacy population of study 3 had bedaquiline susceptibility testing results of paired (baseline and post-baseline, all of which were at Week 24 or later) genotypically identical isolates. Twelve of the 19 had a post-baseline ≥4-fold increase in bedaquiline MIC. Whole genome sequencing of 9 of these 12 post-baseline isolates was done and no mutations were found in the ATP synthase operon. All 9 were found to have a mutation in Rv0678. Eleven of the twelve (11/12) increases in bedaquiline MIC were seen in patients with pre-XDR-TB or with XDR-TB. Pre-XDR- TB is defined as MDR-TB isolates resistant to either a fluoroquinolone or a second line injectable drug, and XDR-TB as MDR-TB isolates resistant to both a fluoroquinolone and a second line injectable drug. Based on available data, response rate (culture conversion at week 120 endpoint) was similar in patients with ≥4-fold increases in bedaquiline MIC (5/12) and patients with < 4-fold increases (3/7).
For specific information regarding susceptibility test criteria and associated test methods and quality control standards recognized by FDA for this drug, please see: www.fda.gov/STIC.
Animal Toxicology And/Or Pharmacology
Bedaquiline is a cationic, amphiphilic drug that induced phospholipidosis (at almost all doses, even after very short exposures) in drug-treated animals, mainly in cells of the monocytic phagocytic system (MPS). All species tested showed drug-related increases in pigment-laden and/or foamy macrophages, mostly in the lymph nodes, spleen, lungs, liver, stomach, skeletal muscle, pancreas and/or uterus. After treatment ended, these findings were slowly reversible. Muscle degeneration was observed in several species at the highest doses tested. For example, the diaphragm, esophagus, quadriceps and tongue of rats were affected after 26 weeks of treatment at doses similar to clinical exposures based on AUC comparisons. These findings were not seen after a 12-week, treatment-free, recovery period and were not present in rats given the same dose biweekly. Degeneration of the fundic mucosa of the stomach, hepatocellular hypertrophy and pancreatitis were also seen.
Clinical Studies
Adult Patients
A placebo-controlled, double-blind, randomized trial (Study 1) was conducted in patients with newly diagnosed sputum smear-positive MDR pulmonary M. tuberculosis. All patients received a combination of five other antimycobacterial drugs used to treat MDR-TB (i.e., ethionamide, kanamycin, pyrazinamide, ofloxacin, and cycloserine/terizidone or available alternative) for a total duration of 18-24 months or at least 12 months after the first confirmed negative culture. In addition to this regimen, patients were randomized to receive 24 weeks of treatment with SIRTURO 400 mg once daily for the first 2 weeks followed by 200 mg 3 times per week for 22 weeks or matching placebo for the same duration. Overall, 79 patients were randomized to the SIRTURO arm and 81 to the placebo arm. A final evaluation was conducted at Week 120.
Sixty-seven patients randomized to SIRTURO and 66 patients randomized to placebo had confirmed MDR-TB, based on susceptibility tests (taken prior to randomization) or medical history if no susceptibility results were available, and were included in the efficacy analyses. Demographics were as follows: 63% of the study population was male, with a median age of 34 years, 35% were Black, and 15% were HIV-positive (median CD4 cell count 468 cells/μL). Most patients had cavitation in one lung (62%); and 18% of patients had cavitation in both lungs.
Time to sputum culture conversion was defined as the interval in days between the first dose of study drug and the date of the first of two consecutive negative sputum cultures collected at least 25 days apart during treatment. In this trial, the SIRTURO treatment group had a decreased time to culture conversion and improved culture conversion rates compared to the placebo treatment group at Week 24. Median time to culture conversion was 83 days for the SIRTURO treatment group compared to 125 days for the placebo treatment group. Table 7 shows the proportion of patients with sputum culture conversion at Week 24 and Week 120.
Table 7: Culture Conversion Status in Patients with MDR-TB at Week 24 and Week 120 in Study 1
| Microbiologic Status | SIRTURO (24 weeks) + combination of other anti mycobacterial drugs N=67 | Placebo (24 weeks) + combination of other anti mycobacterial drugs N=66 | Difference [95% CI] p-value |
| Week 24 | |||
| Sputum Culture Conversion | 78% | 58% | 20.0% [4.5%, 35.6%] 0.014 |
| Treatment failure* | 22% | 42% | |
| Died | 1% | 0% | |
| Lack of conversion | 21% | 35% | |
| Discontinuation | 0% | 8% | |
| Week 120* | |||
| Sputum Culture Conversion | 61% | 44% | 17.3% [0.5%, 34.0%] 0.046 |
| Treatment failure* | 39% | 56% | |
| Died | 12% | 3% | |
| Lack of conversion/relapse | 16% | 35% | |
| Discontinuation | 10% | 18% | |
| * A patient’s reason for treatment failure was counted only in the first row for which a patient qualifies. ** Patients received 24 weeks of SIRTURO or placebo for the first 24 weeks and received a combination of other antimycobacterial drugs for up to 96 weeks. | |||
Study 2 was a smaller placebo-controlled study designed similarly to Study 1 except that SIRTURO or placebo was given for only 8 weeks instead of 24 weeks. Patients were randomized to either SIRTURO and other drugs used to treat MDR-TB (SIRTURO treatment group) (n=23) or placebo and other drugs used to treat MDR-TB (placebo treatment group) (n=24). Twenty-one patients randomized to the SIRTURO treatment group and 23 patients randomized to the placebo treatment group had confirmed MDR-TB based on patients’ baseline M. tuberculosis isolate obtained prior to randomization. The SIRTURO treatment group had a decreased time to culture conversion and improved culture conversion rates compared to the placebo treatment group at Week 8. At Weeks 8 and 24, the differences in culture conversion proportions were 38.9% (95% CI: [12.3%, 63.1%] and p-value: 0.004), 15.7% (95% CI: [-11.9%, 41.9%] and p-value: 0.32), respectively.
Study 3 was a Phase 2b, uncontrolled study to evaluate the safety, tolerability, and efficacy of SIRTURO as part of an individualized MDR-TB treatment regimen in 233 patients with sputum smear positive (within 6 months prior to screening) pulmonary MDR-TB. Patients received SIRTURO for 24 weeks in combination with antibacterial drugs. Upon completion of the 24-week treatment with SIRTURO, all patients continued to receive their background regimen in accordance with national TB program (NTP) treatment guidelines. A final evaluation was conducted at Week 120. Treatment responses to SIRTURO at week 120 were generally consistent with those from Study 1.
Pediatric Patients (5 Years To Less Than 18 Years Of Age)
The pediatric trial, (Study 4, NCT02354014), was designed as a single-arm, open-label, multi-cohort trial to evaluate the pharmacokinetics, safety and tolerability of SIRTURO in combination with a background regimen in patients 5 to less than 18 years of age with confirmed or probable pulmonary MDR-TB infection.
Pediatric Patients (12 Years To Less Than 18 Years Of Age)
Fifteen patients ages 14 years to less than 18 years of age were enrolled in the first cohort. The median age was 16 years, 80% were female, 53% were Black, 33% were White and 13% were Asian. No patient 12 years to less than 14 years of age was enrolled in this cohort. SIRTURO was administered as 400 mg once daily for the first 2 weeks and 200 mg 3 times/week for the following 22 weeks using the 100 mg tablet.
In the subset of patients with culture positive pulmonary MDR-TB at baseline, treatment with bedaquiline resulted in conversion to a negative culture in 75.0% (6/8 patients) at Week 24.
Pediatric Patients (5 Years To Less Than 12 Years Of Age)
Fifteen patients ages 5 years to 10 years of age were enrolled in the second cohort. The median age was 7 years, 60% were female, 60% were Black, 33% were White and 7% were Asian. No patient older than 10 years to less than 12 years of age was enrolled in this cohort. The body weight range was 14 kg to 36 kg; only 1 patient weighing 14 kg was enrolled. SIRTURO was administered as 200 mg once daily for the first 2 weeks and 100 mg 3 times/week for the following 22 weeks using the 20 mg tablet.
In the subset of patients with culture positive pulmonary MDR-TB at baseline, treatment with bedaquiline resulted in conversion to a negative culture in 100% (3/3 patients) at Week 24.
REFERENCES
1. Martin A, Portaels F, Palomino JC. Colorimetric redox-indicator methods for the rapid detection of multidrug resistance in Mycobacterium tuberculosis: a systematic review and meta-analysis. J Antimicrob Chemother. 2007; 59 (2): 175-83.
Medication Guide
PATIENT INFORMATION
SIRTURO®
(ser-too-roh)
(bedaquiline) tablets, for oral use
Read this Medication Guide before you start taking SIRTURO® and each time you get a refill. There may be new information. This information does not take the place of talking with your healthcare provider about your medical condition or your treatment.
What is the most important information I should know about SIRTURO?
SIRTURO can cause serious side effects, including:
- Increased risk of death. Some people who had pulmonary tuberculosis resistant to other antibiotics (multi-drug resistant tuberculosis) and were treated with SIRTURO, had an increased risk in death.
- A serious heart rhythm problem called QT prolongation. This condition can cause an abnormal heartbeat in people who take SIRTURO and may lead to death. Your healthcare provider should check your heart and do blood tests before and during treament with SIRTURO. Tell your healthcare provider right away if you have a change in your heartbeat (a fast or irregular heartbeat) or if you feel dizzy or faint.
What is SIRTURO?
SIRTURO is a diarylquinoline antibiotic prescription medicine used in people 5 years of age and older with multi-drug resistant tuberculosis (MDR-TB) of the lungs when other effective treatment options are not possible.
It is not known if SIRTURO is safe and effective in:
- Â people who have a tuberculosis (TB) infection, but do not show symptoms of TB (also known as latent TB).
- people who have TB that is not resistant to antibiotics.
- people who have types of TB other than TB of the lungs.
- people who have an infection caused by bacteria other than TB.
- people who are being treated for Human Immunodeficiency Virus (HIV) who also have MDR-TB.
- children under 5 years of age or weighing less than 33 pounds (15 kg).
Before you take SIRTURO, tell your healthcare provider about all your medical conditions including, if you:
- take any other medicines for your heart.
- have had an abnormal heart rhythm (ECG) or other heart problems.
- have a family history of a heart problem called “congenital long QT syndrome” or heart failure.
- have decreased thyroid gland function (hypothyroidism).
- have liver or kidney problems.
- have HIV infection.
- are pregnant or plan to become pregnant. It is not known if SIRTURO will harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if SIRTURO passes into breast milk. Talk to your healthcare provider about the best way to feed your baby while taking SIRTURO.
- If you and your healthcare provider decide for you to breastfeed while taking SIRTURO, tell your healthcare provider right away if your baby has:
- yellowing of their eyes.
- lighter than usual stool color or stool that is pale or light brown.
- darker than usual urine color.
- If you and your healthcare provider decide for you to breastfeed while taking SIRTURO, tell your healthcare provider right away if your baby has:
Tell your healthcare provider about all the medicines you take, including prescription and over-thecounter medicines, vitamins, and herbal supplements.
You should not take certain liver medicines or herbal supplements while taking SIRTURO.
How should I take SIRTURO?
- Take SIRTURO exactly as your healthcare provider tells you to take it.
- You will take SIRTURO for a total of 24 weeks. You may need to take your other TB medicines for longer than 24 weeks. If you are not sure, you should talk with your healthcare provider.
- SIRTURO must always be taken with other medicines to treat TB. Your healthcare provider will decide which other medicines you should take with SIRTURO.
- It is important that you complete the full course of treatment with SIRTURO and not skip doses. Skipping doses may decrease the effectiveness of the treatment and increase the chances that your TB will not be treatable by SIRTURO or other medicines.
Week 1 and Week 2:
Take your prescribed dose 1 time each day.
Week 3 to Week 24:
- Take your prescribed dose 3 times a week.
- Take SIRTURO doses at least 48 hours apart. For example, you may take SIRTURO on Monday, Wednesday and Friday every week from week 3 to week 24.
- Do not skip SIRTURO doses. If you skip doses, or do not complete the total 24 weeks of SIRTURO, your treatment may not work as well, and your TB may be harder to treat.
- If you take more SIRTURO than you should, talk to a healthcare provider right away.
If you miss your SIRTURO dose during Week 1 or Week 2:
- Do not take a double dose to make up for the missed dose. Take the next dose as usual.
If you miss your SIRTURO dose during Week 3 to Week 24:
- Take the missed dose as soon as possible and continue taking SIRTURO on the 3 times a week schedule.
- Make sure that there is at least 24 hours between taking the missed dose and the next scheduled dose.
- Do not take more than the prescribed weekly dose in a 7-day period.
- If you miss a dose and you are not sure what to do, talk to your healthcare provider.
Do not stop taking SIRTURO without first talking to your healthcare provider.
SIRTURO 20 mg tablet
- Always take SIRTURO with food.
- Can swallow whole tablets:
- Swallow tablets whole with water or
- Split tablets in half at the score line in the middle of the tablet into 2 equal parts of 10 mg each, then swallow both half parts of SIRTURO with water.
- Cannot swallow whole tablets:
- Use 1 teaspoon (5ml) of water for a maximum of 5 SIRTURO tablets. Mix well in a drinking cup.
- Swallow mixture immediately, or
- To help with taking SIRTURO you may add at least 1 more teaspoon (5ml) of beverage or soft food and mix. Beverages such as water, milk products, apple juice, orange juice, cranberry juice or carbonated beverages may be used. Soft foods such as yogurt, apple sauce, mashed banana or porridge may be used.
- Swallow mixture immediately.
- Make sure no remaining medicine is left in the drinking cup, rinse with beverage or soft food and swallow mixture immediately.
- If you need more than 5 tablets of SIRTURO to get your total dose, repeat the steps above until you reach your prescribed dose.
- Use 1 teaspoon (5ml) of water for a maximum of 5 SIRTURO tablets. Mix well in a drinking cup.
- Can swallow whole tablets:
or
- Crush tablets and mix with soft food. Soft food such as yogurt, apple sauce, mashed bananas or porridge may be used. Swallow mixture immediately. Make sure no remaining medicine is left in container, add more soft food and swallow mixture immediately.
- SIRTURO 20 mg tablets may also be given through certain nasogastric tubes by your healthcare provider.
SIRTURO 100 mg tablet
- Always take SIRTURO with food. Swallow tablets whole with water.
What should I avoid while taking SIRTURO?
- You should not drink alcohol while taking SIRTURO.
What are the possible side effects of SIRTURO?
SIRTURO may cause serious side effects, including:
- See “What is the most important information I should know about SIRTURO?”
- liver problems (hepatotoxicity). Your healthcare provider should do blood tests to check your liver before you start taking SIRTURO and during treatment. Call your healthcare provider right away if you have unexplained symptoms such as nausea or vomiting, stomach pain, fever, weakness, itching, unusual tiredness, loss of appetite, light colored bowel movements, dark colored urine, yellowing of your skin or the white of your eyes.
The most common side effects of SIRTURO in adults include nausea, joint pain, headache, coughing up blood, or chest pain.
The most common side effects of SIRTURO in children 12 years to less than 18 years of age include joint pain, nausea and stomach pain.
The most common side effect in children 5 years to less than 12 years of age is increased level of liver enzymes in the blood.
These are not all the possible side effects of SIRTURO. For more information, ask your healthcare provider or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA1088.
How should I store SIRTURO?
- Store SIRTURO at room temperature between 68°F to 77°F (20°C to 25°C).
- Keep SIRTURO in the original container.
- Protect SIRTURO from light.
- The SIRTURO 20 mg container contains a desiccant packet to keep your medicine dry (protect it from moisture). Do not throw away (discard) the desiccant.
Keep SIRTURO and all medicines out of reach of children.
General information about the safe and effective use of SIRTURO: This Medication Guide summarizes the most important information about SIRTURO. If you would like more information, talk to your healthcare provider. You can ask your pharmacist or healthcare provider for information about SIRTURO that is written for health professionals.
What are the ingredients in SIRTURO?
Active ingredient: bedaquiline fumarate
SIRTURO 20 mg tablets contain the following inactive ingredients: colloidal silicon dioxide, crospovidone, hypromellose 2910 5 mPa s, polysorbate 20, purified water (removed during processing), silicified microcrystalline cellulose and sodium stearyl fumarate.
SIRTURO 100 mg tablets contain the following inactive ingredients: colloidal silicon dioxide, corn starch, croscarmellose sodium, hypromellose 2910, lactose monohydrate, magnesium stearate, microcrystalline cellulose, polysorbate 20, purified water (removed during processing)
This Medication Guide has been approved by the U.S. Food and Drug Administration.
