Tobradex Drug Description: Detailed Datasheet
- Generic Name: tobramycin and dexamethasone
- Brand Name: Tobradex
side effects drug center tobradex (tobramycin and dexamethasone) drug
Drug Description
What is TobraDex and how is it used?
Tobradex is a prescription medicine used to treat the symptoms of Ocular Inflammation and Bacterial Infection of the eye. Tobradex may be used alone or with other medications.
Tobradex belongs to a class of drugs called Antibiotics/Corticosteroids, Opthalmic.
It is not known if Tobradex is safe and effective in children younger than 2 years of age.
What are the possible side effects of TobraDex?
TobraDex may cause serious side effects including:
- severe eye redness, itching or swelling,
- blurred vision,
- tunnel vision,
- seeing halos around lights,
- pain behind your eyes,
- sudden vision changes,
- slow healing after eye surgery, and
- redness, severe discomfort, crusting or drainage of the eye
Get medical help right away, if you have any of the symptoms listed above.
The most common side effects of Tobradex include:
- minor burning or stinging
Tell the doctor if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of TobraDex. For more information, ask your doctor or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
DESCRIPTION
TOBRADEX® (tobramycin and dexamethasone ophthalmic suspension) is a sterile, multiple dose antibiotic and steroid combination for topical ophthalmic use.
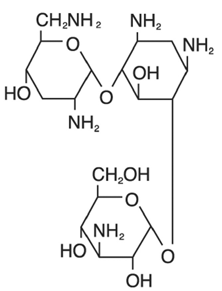
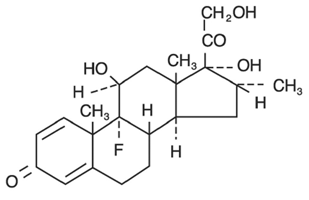
The chemical structures for tobramycin and dexamethasone are presented below:
 |
 |
Tobramycin
Empirical Formula: C18H37N5O9
Chemical Name: O-3-Amino-3-deoxy-α-D-glucopyranosyl-(1→4)-O-[2,6-diamino-2,3,6-trideoxy-α- D-ribo-hexopyranosyl-(1→6)]-2-deoxy-L-streptamine
Dexamethasone
Empirical Formula: C22H29FO5
Chemical Name: 9-Fluoro-11β,17,21-trihydroxy-16α-methylpregna-1,4-diene-3,20-dione
Each mL of TOBRADEX® (tobramycin and dexamethasone ophthalmic suspension) contains: Actives: tobramycin 0.3% (3 mg) and dexamethasone 0.1% (1 mg). Preservative: benzalkonium chloride 0.01%. Inactives: tyloxapol, edetate disodium, sodium chloride, hydroxyethyl cellulose, sodium sulfate, sulfuric acid and/or sodium hydroxide (to adjust pH) and purified water.
Indications & Dosage
INDICATIONS
TOBREX® (tobramycin ophthalmic solution) 0.3% is a topical antibiotic indicated in the treatment of external infections of the eye and its adnexa caused by susceptible bacteria. Appropriate monitoring of bacterial response to topical antibiotic therapy should accompany the use of TOBREX. Clinical studies have shown tobramycin to be safe and effective for use in children.
DOSAGE AND ADMINISTRATION
In mild to moderate disease, instill 1 or 2 drops into the affected eye(s) every 4 hours. In severe infections, instill 2 drops into the eye(s) hourly until improvement, following which treatment should be reduced prior to discontinuation.
HOW SUPPLIED
TOBREX (tobramycin ophthalmic solution) 0.3% is supplied as a 5 mL sterile solution, packaged in a 8 mL low density polyethylene white bottle and natural dispensing plug and white polypropylene closure as follows:
5 mL containing tobramycin 0.3% (3 mg/mL) - NDC 0065-0643-05
Storage: Store at 2°C to 25°C (36°F to 77°F).
After opening, TOBREX (tobramycin ophthalmic solution) 0.3% can be used until the expiration date on the bottle.
Distributed by: Novartis Pharmaceuticals Corporation East Hanover, New Jersey 07936. Revised: May 2021
Side Effects & Drug Interactions
SIDE EFFECTS
The most frequent adverse reactions to TOBREX (tobramycin ophthalmic solution) 0.3% are hypersensitivity and localized ocular toxicity, including lid itching and swelling, and conjunctival erythema. These reactions occur in less than three of 100 patients treated with TOBREX®.
Postmarketing Experience
Additional adverse reactions identified from post-marketing use include anaphylactic reaction, Stevens-Johnson syndrome, and erythema multiforme.
The following additional adverse reactions have been reported with systemic aminoglycosides: Neurotoxicity, ototoxicity and nephrotoxicity have occurred in patients receiving systemic aminoglycoside therapy. Aminoglycosides may aggravate muscle weakness in patients with known or suspected neuromuscular disorders, such as myasthenia gravis or Parkinson’s disease, because of their potential effect on neuromuscular function.
DRUG INTERACTIONS
No Information Provided
Warnings & Precautions
WARNINGS
FOR TOPICAL OPHTHALMIC USE. NOT FOR INJECTION INTO THE EYE. Sensitivity to topically applied aminoglycosides may occur in some patients. Severity of hypersensitivity reactions may vary from local effects to generalized reactions such as erythema, itching, urticaria, skin rash, anaphylaxis, anaphylactoid reactions, or bullous reactions. If a sensitivity reaction to TOBREX® (tobramycin ophthalmic solution) 0.3% occurs, discontinue use.
PRECAUTIONS
General
As with other antibiotic preparations, prolonged use may result in overgrowth of nonsusceptible organisms, including fungi. If superinfection occurs, appropriate therapy should be initiated.
Cross-sensitivity to other aminoglycoside antibiotics may occur; if hypersensitivity develops with this product, discontinue use and institute appropriate therapy. Patients should be advised not to wear contact lenses if they have signs and symptoms of bacterial ocular infection.
Pregnancy
Reproduction studies in 3 types of animals at doses up to 33 times the normal human systemic dose have revealed no evidence of impaired fertility or harm to the fetus due to tobramycin. There are, however, no adequate and well-controlled studies in pregnant women. Because animal studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Nursing Mothers
Because of the potential for adverse reactions in nursing infants from TOBREX, a decision should be made whether to discontinue nursing the infant or discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
Safety and effectiveness in pediatric patients below the age of 2 months has not been established.
Geriatric Use
No overall differences in safety or effectiveness have been observed between elderly and younger patients.
Overdosage & Contraindications
OVERDOSE
No Information Provided
CONTRAINDICATIONS
TOBREX (tobramycin ophthalmic solution) 0.3% is contraindicated in patients with known hypersensitivity to any of its components.
Clinical Pharmacology
In Vitro Data
In vitro studies have demonstrated tobramycin is active against susceptible strains of the following microorganisms: Staphylococci, including S. aureus and S. epidermidis (coagulase-positive and coagulase-negative), including penicillin-resistant strains.
Streptococci, including some of the Group A-beta-hemolytic species, some nonhemolytic species, and some Streptococcus pneumoniae.
Pseudomonas aeruginosa, Escherichia coli, Klebsiella pneumoniae, Enterobacter aerogenes, Proteus mirabilis, Morganella morganii, most Proteus vulgaris strains, Haemophilus influenzae and H. aegyptius, Moraxella lacunata, Acinetobacter calcoaceticus and some Neisseria species. Bacterial susceptibility studies demonstrate that in some cases, microorganisms resistant to gentamicin retain susceptibility to tobramycin.
Medication Guide
PATIENT INFORMATION
Do not touch dropper tip to any surface, as this may contaminate the solution.
