Hydro 35 Drug Description: Detailed Datasheet
- Generic Name: hydrating topical foam
- Brand Name: Hydro 35
side effects drug center hydro 35 (hydrating topical foam) drug
Drug Description
HYDRO 35
Hydrating Topical Foam
For Topical Dermatological Use Only
Caution: Federal Law restricts this product to sale by, or on the order of a licensed healthcare practitioner.
DESCRIPTION
HYDRO 35 Foam is a keratolytic agent delivered in a water & lipid based emollient foam containing lactic acid. This foam gently softens excess tissue to enhance removal from skin and nails, while rehydrating healthy tissue. Each gram contains 35% Urea as the active ingredient.
Urea has the following chemical structure:
Chemical Structure
 |
Indications
For enzymatic debridement and promotion of normal healing of surface lesions, particularly where healing is retarded by local infection, necrotic tissue, fibrinous or purulent debris, or eschar. Topically applied urea is useful for the treatment of hyperkeratotic conditions such as dermatitis, psoriasis, xerosis, ichthyosis, eczema, keratosis, keratoderma, and dry, rough skin, as well as corns and calluses and damaged, ingrown and devitalized nails.
Dosage
DOSAGE AND ADMINISTRATION
Apply to affected area twice a day unless otherwise directed by a prescribing healthcare practitioner. HYDRO 35 Foam should be rubbed gently into the skin until it is completely absorbed.
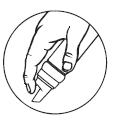
Follow these important directions to ensure proper foaming and maximum delivery of product:
- Shake canister vigorously before each use.
- Turn upside down (nozzle down) to dispense.
- Depress ridged portion of dispenser, as illustrated at right.
 |
Ingredients
Urea 35%, dimethicone, ethylparaben, glycerin, lactic acid, methylparaben, phenoxyethanol, polysorbate 20, povidone, propylene glycol, propylparaben, purified water, stearic acid, trolamine, and as propellants isobutane and propane.
HOW SUPPLIED
HYDRO 35 Foam is supplied in a 5.3 ounce (150g) pressurized canister bearing the NDC Number 23710-035-15 and in a 0.79 ounce (22g) pressurized canister bearing the NDC Number 23710-035-20.
Store at controlled room temperature 15° to 25°C (59° to 77°F).
Manufactured in the USA for: Quinnova Pharmaceuticals, LLC., Jamison, PA 18929. (877) 660-6263. www.QUINNOVA.com. Revised: December 2012
Side Effects & Drug Interactions
SIDE EFFECTS
No information provided.
DRUG INTERACTIONS
No information provided.
Warnings & Precautions
WARNINGS
For external use only. Not for ophthalmic, oral, anal or intravaginal use. Avoid contact with the eyes, lips, and other mucous membranes.
KEEP THIS AND OTHER MEDICATIONS OUT OF THE REACH OF CHILDREN.
Contains flammable materials. Contents under pressure. Do not puncture or incinerate. Do not expose to temperatures over 120°F (48°C) even when empty.
PRECAUTIONS
Use only as directed by a healthcare practitioner. Do not use to treat any condition other than that for which it is prescribed. If redness or irritation occurs, discontinue use and contact the prescribing healthcare practitioner.
Pregnancy
(Category C)
Animal reproduction studies have not been conducted with Hydro 35 Foam. It is also not known whether Hydro 35 Foam can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Hydro 35 Foam should be given to a pregnant woman only if clearly needed.
Nursing Mothers
It is not known whether topically applied urea is excreted in human milk. Due to the fact that many drugs are excreted in human milk, caution should be exercised by physicians when administering HYDRO 35 Foam to nursing mothers.
Overdosage & Contraindications
OVERDOSE
No information provided.
CONTRAINDICATIONS
HYDRO 35 Foam should not be used by persons who have a known hypersensitivity to urea or any of the listed ingredients.
Clinical Pharmacology
Topically applied urea dissolves the intercellular matrix of the skin which results in softening of the hyperkeratotic tissue, and thus enhances shedding of scaly, dry skin. Urea topically applied to the nail plate has a similar effect on the intercellular matrix of the nail plate.
Pharmacokinetics
The mechanism of action of topical urea is not yet known.
Medication Guide
PATIENT INFORMATION
No information provided. Please refer to the WARNINGS and PRECAUTIONS sections.
