Infanrix Hexa Drug Description: Detailed Datasheet
- Generic Name: combined diphtheria, tetanus toxoids, acellular pertussis, hepatitis b, inactivated poliomyelitis, adsorbed conjugated haemophilus influenzae
- Brand Name: Infanrix Hexa
side effects drug center infanrix hexa (combined diphtheria, tetanus toxoids, acellular pertussis, hepatitis b, inactivated poliomyelitis, adsorbed conjugated haemophilus influenzae ) drug
Drug Description
What is Infanrix Hexa and how is it used?
Infanrix Hexa is a prescription medicine used as a vaccine for active immunization against diphtheria, tetanus, and pertussis. Infanrix Hexa may be used alone or with other medications.
Infanrix Hexa belongs to a class of drugs called Vaccines, Combos.
It is not known if Infanrix Hexa is safe and effective in children younger than 6 weeks of age.
What are the possible side effects of Infanrix Hexa?
Infanrix Hexa may cause serious side effects including:
- hives,
- difficulty breathing,
- swelling of your face, lips, tongue, or throat,
- altered mental state,
- confusion, and
- behavioral changes
Get medical help right away, if you have any of the symptoms listed above.
The most common side effects of Infanrix Hexa include:
- injection site reactions (pain, redness, and swelling),
- fever,
- drowsiness,
- irritability,
- fussiness, and
- loss of appetite
Tell the doctor if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of Infanrix Hexa. For more information, ask your doctor or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
SUMMARY PRODUCT INFORMATION
| Route of Administration | Dosage Form/ Strength | Clinically Relevant Nonmedicinal Ingredients |
| Intramuscular injection | Sterile suspension for injection/ After reconstitution, 1 dose (0.5 ml) contains 25 limit of flocculation (Lf) [30 International Units (IU)] diphtheria toxoid; 10 Lf (40 IU) tetanus toxoid; 25 μg pertussis toxoid (PT); 25 μg filamentous haemagglutinin (FHA); 8 μg pertactin; 10 μg hepatitis B surface antigen (HBsAg); 40 D-antigen units (DU) of type 1 poliovirus, 8 DU type 2 poliovirus, and 32 DU type 3 poliovirus; 10 μg of adsorbed purified capsular polysaccharide of Haemophilus influenzae type b (Hib) (PRP) covalently bound to approximately 25 μg of tetanus toxoid per 0.5 mL dose. | lactose, sodium chloride, aluminum adjuvant (as aluminum salts), water for injection, residual formaldehyde, polysorbate 20 and 80 (Tween 20 and 80), M199, potassium chloride, disodium phosphate, monopotassium phosphate, glycine, neomycin sulphate, polymyxin B sulphate and aluminum phosphate. |
DESCRIPTION
INFANRIX hexa® (combined diphtheria and tetanus toxoids, acellular pertussis, hepatitis B (recombinant), inactivated poliomyelitis, and adsorbed conjugated Haemophilus influenzae type b vaccine) contains diphtheria toxoid, tetanus toxoid, three purified pertussis antigens [pertussis toxoid (PT), filamentous haemagglutinin (FHA) and pertactin (69 kiloDalton outer membrane protein)], hepatitis B virus surface antigen recombinant, adsorbed onto aluminum salts, purified, inactivated poliovirus types 1, 2 and 3, Haemophilus influenzae type b polysaccharide conjugated to tetanus toxoid.
Indications
Pediatrics
Primary Immunization
INFANRIX hexa® (combined diphtheria and tetanus toxoids, acellular pertussis, hepatitis B (recombinant), inactivated poliomyelitis, and adsorbed conjugated Haemophilus influenzae type b vaccine) is indicated for:
- active primary immunization against diphtheria, tetanus, pertussis, hepatitis B, poliomyelitis, and disease caused by Haemophilis influenzae type b in infants and children 6 weeks to 2 years.
INFANRIX hexa® will not prevent hepatitis caused by other agents, such as hepatitis A, C and E viruses, or other pathogens known to infect the liver. As hepatitis D (caused by the delta virus) does not occur in the absence of hepatitis B infection, it can be expected that hepatitis D will also be prevented by INFANRIX hexa® vaccination.
Where a dose of hepatitis B vaccine is given at birth, INFANRIX hexa® can be used for the second dose from the age of six weeks. If a second dose of hepatitis B vaccine is required before this age, monovalent Hepatitis B vaccine should be used.
INFANRIX hexa® has not been evaluated in the Canadian Native Population.
Booster Vaccination
The administration of the booster dose should be given at 18 months as stated in the Canadian Immunization Guide.
INFANRIX hexa® can be used for the booster dose provided that the infant has received a full primary vaccination course of each of the antigens contained in INFANRIX hexa®, regardless of whether these were administered as monovalent or combination vaccines.
Other combinations of antigens have been studied in clinical trials following primary vaccination with INFANRIX hexa® and may be used for a booster dose, these include diptheria, tetanus, acellular pertussis (DTaP) and DTaP-Hib.
Dosage
DOSAGE AND ADMINISTRATION
The use of reduced volume (fractional doses) is not recommended. The effect of such practices on the frequency of serious adverse events and on protection against disease has not been determined.
Pre-term infants should be vaccinated according to their chronological age from birth.
INFANRIX hexa® (combined diphtheria and tetanus toxoids, acellular pertussis, hepatitis B (recombinant), inactivated poliomyelitis, and adsorbed conjugated Haemophilus influenzae type b vaccine) has not been evaluated in the Canadian Native Population.
Recommended Dose
Primary Immunization
The primary immunization course for infants born of HBsAg-negative mothers is 3 doses of INFANRIX hexa® 0.5 mL, given intramuscularly, at 2, 4, 6 months of age. INFANRIX hexa® should not be administered to any infant before the age of 6 weeks.
Children Previously Vaccinated with One or More Doses of Hepatitis B Vaccine
Children who receive one dose of Hepatitis B vaccine at or shortly after birth may be administered a 3 dose series of INFANRIX hexa® vaccine starting as early as 6 weeks of age. There is no data to support the use of a 3 dose series of INFANRIX hexa® in infants who have previously received more than one dose of Hepatitis B vaccine. INFANRIX hexa® may be administered to infants otherwise scheduled to receive concurrent INFANRIX® (diphtheria, tetanus and acellular pertussis vaccine) and Hepatitis B vaccine and in whom vaccination against poliovirus is also desired.
Booster Immunization
The administration of the booster dose should be given at 18 months as stated in the Canadian Immunization Guide.
Missed Dose
Interruption of the recommended schedule with a delay between doses should not interfere with the final immunity achieved with INFANRIX hexa®. There is no need to start the series over again regardless of the time elapsed between doses.
Additional Dosing Information
If any recommended dose of pertussis vaccine cannot be given, diphtheria and tetanus toxoids (DT) for pediatric use should be given as needed to complete the series.
For persons 7 years of age or older, Tetanus and Diphtheria Toxoids (Td) for adult use should be given for routine booster immunization against tetanus and diphtheria.
Administration
Preparation for Administration
The vaccine is reconstituted by adding the entire contents of the syringe (PEDIARIX™) to the vial containing the Hib pellet.
Do not remove the white back-stop from the syringe. Prior to administration, ensure that the plunger rod is firmly attached to the rubber stopper by turning the plunger clockwise until slight resistance is felt. Do not over tighten. Remove syringe LUER Tip-cap and needle cap. Attach needle by pressing and twisting in a clockwise rotation until secured to the syringe.
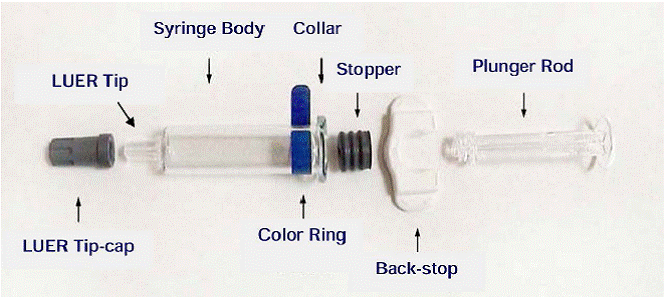 |
Specific instructions for the pre-filled syringe with a LUER lock adaptor
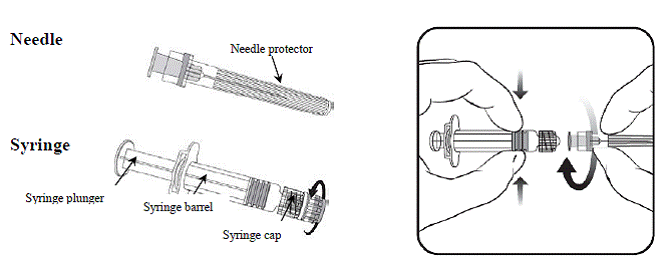 |
Holding the syringe barrel in one hand (avoid holding the syringe plunger), unscrew the syringe cap by twisting it anticlockwise. To attach the needle to the syringe, twist the needle clockwise into the syringe until you feel it lock (see picture). Remove the needle protector, which on occasion can be a little stiff.
Reconstitution
Upon storage, a white deposit and clear supernatant may be observed in the syringe. This is a normal observation and does not constitute a sign of deterioration. Shake the syringe well before use. With thorough agitation, DTaP-HB-IPV (combined diphtheria and tetanus toxoids, acellular pertussis, hepatitis B (recombinant) and inactivated poliomyelitis vaccine, tradename PEDIARIX™) is a homogeneous white turbid suspension. The syringe and the vial containing the Hib pellet should be inspected visually for any foreign particulate matter and/or variation of physical aspect. In the event of either being observed, discard the vaccine. The vaccine is reconstituted by adding the entire contents of the syringe (PEDIARIX™) to the vial containing the Hib pellet. After the addition of the PEDIARIX™ vaccine to the pellet, the mixture should be well shaken until the pellet is completely dissolved. The vaccine should not be mixed with other vaccines.
It is good clinical practice to only inject a vaccine when it has reached room temperature. In addition, a vial at room temperature ensures sufficient elasticity of the rubber closure to minimise any coring of rubber particles. To achieve this, the vial should be kept at room temperature (25 ± 3 °C) for at least five minutes before connecting the syringe and reconstituting the vaccine.
The reconstituted vaccine presents as a slightly more cloudy suspension than the liquid component alone. This is a normal observation. The reconstituted vaccine should be inspected visually for any foreign particulate matter and/or abnormal physical appearance. In the event of either being observed, discard the vaccine.
Since this product is a suspension containing an adjuvant, shake vigorously to obtain a uniform suspension prior to withdrawal from the vial. Do not use if resuspension does not occur with vigorous shaking. Withdraw the entire contents of the vial.
INFANRIX hexa® should be administered by intramuscular injection. The preferred sites are the anterolateral aspects of the thigh or the deltoid muscle of the upper arm. The vaccine should not be injected in the gluteal area or areas where there may be a major nerve trunk. Before injection, the skin at the injection site should be cleaned and prepared with a suitable germicide. After insertion of the needle, aspirate to ensure that the needle has not entered a blood vessel.
Do not administer this product subcutaneously or intravenously.
After reconstitution, the vaccine should be injected promptly. However the vaccine may be kept for up to 8 hours at room temperature (21°C).
HOW SUPPLIED
Storage And Stability
Store INFANRIX hexa® (combined diphtheria and tetanus toxoids, acellular pertussis, hepatitis B (recombinant), inactivated poliomyelitis, and adsorbed conjugated Haemophilus influenzae type b vaccine) at 2° to 8°C. Do not use after the expiration date shown on the label. After reconstitution, immediate use is recommended. However, stability of the vaccine has been demonstrated for 8 hours at + 21°C after reconstitution.
Do not freeze. Discard if the vaccine has been frozen.
Protect from light.
During transport, recommended conditions of storage must be respected.
Stability data indicate that the vaccine components are stable at temperatures up to 25°C for 72 hours. These data are intended to guide healthcare professionals in case of temporary temperature excursion only.
Dosage Forms, Composition And Packaging
Dosage Forms
Syringe and Vial
Haemophilus influenzae type b vaccine is supplied as a pellet in a 3.0 mL vial (Type I glass) with stopper (butyl).
PEDIARIX™ (combined diphtheria and tetanus toxoids, acellular pertussis, hepatitis B (recombinant) and inactivated poliomyelitis vaccine) is supplied as a turbid suspension in a pre-filled syringe (Type I glass) (0.5 mL) with plunger stoppers (butyl).
Composition
After reconstitution, each 0.5 mL dose is formulated to contain 25 Lf (30 IU) diphtheria toxoid, 10 Lf (40 IU) tetanus toxoid, 25 μg PT, 25 μg FHA, 8 μg pertactin, 10 μg HBsAg, 40 D-antigen Units (DU) of type 1 poliovirus, 8 DU type 2 poliovirus, 32 DU type 3 poliovirus, and 10 μg of adsorbed purified capsular polysaccharide of Hib (PRP) covalently bound to approximately 25 μg of tetanus toxoid.
After reconstitution, each 0.5 mL dose also contains 12.6 mg lactose, 4.5 mg sodium chloride and 0.7 mg aluminum adjuvants (as aluminum salts), 0.12 mg aluminum (AlPO4), water for injection. The vaccine contains residual formaldehyde, polysorbate and 80 (Tween 20 and 80), M199 (as stabilizer), potassium chloride and disodium phosphate, monopotassium phosphate, glycine, neomycin sulphate, polymyxin B sulphate from the manufacturing process. The procedures used to manufacture the antigen result in a product that contains ≤ 5% yeast protein.
Packaging
Pack sizes of:
Syringe and Vial: Supplied as a kit in pack sizes of 10 with or without needles.
This document plus the full product monograph, prepared for health professionals can be found at: http://www.gsk.ca or by contacting the sponsor, GlaxoSmithKline Inc., 7333 Mississauga Road Mississauga, Ontario L5N 6L4 1-800-387-7374. Revised: May 2015.
Side Effects
Adverse Drug Reaction Overview
INFANRIX hexa® (combined diphtheria and tetanus toxoids, acellular pertussis, hepatitis B (recombinant), inactivated poliomyelitis, and adsorbed conjugated Haemophilus influenzae type b vaccine) is generally well tolerated.
Clinical Trial Adverse Drug Reactions
Because clinical trials are conducted under very specific conditions the adverse reaction rates observed in the clinical trials may not reflect the rates observed in practice and should not be compared to the rates in the clinical trials of another drug. Adverse drug reaction information from clinical trials is useful for identifying drug-related adverse events and for approximating rates.
During a study conducted in the United States, a total of 785 documented doses of study vaccines were given to 267 subjects included in the According To Protocol (ATP) reactogenicity analysis. Solicited and unsolicited symptoms occurring during the 8-day follow-up period after vaccination were reported. Most reported solicited local symptoms and solicited general symptoms were mild to moderate in intensity. There were no statistically significant differences between the two groups in the incidence of soreness, redness or swelling at the injection site (regardless of side/site/dose) or fever. The percentage of subjects per group experiencing symptoms (both solicited and unsolicited) during the 8 days after vaccination is outlined in Table 1.
Table 1 : Percentage of U.S.
Infants with Local or Systemic Reactions within 8 Days of Primary Vaccination
with either INFANRIX hexa® or Commercially Available INFANRIX®, ENGERIX®-B, and
OPV Administered Simultaneously with Hib at Separate Sites (Per subject
analysis).
| Event | INFANRIX hexa® (N=134) |
INFANRIX®, ENGERIX® -B, H1b vaccine, OPV (N=133) |
| Local | % | % |
| Pain, any | 42.54 | 52.63 |
| Pain, severe | 1.49 | 2.26 |
| Redness, any | 48.51 | 47.37 |
| Redness, > 20 mm | 2.24 | 3.01 |
| Swelling, any | 35.82 | 40.60 |
| Swelling, > 20 mm | 3.73 | 4.51 |
| Systemic | % | % |
| Temperature | ||
| ≥ 38°C | 55.97 | 51.88 |
| > 39.5°C | 0.75 | 2.26 |
| Diarrhea, any | 35.82 | 33.08 |
| Grade 3 | 0.75 | 2.26 |
| Eating/drinking less than usual, any | 49.25 | 57.14 |
| Grade 3 | 2.24 | 2.26 |
| Irritability/fussiness, any | 82.84 | 86.47 |
| Grade 3 | 6.72 | 6.02 |
| Sleeping less than usual, any | 50.75 | 56.39 |
| Grade 3 | 2.24 | 3.76 |
| Sleeping more than usual, any | 62.69 | 67.67 |
| Grade 3 | 3.73 | 1.50 |
| Unusual crying for more than one hour, any | 42.54 | 41.35 |
| Grade 3 | 3.73 | 2.26 |
| Vomiting, any | 25.37 | 20.30 |
| Grade 3 | 0.75 | 0.75 |
| N= number of infants | ||
The safety profile presented below is based on data from more than 16,000 subjects.
As has been observed for DTaP and DTaP-containing combinations, an increase in local reactogenicity and fever was reported after booster vaccination with INFANRIX hexa® with respect to the primary course.
Frequencies per dose as defined by CIOMS:
Very common: ≥ 10%
Appetite lost, irritability, crying abnormal, restlessness, pain, redness, local swelling at the injection site ( ≤ 50 mm), fever ≥ 38°C, and fatigue.
Common: ≥ 1% and < 10%
Nervousness, vomiting, diarrhea, local swelling at the injection site ( > 50 mm)*, fever > 39.5°C, pruritis** and injection site reactions, including induration.
Uncommon: ≥ 0.1% and < 1%
Upper respiratory tract infection, somnolence, cough** and diffuse swelling of the injected limb, sometimes involving the adjacent joint*.
Rare: ≥ 0.01% and < 0.1%
Bronchitis and rash.
Very rare: < 0.01%
Convulsions (with or without fever)***, dermatitis, bronchospasm, and urticaria**.
* Children primed with acellular pertussis vaccines are more likely to experience swelling reactions after booster administration in comparison with children primed with whole cell vaccines. These reactions resolve over an average of 4 days.
**Observed with other GSK DTaP-containing vaccines
*** Analysis of post-marketing reporting rates suggests a potential increased risk of convulsions (with or without fever) and hypotonic hyporesponsive episode when comparing groups which reported use of INFANRIX hexa® with Prevnar® 13 to those which reported use of INFANRIX hexa® alone.
Local reactions after immunization usually consist of swelling or induration, tenderness, and redness or erythema at the injection site. More severe local reactions occasionally occur, such as inflammatory cellulitis without bacterial infection after DTP-containing vaccines.
Post-Marketing Adverse Drug Reaction
Over 12 million doses of INFANRIX hexa® have been distributed overall for primary and booster vaccinations. Extremely rare cases of Sudden Unexpected Death (SUD) in close temporal association to vaccination with INFANRIX hexa® have been reported in the first year of life. However, a causal relationship has not been established. The observed number of SUD cases following INFANRIX hexa® is below the number of cases expected to occur by chance.
Blood And Lymphatic System Disorders
Lymphadenopathy, thrombocytopenia
Immune System Disorders
Allergic reactions (including anaphylactic and anaphylactoid reactions)
Nervous System Disorders
Collapse or shock-like state (hypotonic hyporesponsive episode)***.
Respiratory, Thoracic And Mediastinal Disorders
Apnea** [see section “WARNINGS AND PRECAUTIONS” for apnea in very premature infants ( ≤ 28 weeks of gestation)].
Skin and Subcutaneous Tissue Disorders
Angioneurotic oedema**
General Disorders and Administration Site Conditions
Extensive swelling reactions, swelling of the entire injected limb*, vesicles at the injection site
*Children primed with acellular pertussis vaccines are more likely to experience swelling reactions after booster administration in comparison with children primed with whole cell vaccines. These reactions resolve over an average of 4 days.
**Observed with other GSK DTaP-containing vaccines
*** Analysis of post-marketing reporting rates suggests a potential increased risk of convulsions (with or without fever) and hypotonic hyporesponsive episode when comparing groups which reported use of INFANRIX hexa® with Prevnar® 13 to those which reported use of INFANRIX hexa® alone.
Experience with Hepatitis B Vaccine
Paralysis, neuropathy, Guillain-Barré syndrome, encephalopathy, encephalitis, meningitis, allergic reactions mimicking serum sickness, neuritis, hypotension, vasculitis, lichen planus, erythema multiforme, arthritis and muscular weakness have been reported extremely rarely during post-marketing surveillance following vaccination with ENGERIX®-B (Hepatitis B vaccine, GlaxoSmithKline) in infants < 2 years old. The causal relationship to the vaccine has not been established.
Drug Interactions
Overview
INFANRIX hexa® (Combined diphtheria and tetanus toxoids, acellular pertussis, hepatitis B (recombinant), inactivated poliomyelitis, and adsorbed conjugated Haemophilus influenzae type b vaccine) should not be mixed with any other vaccine in the same syringe or vial.
Drug-Drug Interactions
Tetanus Immune Globulin or Diphtheria Antitoxin, if used, should be given at a separate site, with a separate needle and syringe.
Anticoagulants
As with other intramuscular injections, INFANRIX hexa® should not be given to infants or children on anticoagulant therapy unless the potential benefit clearly outweighs the risk of administration (see WARNINGS AND PRECAUTIONS).
Immunosuppressive Therapies
Immunosuppressive therapies, including irradiation, antimetabolites, alkylating agents, cytotoxic drugs and corticosteroids (used in greater than physiologic doses), may reduce the immune response to vaccines. Although no specific data are available from studies with INFANRIX hexa® under these conditions, if immunosuppressive therapy will be discontinued shortly, it would be reasonable to defer immunization until the patient has been off therapy for 3 months; otherwise, the patient should be vaccinated while still on therapy. If INFANRIX hexa® is administered to a person receiving immunosuppressive therapy, or a recent injection of immune globulin, an adequate immunologic response may not be obtained.
Use with Other Vaccines
INFANRIX hexa® can be given concomitantly with pneumococcal conjugate, MenC conjugate, MenACWY conjugate, rotavirus, measles, mumps, rubella and varicella vaccines. Data have shown no clinically relevant interference in the antibody response to each of the individual antigens in INFANRIX hexa®.
Data from clinical studies indicate that, when INFANRIX hexa® is co-administered with pneumococcal conjugate vaccine, the rate of febrile reactions is higher compared to that occurring following the administration of INFANRIX hexa® (see WARNINGS AND PRECAUTIONS, General). The incidence of fever following administration of the two vaccines in the primary series was lower than that observed after the booster vaccination.
Drug-Food Interactions
Interactions with food have not been established.
Drug-Herb Interactions
Interactions with herbal products have not been established.
Drug-Laboratory Interactions
Interactions with laboratory tests have not been established.
Warnings & Precautions
WARNINGS
Included as part of the PRECAUTIONS section.
PRECAUTIONS
General
INFANRIX hexa® (combined diphtheria and tetanus toxoids, acellular pertussis, hepatitis B (recombinant), inactivated poliomyelitis, and adsorbed conjugated Haemophilus influenzae type b vaccine) should under no circumstances be administered intravascularly or intradermally.
As for all diphtheria, tetanus and pertussis vaccines, each injection should be given deep intramuscularly and each injection of the immunization series should be made at a different site.
As with other injectable vaccines, epinephrine injection (1:1000) and other appropriate agents used for the control of immediate allergic reactions must be immediately available should an acute anaphylactic reaction occur. For this reason, the vaccinee should remain under medical supervision for 30 minutes after immunization.
It is good clinical practice that vaccination should be preceded by a review of the medical history (especially with regard to previous vaccination and possible occurrence of undesirable events) and a clinical examination.
INFANRIX hexa® will not prevent disease caused by pathogens other than Corynebacterium diphtheria, Clostridium tetani, Bordetella pertussis, hepatitis B virus, poliovirus or Haemophilus influenzae type b.
As with any other vaccine, a protective immune response may not be elicited in all vaccinees for all component antigens in the vaccine. This product is not recommended for treatment of actual infections.
Where passive protection is required, Tetanus Immune Globulin and/or Diphtheria Antitoxin may also be administered at separate sites. Because of the substantial risks of complications from pertussis disease, completion of a primary series of vaccine early in life is strongly recommended.
If any of the following events occur in temporal relation to administration of whole-cell DTP or acellular DTP vaccine, the decision to give subsequent doses of vaccine containing the pertussis component should be carefully considered:
- Temperature of > 40.5° C within 48 hours of vaccination not due to another identifiable cause.
- Collapse or shock-like state (hypotonic hyporesponsive episode) within 48 hours of vaccination.
- Persistent, inconsolable crying lasting ≥ 3 hours, occurring within 48 hours of vaccination.
- Convulsions with or without fever occurring within 3 days of vaccination.
There may be circumstances, such as high incidence of pertussis, in which the potential benefits outweigh possible risks, particularly since these events have not been proven to cause permanent sequelae.
Data from clinical studies indicate that, when INFANRIX hexa® is co-administered with pneumococcal conjugate vaccine (Prevnar®, Prevnar® 13 or SYNFLORIX®), the rate of febrile reactions is higher compared to that occurring following the administration of INFANRIX hexa® alone.
Increased reporting rates of convulsions (with or without fever) and hypotonic hyporesponsive episode were observed with concomitant administration of INFANRIX hexa® and Prevnar®13 (see ADVERSE REACTIONS).
Antipyretic treatment should be initiated according to local treatment guidelines.
Syncope (fainting) can occur following, or even before, any vaccination as a psychogenic response to the needle injection. It is important that procedures are in place to avoid injury from faints.
Hematologic
INFANRIX hexa® should be administered with caution to subjects with thrombocytopenia or a bleeding disorder since bleeding may occur following intramuscular administration to these subjects.
Immune
Hepatitis B has a long incubation period. Hepatitis B vaccination may not prevent hepatitis B infection in individuals who had an unrecognized hepatitis B infection at the time of vaccine administration.
INFANRIX hexa® is not contraindicated for use in individuals with HIV infection. The expected immunological response may not be obtained after vaccination of immunosuppressed patients.
Immunosuppressive therapies, including irradiation, antimetabolites, alkylating agents, cytotoxic drugs and corticosteroids (used in greater than physiologic doses), may reduce the immune response to vaccines (See DRUG INTERACTIONS).
Hepatitis B
Infants born of HBsAg-positive mothers should receive hepatitis B immune globulin (HBIG) and Hepatitis B vaccine at birth and should complete the Hepatitis B vaccination series given according to a particular schedule. Infants born of mothers of unknown HBsAg status should receive Hepatitis B vaccine at birth and should complete the Hepatitis B vaccination series given according to a particular schedule (see Manufacturer's package insert for Hepatitis B vaccine).
The subsequent administration of INFANRIX hexa® for completion of the Hepatitis B vaccination series in infants who were born of HBsAg-positive mothers and received HBIG, or infants born of mothers of unknown status has not been studied.
Neurologic
Experience with INFANRIX® (DTaP) and other INFANRIX®-based combinations has not revealed any cases of encephalopathy or permanent neurologic damage causally linked to vaccination. While acute encephalopathy and permanent neurologic damage have not been reported to be causally linked nor in a temporal association with administration of INFANRIX hexa® data is limited at this time.
In children with progressive neurological disorders, including infantile spasms, uncontrolled epilepsy or progressive encephalopathy, it is better to defer pertussis (Pa or Pw) immunization until the condition is corrected or stable. However, the decision to give pertussis vaccine must be made on an individual basis after careful consideration of the risks and benefits.
A history of convulsions or other central nervous system disorders in parents or siblings is not a contraindication for INFANRIX hexa®, an acellular DTP vaccine. Vaccinees with a history of febrile convulsions should be closely followed up as such adverse events may occur within 2 to 3 days post vaccination.
Studies suggest that when given whole-cell DTP vaccine, infants and children with a history of convulsions in first-degree family members (i.e., siblings and parents) have a 2.4-fold increased risk for neurologic events compared to those without such histories.
Respiratory
Although a moderate or severe illness with or without fever is a reason to defer vaccination, minor illnesses such as mild upper respiratory infections with or without low-grade fever are not a contraindication.
Special Populations
Pregnant Women: As INFANRIX hexa® is not intended for use in adults, adequate human data on use during pregnancy and adequate animal reproduction studies are not available.
Nursing Women: As INFANRIX hexa® is not intended for use in adults, adequate human data on use during lactation and adequate animal reproduction studies are not available.
Pediatrics: Limited data in 169 premature infants indicate that INFANRIX hexa® can be given to premature children. However, a lower immune response may be observed and the level of clinical protection remains unknown. The potential risk of apnea and the need for respiratory monitoring for 48-72h should be considered when administering the primary immunization series to very premature infants (born ≤ 28 weeks of gestation) and particularly for those with a previous history of respiratory immaturity. As the benefit of vaccination is high in this group of infants, vaccination should not be withheld or delayed. Safety and effectiveness of INFANRIX hexa® have not been established in infants below the age of 6 weeks and children over 2 years of age.
Interference With Laboratory Testing
The Hib component of the vaccine does not protect against diseases due to capsular serotypes other than type b of Haemophilus influenzae or against meningitis caused by other organisms. Excretion of capsular polysaccharide antigen in the urine has been described following administration of Hib vaccines, and therefore antigen detection may not have a diagnostic value in suspected Hib disease within 1-2 weeks of vaccination. Other tests should be performed in order to confirm Hib infection during this period.
Overdosage & Contraindications
OVERDOSE
For management of a suspected drug overdose, contact your regional Poison Control Centre.
CONTRAINDICATIONS
INFANRIX hexa® (combined diphtheria and tetanus toxoids, acellular pertussis, hepatitis B (recombinant), inactivated poliomyelitis, and adsorbed conjugated Haemophilus influenzae type b vaccine):
- should not be administered to subjects with known hypersensitivity to any component of this vaccine (see Dosage Forms, Composition And Packaging) or to subjects having shown signs of hypersensitivity after a previous dose of this vaccine or any injection containing diphtheria, tetanus, pertussis, hepatitis B, poliovirus or Haemophilus influenzae type b (see WARNINGS AND PRECAUTIONS, General section for information on treatment of immediate allergic reactions).
- should be used with caution in subjects with known hypersensitivity to the antibiotics neomycin and polymyxin, as INFANRIX hexa® contains traces of these antibiotics.
- is contraindicated for use after an immediate anaphylactic reaction temporally associated with a previous dose of this vaccine or any injection containing diphtheria, tetanus, pertussis, hepatitis B, poliovirus, or Haemophilus influenzae type b. Because of the uncertainty as to which component of the vaccine might be responsible, no further vaccination with any of these components should be given. Alternatively, because of the importance of tetanus vaccination, such individuals may be referred to an allergist for evaluation.
- should not be administered to persons 7 years of age or older because diphtheria toxoid may cause severe but transient local and febrile reactions in children and adults, the frequency increasing with age, the dose of toxoid and the number of doses given.
- is contraindicated if the infant has experienced an encephalopathy of unknown etiology, occurring within 7 days following previous vaccination with a pertussis containing vaccine. In these circumstances, pertussis vaccination should be discontinued and the vaccination should be continued with diphtheria-tetanus, Hepatitis B, polio, and Hib vaccines.
Immunization should be deferred during the course of a moderate or severe acute febrile illness or acute infection (see WARNINGS AND PRECAUTIONS). The presence of a minor infection, however is not a contraindication.
Elective immunization of individuals over 6 months should be deferred during an outbreak of poliomyelitis.
Clinical Pharmacology
Action And Clinical Pharmacology
Diphtheria
Diphtheria is a serious communicable disease, primarily a localized and generalized intoxication caused by diphtheria toxin, an extracellular protein metabolite of toxigenic strains of Corynebacterium diphtheriae. The disease occurs most frequently in unimmunized or partially immunized individuals. The incidence of diphtheria in Canada has decreased from 9,000 cases reported in 1924 to extremely low levels. Only 1 or 2 cases have been reported annually in recent years. The case fatality rate remains 5% to 10 %, with the highest death rates in the very young and elderly. If immunization levels are allowed to fall and adults do not receive booster doses, disease re-emergence may appear as demonstrated in the Commonwealth of Independent States (former Soviet Union), where tens of thousands of cases with substantial mortality have been reported. Protection against disease is due to the development of neutralizing antibodies to the diphtheria toxin. Following adequate immunization with diphtheria toxoid, it is thought that protection persists for at least 10 years. Serum antitoxin levels of at least 0.01 antitoxin units per mL are generally regarded as protective.
This significantly reduces both the risk of developing diphtheria and the severity of clinical illness. Immunization with diphtheria toxoid does not, however, eliminate carriage of C. diphtheriae in the pharynx, nose or on the skin.
Tetanus
Tetanus is an intoxication manifested primarily by neuromuscular dysfunction caused by a potent exotoxin released by Clostridium tetani. Immunization is highly effective, provides long-lasting protection and is recommended for the whole population. Only 1 to 7 with an average of 5 cases of tetanus are now reported annually in Canada, while no deaths have been recorded since 1995. The disease continues to occur almost exclusively among persons who are unvaccinated, inadequately vaccinated or whose vaccination histories are unknown or uncertain.
Spores of C. tetani are ubiquitous. Naturally acquired immunity to tetanus toxin does not occur. Thus, universal primary immunization and timed booster doses to maintain adequate tetanus antitoxin levels are necessary to protect all age groups. Protection against disease is due to the development of neutralizing antibodies to the tetanus toxin. Tetanus toxoid is a highly effective antigen and a completed primary series generally induces serum antitoxin levels of at least 0.01 antitoxin units per mL, a level which has been reported to be protective. It is thought that protection persists for at least 10 years.
Pertussis
Pertussis (whooping cough) is a disease of the respiratory tract caused by Bordetella pertussis. Pertussis is highly communicable (attack rates in unimmunized household contacts of up to 90% have been reported) and can affect individuals of any age; however, severity is greatest among young infants. Precise epidemiologic data do not exist, since bacteriological confirmation of pertussis can be obtained in less than half of the suspected cases. Most reported illness from B. pertussis occurred in infants and young children in whom complications can be severe. Older children, adolescents and adults, in whomic signs are often absent, may go undiagnosed and may serve as reservoirs of disease. Pertussis epidemics are cyclic and occur every 3 to 4 years. Pertussis has been controlled in Canada through immunization. During the last 40 years, the incidence of pertussis has decreased by > 90% although outbreaks continue to occur.
A recent study was conducted in Germany to assess the efficacy of pertussis vaccine after partial and completed primary vaccination series for preventing hospitalizations due to pertussis under field conditions. Data was acquired by a nationwide, hospital based, active surveillance system. After one dose of the vaccine, vaccine effectiveness was as high as 68%, increasing to 91.8% after receipt of the second dose. Vaccine effectiveness of 3 and 4 doses of acellular vaccine were estimated to be 99.8% and 98.6%, respectively.
Antigenic components of B. pertussis believed to contribute to protective immunity include: pertussis toxin (PT); filamentous hemagglutinin (FHA); and pertactin. Although the role of these antigens in providing protective immunity in humans is not well understood, clinical trials which evaluated candidate acellular DTP vaccines manufactured by GlaxoSmithKline supported the efficacy of 3 component INFANRIX® (DTaP). Recently published data suggests a higher importance of the PT and pertactin components in providing protection against pertussis.
INFANRIX® contains 3 pertussis antigens (PT, FHA and pertactin), and has been shown to be effective in preventing World Health Organization (WHO)-defined pertussis as well as clinically milder disease in two published clinical trials when administered as a primary series.
A double-blind, randomized, placebo (DT)-controlled trial conducted in Italy, sponsored by the U.S. National Institutes of Health (NIH), assessed the absolute protective efficacy of INFANRIX® when administered at 2, 4 and 6 months of age. A total of 15,601 infants were immunized with 1 of 2 tri-component acellular DTP vaccines (containing inactivated PT, FHA and pertactin), or with a whole-cell DTP vaccine manufactured by Sanofi Pasteur, or with DT vaccine alone. The mean length of follow-up was 17 months, beginning 30 days after the third dose of vaccine. The population used in the primary analysis of vaccine efficacy included 4,481 INFANRIX® vaccinees, 4,348 whole-cell DTP vaccinees and 1,470 DT vaccinees. After 3 doses, the protective efficacy of INFANRIX® against WHO-defined typical pertussis (21 days or more of paroxysmal cough with infection confirmed by culture and/or serologic testing) was 84% (95% CI: 76% to 89%) while the efficacy of the whole-cell DTP vaccine was 36% (95% CI: 14% to 52%). When the definition of pertussis was expanded to include clinically milder disease with respect to type and duration of cough, with infection confirmed by culture and/or serologic testing, the efficacy of INFANRIX® was calculated to be 71% (95% CI: 60% to 78%) against > 7 days of any cough and 73% (95% CI: 63% to 80%) against ≥ 14 days of any cough. A longer follow-up of the Italian trial showed that after 3 doses, the absolute efficacy of INFANRIX® against WHO-defined pertussis remained high at 84% among children up to 4 years of age.
A prospective, blinded efficacy trial was also conducted in Germany employing a household contact study design. In preparation for this study, 3 doses of INFANRIX® were administered at 3, 4 and 5 months of age to more than 22,000 children living in 6 areas of Germany in a large safety and immunogenicity trial. Infants who did not participate in this trial could have received whole-cell DTP vaccine (manufactured by Chiron Behring, Germany) or DT vaccine. Calculation of vaccine efficacy was based on attack rates of pertussis in household contactsified by vaccination status. Of the 173 unvaccinated household contacts, 96 developed WHO-defined pertussis (21 days or more of paroxysmal cough with infection confirmed by culture and/or serologic testing), as compared to 7 of 112 contacts vaccinated with INFANRIX® and 1 of 75 contacts vaccinated with whole-cell DTP vaccine. The protective efficacy of INFANRIX® was calculated to be 89% (95% CI: 77% to 95%), with no indication of waning of immunity up until the time of the booster. The protective efficacy of the whole-cell DTP vaccine was calculated to be 98% (95% CI: 83% to 100%). When the definition of pertussis was expanded to include clinically milder disease, with infection confirmed by culture and/or serologic testing, the efficacy of INFANRIX® against ≥ 7 days of any cough was 67% (95% CI: 52% to 78%) and against ≥ 7 days of paroxysmal cough was 81% (95% CI: 68% to 89%). The corresponding efficacy rates of INFANRIX® against ≥ 14 days of any cough or paroxysmal cough were 73% (95% CI: 59% to 82%) and 84% (95% CI: 71% to 91%), respectively.
Hepatitis B
Several hepatitis viruses are known to cause a systemic infection resulting in major pathologic changes in the liver (e.g., A, B, C, D, E). Hepatitis B infection can have serious consequences including acute massive hepatic necrosis, chronic active hepatitis and cirrhosis of the liver. It has been estimated that more than 350 million people in the world are persistently infected with hepatitis B virus.
Among infected infants, very few (5 -10%) recover completely; the majority (up to 90%) become chronic carriers with the risk of becoming a chronic carrier decreasing with age (children < 5 years 25% to 50%, adults 6% to 10%). Those patients who become chronic carriers can infect others and are at increased risk of developing either cirrhosis or primary hepatocellular carcinoma.
Among other factors, infection with hepatitis B may be the single most important factor for development of this carcinoma. Considering the serious consequences of infection, immunization should be considered for all persons.
Mothers infected with hepatitis B virus can infect their infants at, or shortly after, birth if they are carriers of the hepatitis B surface antigen (HBsAg) or develop an active infection during the third trimester of pregnancy. Infected infants usually become chronic carriers. Therefore, screening of pregnant women for hepatitis B is recommended. According to the Canadian Immunization Guide, hepatitis B prevention should include programs for universal immunization of children, pre-exposure vaccination of high-risk groups, universal HBsAg screening of all pregnant women and post-exposure intervention for those exposed to disease, particularly infants born to hepatitis B-infected mothers. There is no specific treatment for acute hepatitis B infection. However, those who develop anti-HBs antibodies after active infection are usually protected against subsequent infection. Antibody titers ≥ 10 mIU/mL against HBsAg are recognized as conferring protection against hepatitis B. Seroconversion is defined as antibody titers ≥ 1 mIU/mL.
Poliomyelitis
Poliovirus is an enterovirus that belongs to the picornavirus family. Three serotypes of poliovirus have been identified (types 1, 2 and 3). Poliovirus is highly contagious with the predominant mode of transmission being person-to-person via the fecal-oral route. Infection may be spread indirectly through contact with infectious saliva or feces or by contaminated water or sewage.
Replication of poliovirus in the pharynx and intestine is followed by a viremic phase where involvement of the central nervous system can occur. While poliovirus infections are asymptomatic or cause nonspecific symptoms (low-grade fever, malaise, anorexia and sore throat) in 90% to 95% of individuals, 1% to 2% of infected persons will develop paralytic disease.
Following the introduction of inactivated poliovirus vaccines (IPV) in Canada in 1955, the indigenous disease has been eliminated. Since 1980, 12 paralytic cases have been reported in Canada, 11 of which were determined to be vaccine-associated paralytic poliomyelitis (VAPP), with Oral Polio Vaccine (OPV). The last reported case of VAPP occurred in 1995.
Forty seven studies involving over 19,000 infants and children have been conducted in developed and developing countries with GlaxoSmithKline's enhanced inactivated poliovirus vaccine, as trivalent IPV vaccine or as a part of DTaP-IPV based combinations.
Haemophilus Influenzae Type b
Haemophilus influenzae type b (Hib) was the most common cause of bacterial meningitis and a leading cause of other serious invasive infections in young children prior to the introduction of other Hib vaccines. About 55% to 65% of affected children had meningitis while the remainder had epiglottitis, bacterimia, cellulitis, pneumonia or septic arthritis. The case fatality rate of meningitis is about 5%. Severe neurologic sequelae occur in 10% to 15% of survivors and deafness in 15% to 20% (severe in 3% to 7%).
Before the introduction of Hib conjugate vaccines in Canada in 1988, there were approximately 2,000 cases of Hib disease annually. Since then the overall incidence has fallen by more than 99%. The majority of cases occur now in children too old to have received primary vaccination. In 1998, only 15 cases were reported in children < 5 years of age.
SCIENTIFIC AND PHARMACEUTICAL INFORMATION
Drug Substance
Proper name: combined diphtheria and tetanus toxoids, acellular pertussis, hepatitis B (recombinant), inactivated poliomyelitis, and adsorbed conjugated Haemophilus influenzae type b vaccine
Product Characteristics
INFANRIX hexa® (combined diphtheria and tetanus toxoids, acellular pertussis, hepatitis B (recombinant), inactivated poliomyelitis, and adsorbed conjugated Haemophilus influenzae type b vaccine) contains diphtheria toxoid, tetanus toxoid, three purified pertussis antigens [pertussis toxoid (PT), filamentous haemagglutinin (FHA) and pertactin (69 kiloDalton outer membrane protein)], hepatitis B virus surface antigen recombinant, adsorbed onto aluminum salts, purified, inactivated poliovirus types 1, 2 and 3, Haemophilus influenzae type b polysaccharide conjugated to tetanus toxoid.
Clinical Trials
Study Results
Immune Response to INFANRIX hexa® Administered as a 3 Dose Primary Series
A total of 13,500 doses of INFANRIX hexa® (combined diphtheria and tetanus toxoids, acellular pertussis, hepatitis B (recombinant), inactivated poliomyelitis, and adsorbed conjugated Haemophilus influenzae type b vaccine) have been administered to 4,590 infants from 6 weeks of age and up as a primary series in clinical studies.
The immune responses to each of the antigens contained in INFANRIX hexa® were evaluated in sera obtained 1 month after the third dose of vaccine as compared to that following administration of commercially available vaccines (INFANRIX® (diphtheria, tetanus, and acellular pertussis vaccine), ENGERIX®-B (hepatitis B vaccine (recombinant)), Hib vaccine, and Oral Polio Virus vaccine) simultaneously at separate sites, in a study conducted in the U.S. The schedule of administration was 2, 4, and 6 months of age. One month after the third dose of INFANRIX hexa®, immune response rates to each antigen were comparable to rates seen following separately administered vaccines (see Table 2).
Table 2 : Antibody Responses to Each Antigen Following
INFANRIX hexa® as Compared to INFANRIX®, ENGERIX®-B, Hib vaccine, and OPV (One
Month After Administration of Dose 3)
| Anti-Diphtheria % ≥ 0.1 IU/mL | 100.0 | 99.0 |
| GMT | 1.431 | 1.009 |
| Anti-Tetanus | ||
| % ≥ 0.1 IU/mL | 100.0 | 100.0 |
| GMT | 1.979 | 1.486 |
| Anti-PT (V.R.) | ||
| % R | 99.0 | 97.9 |
| GMT | 67.4 | 41.8 |
| Anti-FHA (V.R.) | ||
| % R | 100.0 | 98.7 |
| GMT | 288.0 | 302.8 |
| Anti-Pertactin (V.R.) | ||
| % R | 96.2 | 95.8 |
| GMT | 168.2 | 136.9 |
| Anti-HBs | ||
| % ≥ 10 mIU/mL | 99.1 | 100.0 |
| GMT | 1239.5 | 934.3 |
| Anti-Polio 1 | ||
| % ≥ 8 | 100.0 | 98.6 |
| GMT | 494.8 | 1278.2 |
| Anti-Polio 2 | ||
| % ≥ 8 | 98.8 | 100.0 |
| GMT | 507.4 | 1350.4 |
| Anti-Polio 3 | ||
| % ≥ 8 | 98.8 | 98.6 |
| GMT | 1275.1 | 367.5 |
| Anti-PRP | ||
| % ≥ 0.15 μg/mL | 100.0 | 96.9 |
| Anti-PRP | ||
| % ≥ 1.0 μg/mL | 84.0 | 91.8 |
| GMT | 2.648 | 5.527 |
| OPV manufactured by Wyeth OmniHib manufactured by Sanofi Pasteur % R = in initially seronegative subjects, appearance of antibodies (titre 35 EL.U./mL); in initially seropositive subjects, at least maintenance of prevaccination titre GMT = Geometric mean antibody titre PT = Pertussis Toxoid FHA = Filamentous Haemagglutinin HBs = Hepatitis B surface (antigen) V.R. = Vaccine Response (Vaccine response is defined as appearance of antibodies in initially seronegative subjects or as at least maintenance of pre-vaccination antibody titres in initially seropositive subjects. Polio = Poliovirus PRP = Polyribosyl-ribitol-phosphate |
||
Clinical trials have investigated the tolerability and immunogenicity of the vaccine in various schedules (i.e. 2, 3, 4 months; 3, 4, 5 months; 2, 4, 6 months; 3, 5, 11 months; 1.5, 2.5, 3.5 months). Results obtained in all of the clinical studies for each of the components are summarized below:
DTaP Component
Immunological Data
One month after the 3 dose primary vaccination course, 98.5 to 100% of infants vaccinated with INFANRIX hexa® had antibody titres of ≥ 0.1 IU/mL for both tetanus and diphtheria.
Following administration of a 4th dose of INFANRIX hexa® in the second year of life, 100% of infants had antibody titres of ≥ 0.1 IU/mL for both tetanus and diphtheria.
One month after the 3 dose primary vaccination course, the overall response rate for each of the 3 individual pertussis antigens (PT, FHA, and pertactin) was between 97.2-99.3%, 95.2-100% and 95.9-99.3%, respectively.
Following administration of a 4th dose of INFANRIX hexa® in the second year of life, a booster response was seen in at least 97.2%, 94.1%, and 100% of vaccinated infants against the respective pertussis antigens. Since a serological correlation for protection against pertussis disease does not exist, the efficacy of the pertussis component presently relies on efficacy trials described below.
Protective Efficacy Data
The efficacy of the DTaP component, against WHO-defined typical pertussis ( ≥ 21 days of paroxysmal cough) was demonstrated in 2 studies.
The first was a perspective blinded household contact study performed in Germany (3, 4, 5 months vaccination schedule). Based on data collected from secondary contacts in households where there was an index case with typical pertussis, the protective efficacy of the vaccine was 88.7%.
The second was a National Institutes of Health (NIH) sponsored efficacy study performed in Italy (2, 4, 6 months vaccination schedule). The vaccine efficacy was found to be 84%. In a follow-up of the same cohort, efficacy was confirmed up to 60 months after completion of primary vaccination without administration of a booster dose of pertussis.
Hepatitis B component
After the primary vaccination course with INFANRIX hexa®, 98.5 to 100% of infants developed protective antibody titres of ≥ 10 mIU/mL.
At one month after the booster dose, administered 18 months after primary vaccination, 97 to 100% of these subjects had protective titres of ≥ 10 mIU/mL.
IPV Component
One month after the primary vaccination, the seroprotection rates for each of the three serotypes (types 1, 2, and 3) were 99.2 to 100%, 94.5 to 99.0%, and 98.8 to 100%, respectively.
Following administration of the booster dose, at least 98.5%, 98.5%, and 100% of infants were seroprotected for the three serotypes, respectively.
Hib Component
One month after completion of the primary vaccination course, the Geometric Mean Concentration (GMC) of antibodies ranged from 1.52 to 3.53 μg/mL, with between 93.5 and 100% of the subjects reaching antibody titres ≥ 0.15 μg/mL.
One month after the booster dose given in the second year of life, the GMC ranged from 19.1 to 94.0 μg/mL, with 99.5 to 100% of the subjects reaching antibody titres ≥ 0.15 μg/mL.
These GMCs are numerically lower when compared to GMCs resulting from separate administration of the Hib component, however they are not different from those elicited by comparator vaccines DTaP-Hib and DTaP-IPV-Hib vaccines.
Induction of immunological memory has been shown to be an important and intrinsic part of the protective immune response following administration of Hib conjugate vaccines. Children primed with INFANRIX hexa® had an anamnestic response (defined as a rapid and substantial increase in antibody level) on subsequent exposure to the antigen.
The effectiveness of the GlaxoSmithKline Hib component (when combined with DTaP or DTaP-IPV) has been investigated through an extensive post-marketing surveillance study conducted in Germany. Over a 4 ½ year follow-up period, the effectiveness of 3 primary doses of DTaP-Hib or DTaP-IPV-Hib was 96.7%.
Detailed Pharmacology
Not applicable.
Microbiology
Not applicable.
Toxicology
Not applicable.
REFERENCES
1. National Advisory Committee on Immunization: Canadian Immunization Guide, Sixth Edition. Minister of Public Works and Government Services Canada, 2002.
2. Centers for Disease Control. Diphtheria, tetanus, and pertussis: Recommendations for vaccine use and other preventive measures. Recommendations of the Immunization Practices Advisory Committee (ACIP). MMWR. 1991;Vol. 40 (RR-10):1-28.
3. Centers for Disease Control and Prevention. Tetanus Surveillance-United States, 1995-1997. MMWR.1998;Vol. 47(No. SS-2):1-13.
4. Biological products; bacterial vaccines and toxoids; implementation of efficacy review. Federal Register. Friday, December 13, 1985; Vol. 50 (No. 240):51002-51117.
5. Kendrick PL. Secondary familial attack rates from pertussis in vaccinated and unvaccinated children. Am J Hygiene. 1940;32:89-91.
6. Nennig ME, Shinefield HR, Edwards KM, Black SB, Fireman BH. Prevalence and incidence of adult pertussis in an urban population. JAMA. 1996; Vol. 275 (No. 21):1672-1674.
7. Cowell JL, Oda M, Burstyn DG, Manclark CR. Prospective protective antigens and animal models for pertussis. In: Leive L and Schlessinger D, eds. Microbiology-1984. Washington, DC: American Society for Microbiology, 1984, pp. 172-175.
8. Shahin RD, Brennan MJ, Li ZM, Meade BD, Manclark CR. Characterization of the protective capacity and immunogenicity of the 69-kD outer membrane protein of Bordetella pertussis. J Exper Med. 1990;171(1):63-73.
9. Gustafsson L, Hallander HO, Olin P, Reizenstein E, Storsaeter J. A controlled trial of a two-component acellular, a five-component acellular, and a whole-cell pertussis vaccine. N Engl J Med. 1996;334(6):349-355.
10. Greco D, Salmaso S, Mastrantonio P, Giuliano M, Tozzi AE, Anemona A, et al. A controlled trial of two acellular vaccines and one whole-cell vaccine against pertussis. N Engl J Med. 1996;334(6):341-348.
11. Schmitt HJ, Von Konig CH, Neiss A, Bogaerts H, Bock HL, Schulte-Wissermann H, et al. Efficacy of acellular pertussis vaccine in early childhood after household exposure. JAMA. 1996;275(1):37-41.
12. Cherry JD, Gornbein J, Heininger U, Stehr K. A search for serologic correlates of immunity to Bordetella pertussis cough illness. Vaccine 1998; 20: 1901-6.
13. Storsaeter J, Hallander HO, Gustafsson L, Olin P. Levels of anti-pertussis antibodies related to protection after household exposure to Bordetella pertussis. Vaccine 1998; 20: 1907-16.
14. Salmaso S, Anemona A, Mastrantonio P, Stefanelli P, Tozzi AE, Ciofidegli Atti ML. Long-term efficacy of pertussis vaccines in Italy. In Plotkin S, Brown F, Horatud F, eds. Preclinical and Clinical Development of New Vaccines. Dev Biol Stand. Basel, Karger. 1998; vol. 95. p. 189-194.
15. Kane M. Global programme for control of hepatitis B infection. Vaccine. 1995;13(Suppl 1):S47-49.
16. Lee WM. Hepatitis B virus infection. NEJM. 1997;337(24):1733-1745.
17. Centers for Disease Control: Protection against viral hepatitis: recommendations of the Immunization Practices Advisory Committee (ACIP). MMWR. 1990;39(No. RR-2):1-26.
18. Ambrosch F. Persistence of vaccine-induced antibodies to hepatitis B surface antigen--the need for booster vaccination in adult subjects. Postgrad Med J. 1987;63(Suppl. 2):129-135.
19. Faden HS. Polioviruses. Ped Infect Dis. 1977;249:1282-1287.
20. Centers for Disease Control and Prevention. Poliomyelitis Prevention in the United States: Introduction of a sequential vaccination schedule of inactivated poliovirus vaccine followed by oral poliovirus vaccine. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR. 1997;46 (No.RR-3):1-25.
21. Centers for Disease Control and Prevention. General recommendations on immunization. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR. 1994; Vol. 43 (RR-1):1-38.
22. Centers for Disease Control and Prevention. Update: Vaccine side effects, adverse reactions, contraindications, and precautions. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR. 1996;Vol. 45 (RR-12):1-35.
23. Centers for Disease Control and Prevention. Use of vaccines and immune globulins for persons with altered immunocompetence. Recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR. 1993;Vol. 42 (RR-4):1-3.
24. Expanded programme on Immunization, Injections and Paralytic Poliomyelitis. Wkly Epidem. Rec. 1980; 55:38-39.
25. Livengood JR, Mullen JR, White JW, Brink EW, Orenstein WA. Family history of convulsions and use of pertussis vaccine. J Pediatr. 1989;115(4):527-531.
26. Centers for Disease Control and Prevention. Sudden Infant Death Syndrome-United States 1983-94. MMWR. 1996;45(40):859-863. 3.
27. Roth R, Sudden Infant Death- who is at risk? 1998; 116 (14): 4-8.
28. Stratton KR, Howe CJ, Johnston RB Jr. Adverse events associated with childhood vaccines. Evidence bearing on causality. Institute of Medicine (IOM). Washington, DC: National Academy Press, 1994.
29. Hamati-Haddad-A, Fenichel-GM. Brachial Neuritis Following Routine Childhood Immunization for Diphtheria, Tetanus, and Pertussis (DTP): Report of Two Cases and Review of the Literature. Pediatrics; 1997; 9(4): 602-603.
30. Centers for Disease Control. Hepatitis B virus: a comprehensive strategy for eliminating transmission in the United States through universal childhood vaccination: recommendations of the Immunization Practices Advisory Committee (ACIP). MMWR. 1991;40 (RR-13):1-25.
31. Schmitt H, von Kries R, Siedler A. Surveillance for invasive Haemophilus influenzae disease and vaccination status in Germany: A study through the ESPED reporting system. July 2000.
32. Juretzko P, Von Kries R, Hermann M, Wirsing Von Konig CH, Weil J, Giani G. Effectiveness of acellular pertussis vaccine assessed by hospital-based active surveillance in Germany. Clin Infect Dis 2002; 35 (2): 162-167.
33. Wiebe T, Fergusson P, Home D. Hepatitis B Immunization Strategies with Low-Incidence Province of Canada: Comparing Alternative Strategies. Med Decision Making; 1997, 17(4): 472-482.
34. Schmitt H, Von Kries R, HassenPlug B, Hermann M, Siedler A, Niessing W, et al. Haemophilus influenzae type b disease: impact and effectiveness of diphtheria-tetanus toxoids-acellular pertussis (inactivated poliovirus) H. influenza type b combination vaccines. Ped Infect Dis. 2001;20;767-774.
35. Kalies H, Verstraeten T, Grote V, Meyer N, Siedler A, Schmitt H, Breuer T, Moulton L, von Kries R. Four and one-half-year follow-up of the effectiveness of diphtheria-tetanus toxoids-acellular pertussis/Haemophilus influenzae Type b and diphtheria-tetanus toxoids-acellular pertussis-inactivated poliovirus/H. influenzae Type b combination vaccines in Germany. Ped Infect Dis. 2004;23;944-950.
36. Tichmann-Schumann I, Soemantri P, Behre U, Disselhoff J, Mahler H, Maechler G, et al. Immunogenicity and Reactogenicity of Four Doses of Diphtheria-Tetanus-Three-Component Acellular Pertussis-Hepatitis B-Inactivated Polio Virus-Haemophilus influenzae Type b Vaccine Coadministered with 7-Valent Pneumococcal Conjugate Vaccine. Pediatr Infect Dis J 2005;24: 70-77.
Medication Guide
PATIENT INFORMATION
INFANRIX hexa®
Combined diphtheria and tetanus toxoids, acellular pertussis, hepatitis B
(recombinant), inactivated poliomyelitis, and adsorbed conjugated Haemophilus
influenzae type b vaccine
This leaflet is part III of a three-part ”Product Monograph“ published for INFANRIX hexa® (Combined diphtheria and tetanus toxoids, acellular pertussis, hepatitis B (recombinant), inactivated poliomyelitis, and adsorbed conjugated Haemophilus influenzae type b vaccine) approved for sale in Canada, and is designed specifically for Consumers. This leaflet is a summary and will not tell you everything about INFANRIX hexa®. Contact your doctor or pharmacist if you have any questions about the drug.
ABOUT THIS VACCINE
What the vaccine is used for
INFANRIX hexa® is a vaccine used in children for protection against diphtheria, tetanus (lockjaw), pertussis (whooping cough), hepatitis B, poliomyelitis (Polio) and Haemophilus influenzae type b diseases.
Vaccination is the best way to protect against these diseases.
What it does
INFANRIX hexa® works by helping the body to make its own protection (antibodies) which protect your child against these diseases.
When it should not be used
INFANRIX hexa® should not be used:
- in children who have a known allergy to any component of the vaccine (see “What the important nonmedicinal ingredients are” section) or children having shown signs of an allergic reaction after a previous dose of this vaccine or any injection containing diphtheria, tetanus, pertussis, hepatitis B, poliovirus, or Haemophilus influenzae type b. Signs of an allergic reaction may include skin rash, shortness of breath and swelling of the face or tongue.
- in persons 7 years of age or older.
- in infants who experienced problems of the nervous system within 7 days following previous vaccination with a pertussis (whooping cough) vaccine.
- if your child has an infection with a high temperature (over 38°C). A minor infection such as a cold should not be a problem, but talk to your doctor first.
- if your child has breathing difficulties, please contact your doctor. This may be more common in the first three days following vaccination if your child is born prematurely (before or at 28 weeks of pregnancy).
What the medicinal ingredient is
INFANRIX hexa® contains the following medicinal ingredients: combined diphtheria and tetanus toxoids, three purified pertussis toxoids, [pertussis toxoid, filamentous haemagglutinin and pertactin (69 kiloDalton outer membrane protein)] hepatitis B (recombinant), inactivated polio virus types 1, 2 and 3 and conjugated Haemophilus influenzae type b.
None of the components in the vaccine are infectious. You cannot get the diseases from the INFANRIX hexa® vaccine.
What the important nonmedicinal ingredients are
INFANRIX hexa® contains the following nonmedicinal ingredients: lactose, sodium chloride, aluminum salts, water for injection, residual formaldehyde, polysorbate 20 and 80, M199, potassium chloride, disodium phosphate, monopotassium phosphate, glycine, neomycin sulphate, polymyxin B sulphate and aluminum phosphate
What dosage forms it comes in
INFANRIX hexa® is a sterile suspension for injection, with the following components:
- PEDIARIXTM, supplied as a sterile, cloudy suspension for injection in a pre-filled glass syringe.
- Haemophilus influenzae type b vaccine, supplied as a pellet in a glass vial.
The 2 components are mixed together before they are given to your child.
WARNINGS AND PRECAUTIONS
- your child has a weakened immune system, for example due to HIV infection or due to medicines that suppress the immune system, as your child may not get the full benefit from INFANRIX hexa®.
- you have a family history of convulsions.
- your child is suffering from neurological disorders, including infantile spasms, uncontrolled epilepsy or progressive encephalopathy (disease of brain).
- your child has a bleeding problem or bruises easily. INFANRIX hexa® should be given with caution since bleeding may occur following vaccination.
- your child has a high temperature (over 38°C).
- your child has any known allergies.
- your child is taking any other medicine or has recently received any other vaccine.
- your child has any serious health problem.
- your child is younger than 6 weeks of age.
Fainting can occur following, or even before, any needle injection; therefore, tell the doctor or nurse if your child fainted with a previous injection.
High incidence of fever ( > 39.5°C) was reported in infants receiving INFANRIX hexa® and pneumococcal conjugate vaccine (Prevnar®, Prevnar ®13 or SYNFLORIX®) compared to infants receiving INFANRIX hexa® alone.
Increased reporting rates of fits (with or without fever) and collapse or shock-like state were observed with concomitant administration of INFANRIX hexa® and Prevnar®
INTERACTIONS WITH THIS VACCINE
As with other vaccines, INFANRIX hexa® should not be given to children on anticoagulant (medicine that prevents blood from clotting) therapy unless the benefits clearly outweigh the risks. Talk to your doctor.
Patients receiving immunosuppressive therapy (medicine that lowers the body's normal immune system response) should delay receiving INFANRIX hexa® vaccination until they have been off therapy for 3 months; otherwise you may not be fully protected against the diseases.
PROPER USE OF THIS VACCINE
In case of drug overdose, contact a health care practitioner, hospital emergency department or regional Poison Control Centre immediately, even if there are no symptoms.
Usual dose:
Your child will receive 3 doses given intramuscularly (into a muscle) at 2, 4 and 6 months of age. A booster should be given at 18 months.
Missed Dose:
If your child misses a scheduled injection, talk to your doctor and arrange another visit.
Make sure your child finishes the complete vaccination course of 3 injections. If not, your child may not be fully protected against the diseases.
SIDE EFFECTS AND WHAT TO DO ABOUT THEM
Like all vaccines, INFANRIX hexa® may occasionally cause unwanted effects.
As with other vaccines in any age group, allergic reactions may occur very rarely (in less than 1 in 10,000 doses of the vaccine). This can be recognised by symptoms such as itchy rash of the hands and feet, swelling of the eyes and face, difficulty in breathing or swallowing and a sudden drop in blood pressure and loss of consciousness. Such reactions will usually occur before leaving the doctor's office. However, you should seek immediate treatment in any event.
See your doctor straight away if your child has any of the following serious side effects:
- collapse
- times when they lose consciousness or have a lack of awareness
- fits – this may be when they have a fever
These side effects have happened very rarely with other vaccines against whooping cough. They usually happen within 2 to 3 days after vaccination.
Other side effects
Very common side effects (in more than 1 in 10 doses of the vaccine) after having INFANRIX hexa® are loss of appetite, irritability, unusual crying, restlessness, pain, redness and swelling at injection site, fever more than 38°C and feeling tired.
Common side effects (in more than 1 in 100 doses of the vaccine) after having INFANRIX hexa® are nervousness, vomiting, diarrhea, swelling larger than 5 cm at injection site, fever higher than 39.5°C, itching and a hard lump at injection site.
Uncommon side effects (in more than 1 in 1,000 doses of the vaccine) after having INFANRIX hexa® are upper respiratory tract infection, sleepiness, cough and swelling occurring over a large area of the injected limb.
Rare side effects (in more than 1 in 10,000 doses of the vaccine) after having INFANRIX hexa® are bronchitis and rash.
Very rare side effects (in less than 1 in 10,000 doses of the vaccine) after having INFANRIX hexa® are skin rash, hives, wheeziness or cough, swollen glands in the neck, armpit or groin, bleeding or bruising more easily than normal, temporarily stopping breathing, in babies born very prematurely (at or before 28 weeks of gestation) longer gaps than normal between breaths may occur for 2-3 days after vaccination, swelling of the face, lips, mouth, tongue or throat which may cause difficulty in swallowing or breathing, swelling of the entire injected limb, and blisters at the injection site.
If these symptoms continue or become severe, tell the doctor or nurse.
If your child develops any other symptom within days following the vaccination, tell your doctor as soon as possible.
Do not be alarmed by this list of possible side effects. It is possible that your child will have no side effects from vaccination.
This is not a complete list of side effects. For any unexpected effects while taking INFANRIX hexa®, contact your doctor or pharmacist.
HOW TO STORE IT
Store INFANRIX hexa® in a refrigerator at 2° to 8°C. Do not freeze. Discard if the vaccine has been frozen.
Store in the original package in order to protect from light.
After reconstitution immediate use is recommended.
Do not use after expiration date shown on the label. The date for last use corresponds to the last day of the month mentioned.
REPORTING SUSPECTED SIDE EFFECTS
To monitor vaccine safety, the Public Health Agency of Canada collects case reports on adverse events following immunization.
For health care professionals: If a patient experiences an adverse event following immunization, please complete the appropriate Adverse Events following Immunization (AEFI) Form and send it to your local Health Unit in your province/territory.
For the General Public: Should you experience an adverse event following immunization, please ask your doctor, nurse, or pharmacist to complete the Adverse Events following Immunization (AEFI) Form.
If you have any questions or have difficulties contacting your local health unit, please contact Vaccine Safety Section at Public Health Agency of Canada:
By toll-free telephone: 1-866-844-0018
By toll-free fax: 1-866-844-5931
By email: [email protected]
At the following website:
http://www.phac-aspc.gc.ca/im/vs-sv/index-eng.php
By regular mail:
The Public Health Agency of Canada
Vaccine Safety Section
130 Colonnade Road
Ottawa, Ontario
K1A 0K9 Address Locator 6502A
NOTE: Should you require information related to the management of the side effect, please contact your health care provider before notifying the Public Health Agency of Canada. The Public Health Agency of Canada does not provide medical advice.
MORE INFORMATION
This document plus the full product monograph, prepared for health professionals can be found at:
http://www.gsk.ca or by contacting the sponsor, GlaxoSmithKline Inc., 7333 Mississauga Road, Mississauga, Ontario, L5N 6L4
