RyClora Drug Description: Detailed Datasheet
- Generic Name: dexchlorpheniramine maleate oral solution
- Brand Name: RyClora
side effects drug center ryclora (dexchlorpheniramine maleate oral solution) drug
Drug Description
What is RyClora and how is it used?
RyClora (dexchlorpheniramine maleate liquid) is an antihistamine used to treat perennial and seasonal allergic rhinitis, vasomotor rhinitis, allergic conjunctivitis due to inhalant allergens and foods, mild uncomplicated allergic skin manifestations of hives and angioedema, amelioration of allergic reactions to blood or plasma, dermographism, and as therapy for anaphylactic reactions adjunctive to epinephrine and other standard measures after the acute manifestations have been controlled.
What are side effects of RyClora?
Side effects of RyClora include:
- hives,
- drug rash,
- anaphylactic shock,
- photosensitivity,
- excessive sweating,
- chills,
- dry mouth, nose and throat,
- hemolytic anemia,
- thrombocytopenia,
- agranulocytosis,
- sedation,
- sleepiness,
- dizziness,
- disturbed coordination,
- fatigue,
- confusion,
- restlessness,
- excitation,
- nervousness,
- tremor,
- irritability,
- insomnia,
- euphoria,
- numbness and tingling,
- blurred vision,
- double vision,
- spinning sensation (vertigo),
- ringing in the ears (tinnitus),
- acute labyrinthitis,
- nerve inflammation,
- convulsions,
- stomach upset,
- loss of appetite,
- nausea,
- vomiting,
- diarrhea,
- constipation,
- urinary frequency,
- difficult urination,
- urinary retention,
- early menses,
- thickening of bronchial secretions,
- chest tightness,
- wheezing, and
- and stuffy nose
DESCRIPTION
Each 5 mL (Teaspoonful) Contains
Dexchlorpheniramine Maleate, USP 2 mg
Dexchlorpheniramine Maleate, USP, an antihistamine agent, is a white, odorless crystalline powder that is freely soluble in water. The molecular formula is C16H19ClN2· C4H4O4, designated chemically as (+)-2-[p-Chloro-α- [2-(dimethylamino)ethyl]benzyl] pyridine maleate (1:1).
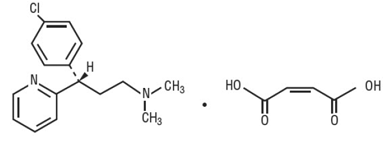 |
M.W. = 390.86
Inactive Ingredients
Citric acid, cherry flavoring, FD&C Red No. 40, glycerin, menthol, methylparaben, propylene glycol, propylparaben, purified water, sodium citrate dihydrate, and sugar.
Indications & Dosage
INDICATIONS
Perennial and seasonal allergic rhinitis
Vasomotor rhinitis
Allergic conjunctivitis due to inhalant allergens and foods
Mild, uncomplicated allergic skin manifestations of urticaria and angioedema
Amelioration of allergic reactions to blood or plasma
Dermographism
As therapy for anaphylactic reactions adjunctive to epinephrine and other standard measures after the acute manifestations have been controlled.
DOSAGE AND ADMINISTRATION
DOSAGE SHOULD BE INDIVIDUALIZED ACCORDING TO THE NEEDS AND THE RESPONSE OF THE PATIENT.
Recommended Dosage
Adults and Children 12 years of age and older: 2 mg (1 teaspoonful)
Children 6 to 11 years: 1 mg (1/2 teaspoonful)
Children 2 to 5 years: 0.5 mg (1/4 teaspoonful)
Doses are generally given every 4 to 6 hours.
HOW SUPPLIED
RYCLORA™ Oral Solution is supplied as a red colored, cherry flavored liquid in the following sizes:
4 fl oz (118 mL), NDC 15370-150-04
16 fl oz (473 mL), NDC 15370-150-16
0.7 fl oz (20mL), NDC 15370-150-99 (as physician sample)
Recommended Storage
Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature].
Dispense in a tight, light-resistant container as defined in the USP, with child-resistant closure.
Manufactured for: Carwin Pharmaceutical Associates, LLC, Hazlet, NJ 07730. Revised: Mar 2018
Side Effects & Drug Interactions
SIDE EFFECTS
- General: Urticaria, drug rash, anaphylactic shock, photosensitivity, excessive perspiration, chills, dryness of mouth, nose and the throat.
- Cardiovascular System: Hemolytic anemia, thrombocytopenia, agranulocytosis.
- Hematologic System: Hemolytic anemia, thrombocytopenia, agranulocytosis.
- Nervous System: Sedation, sleepiness, dizziness, disturbed coordination, fatigue, confusion, restlessness, excitation, nervousness, tremor, irritability, insomnia, euphoria, paresthesias, blurred vision, diplopia, vertigo, tinnitus, acute labyrinthitis, hysteria, neuritis, convulsions.
- G.I. System: Epigastric distress, anorexia, nausea, vomiting, diarrhea, constipation.
- G.U. System: Urinary frequency, difficult urination, urinary retention, early menses.
- Respiratory System: Thickening of bronchial secretions, tightness of chest and wheezing, nasal stuffiness.
Call your doctor for medical advice about side effects. You may voluntarily report side effects to FDA at 1-800-FDA-1088. Questions or comments? Call Carwin Pharmaceutical Associates, LLC at 1-844-700-5011.
DRUG INTERACTIONS
MAO inhibitors prolong and intensify the anticholinergic (drying) effects of antihistamines.
Warnings & Precautions
WARNINGS
Antihistamines should be used with considerable caution in patients with:
Narrow angle glaucoma
Stenosing peptic ulcer
Pyloroduodenal obstruction
Symptomatic prostatic hypertrophy
Bladder neck obstruction
Use In Children
In infants and children, especially, antihistamines in overdosage may cause hallucinations, convulsions, or death.
As in adults, antihistamines may diminish mental alertness in children. In the young child, particularly, they may produce excitation.
Use In Pregnancy
Experience with this drug in pregnant women is inadequate to determine whether there exists a potential for harm to the developing fetus.
Use With CNS Depressants
RYCLORA™ Oral Solution has additive effects with alcohol and other CNS depressants (hypnotics, sedatives, tranquilizers, etc.).
Use In Activities Requiring Mental Alertness
Patients should be warned about engaging in activities requiring mental alertness such as driving a car or operating appliances, machinery, etc.
Use In The Elderly (Approximately 60 Years Or Older)
Antihistamines are more likely to cause dizziness, sedation, and hypotension in elderly patients.
PRECAUTIONS
RYCLORA™ Oral Solution has an atropine-like action and, therefore, should be used with caution in patients with:
History of bronchial asthma
Increased intraocular pressure Hyperthyroidism
Cardiovascular disease
Hypertension
Overdosage & Contraindications
OVERDOSE
Antihistamine overdosage reactions may vary from central nervous system depression to stimulation. Stimulation is particularly likely in children. Atropine-like signs and symptoms—dry mouth, fixed, dilated pupils, flushing, and gastrointestinal symptoms may also occur.
If vomiting has not occurred spontaneously the patient should be induced to vomit. This is best done by having the patient drink a glass of water or milk after which the patient should be made to gag. Precautions against aspiration must be taken, especially in infants and children.
Saline cathartics, such as milk of magnesia, draw water into the bowel by osmosis and therefore, are valuable for their action in rapid dilution of bowel content.
Stimulants should not be used.
Vasopressors may be used to treat hypotension.
CONTRAINDICATIONS
Use In Newborn Or Premature Infants
This drug should not be used in newborn or premature infants.
Use In Nursing Mothers
Because of the higher risk of antihistamines for infants generally and for newborns and prematures in particular, antihistamine therapy is contraindicated in nursing mothers.
Use In Lower Respiratory Disease
Antihistamines should NOT be used to treat lower respiratory tract symptoms including asthma.
Antihistamines are also contraindicated in the following conditions:
Hypersensitivity to dexchlorpheniramine maleate or other antihistamines of similar chemical structure
Monoamine oxidase inhibitor therapy (See DRUG INTERACTIONS)
Clinical Pharmacology
Dexchlorpheniramine maleate is an antihistamine with anticholinergic (drying) and sedative side effects. Antihistamines appear to compete with histamine for cell receptor sites on effector cells.
Medication Guide
PATIENT INFORMATION
No information provided. Please refer to the WARNINGS AND PRECAUTIONS section.
