Pyridium Drug Description: Detailed Datasheet
- Generic Name: phenazopyridine
- Brand Name: Pyridium
side effects drug center pyridium (phenazopyridine) drug
Drug Description
What is Pyridium and how is it used?
Pyridium is a prescription and over the counter medicine used to prevent treat the symptoms of the lower urinary tract. Pyridium may be used alone or with other medications.
Pyridium is an Analgesics.
It is not known if Pyridium is safe and effective in children younger than 6 years.
What are the possible side effects of Pyridium?
Pyridium may cause serious side effects including:
- little or no urination
- swelling
- rapid weight gain
- confusion
- loss of appetite
- pain in your side or lower back
- fever
- pale or yellowed skin
- stomach pain
- nausea
- vomiting
- blue or purple appearance of your skin
Get medical help right away, if you have any of the symptoms listed above.
The most common side effects of Pyridium include:
- headache
- dizziness
- upset stomach
Tell the doctor if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of Pyridium. For more information, ask your doctor or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
CAUTION: Federal law prohibits dispensing without prescription.
DESCRIPTION
Pyridium® (Phenazopyridine Hydrochloride) is light or dark red to dark violet, odorless, slightly bitter, crystalline powder. It has a specific local analgesic effect in the urinary tract, promptly relieving burning and pain. It has the following structural formula:
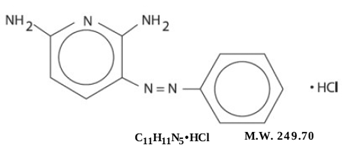 |
Pyridium (Phenazopyridine HCl Tablets, USP) contains the following inactive ingredients: carnauba wax, croscarmellose sodium, hypromellose, magnesium stearate, microcrystalline cellulose, polyethylene glycol, povidone and pregelatinized starch.
Indications
Pyridium is indicated for the symptomatic relief of pain, burning, urgency, frequency, and other discomforts arising from irritation of the lower urinary tract mucosa caused by infection, trauma, surgery, endoscopic procedures, or the passage of sounds or catheters. The use of Phenazopyridine HCl for relief of symptoms should not delay definitive diagnosis and treatment of causative conditions. Because it provides only symptomatic relief, prompt appropriate treatment of the cause of pain must be instituted and Phenazopyridine HCl should be discontinued when symptoms are controlled.
The analgesic action may reduce or eliminate the need for systemic analgesics or narcotics. It is, however, compatible with antibacterial therapy and can help to relieve pain and discomfort during the interval before antibacterial therapy controls the infection. Treatment of a urinary tract infection with Phenazopyridine HCl should not exceed two days because there is a lack of evidence that the combined administration of Phenazopyridine HCl and an antibacterial provides greater benefit than administration of the antibacterial alone after two days. (See DOSAGE AND ADMINISTRATION section.)
Dosage
DOSAGE AND ADMINISTRATION
100 mg Tablets: Average adult dosage is two tablets 3 times a day after meals.
200 mg Tablets: Average adult dosage is one tablet 3 times a day after meals.
When used concomitantly with an antibacterial agent for the treatment of a urinary tract infection, the administration of Phenazopyridine HCl should not exceed 2 days.
HOW SUPPLIED
100 mg Tablets: Supplied in bottles of 100 (NDC 60846-517-01) counts.
Appearance: Deep brown to maroon colored, round, film coated tablets debossed “AN” above “1” on one side and plain on the other.
200 mg Tablets: Supplied in bottles of 100 (NDC 60846-520-01) counts.
Appearance: Deep brown to maroon colored, round, film coated tablets debossed “AN” above “2” on one side and plain on the other.
DISPENSE contents with a child-resistant closure (as required) and in a tight container as defined in the USP.
STORE at 20° to 25°C (68° to 77°F); excursions permitted to 15° to 30°C (59° to 86°F) [See USP Controlled Room Temperature].
Manufactured for: Gemini Laboratories , LLC, Bridgewater, NJ 08807. Rev. Feb 2014
Side Effects & Drug Interactions
SIDE EFFECTS
Headache, rash, pruritus and occasional gastrointestinal disturbance. An anaphylactoid-like reaction has been described. Methemoglobinemia, hemolytic anemia, renal and hepatic toxicity have been reported, usually at overdosage levels (see OVERDOSAGE section).
DRUG INTERACTIONS
No information provided.
Warnings & Precautions
WARNINGS
No information provided.
PRECAUTIONS
General
A yellowish tinge of the skin or sclera may indicate accumulation due to impaired renal excretion and the need to discontinue therapy. The decline in renal function associated with advanced age should be kept in mind.
NOTE: Patients should be informed that Phenazopyridine HCl produces a reddish-orange discoloration of the urine and may stain fabric. Staining of contact lenses has been reported.
Laboratory Test Interaction
Due to its properties as an azo dye, Phenazopyridine HCl may interfere with urinalysis based on spectrometry or color reactions.
Carcinogenesis, Mutagenesis, Impairment Of Fertility
Long-term administration of Phenazopyridine HCl has induced neoplasia in rats (large intestine) and mice (liver). Although no association between Phenazopyridine HCl and human neoplasia has been reported, adequate epidemiological studies along these lines have not been conducted.
Pregnancy Category B
Reproduction studies have been performed in rats at doses up to 50 mg/kg/day and have revealed no evidence of impaired fertility or harm to the fetus due to Phenazopyridine HCl. There are, however, no adequate and well controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Nursing Mothers
No information is available on the appearance of Phenazopyridine HCl, or its metabolites in human milk.
Overdosage & Contraindications
OVERDOSE
Exceeding the recommended dose in patients with good renal function or administering the usual dose to patients with impaired renal function (common in elderly patients) may lead to increased serum levels and toxic reactions. Methemoglobinemia generally follows a massive, acute overdose. Methylene blue, 1 to 2 mg/kg/body weight intravenously or ascorbic acid 100 to 200 mg given orally should cause prompt reduction of the methemoglobinemia and disappearance of the cyanosis which is an aid in diagnosis. Oxidative Heinz body hemolytic anemia may also occur, and “bite cells” (degmacytes) may be present in a chronic overdosage situation. Red blood cell G-6-PD deficiency may predispose to hemolysis. Renal and hepatic impairment and occasional failure, usually due to hypersensitivity, may also occur.
CONTRAINDICATIONS
Phenazopyridine HCl should not be used in patients who have previously exhibited hypersensitivity to it. The use of Phenazopyridine HCl is contraindicated in patients with renal insufficiency.
Clinical Pharmacology
Phenazopyridine HCl is excreted in the urine where it exerts a topical analgesic effect on the mucosa of the urinary tract. This action helps to relieve pain, burning, urgency and frequency. The precise mechanism of action is not known.
The pharmacokinetic properties of Phenazopyridine HCl have not been determined. Phenazopyridine HCl is rapidly excreted by the kidneys, with as much as 66% of an oral dose being excreted unchanged in the urine.
Medication Guide
PATIENT INFORMATION
No information provided. Please refer to the PRECAUTIONS section.
