Topicort Drug Description: Detailed Datasheet
- Generic Name: desoximetasone
- Brand Name: Topicort
side effects drug center topicort (desoximetasone) drug
Drug Description
What is Topicort and how is it used?
Topicort is a prescription medicine used to treat the symptoms of Corticosteroid-Responsive Dermatoses. Topicort may be used alone or with other medications.
Topicort belongs to a class of drugs called Corticosteroids, Topical.
What are the possible side effects of Topicort?
Topicort may cause serious side effects including:
- hives,
- difficulty breathing,
- swelling of your face, lips, tongue, or throat,
- worsening of your skin condition,
- redness, warmth, swelling, oozing, or severe irritation of any treated skin,
- blurred vision,
- tunnel vision,
- eye pain,
- seeing halos around lights,
- increased thirst,
- increased urination,
- dry mouth,
- fruity breath odor,
- weight gain (especially in your face or you upper back and torso),
- slow wound healing,
- thinning skin,
- discolored skin,
- increased body hair,
- muscle weakness,
- nausea,
- diarrhea,
- tiredness,
- mood changes,
- menstrual changes, and
- sexual changes
Get medical help right away, if you have any of the symptoms listed above.
The most common side effects of Topicort include:
- rash,
- itching,
- irritation around your mouth,
- burning, irritation, itching, or dryness of treated skin,
- redness or crusting around your hair follicles,
- increased hair growth,
- blisters,
- pimples,
- crusting of treated skin,
- color changes of treated skin,
- white or "pruned" appearance of the skin, and
- stretch marks
Tell the doctor if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of Topicort. For more information, ask your doctor or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
For topical use only. Not for oral, ophthalmic, or intravaginal use.
DESCRIPTION
Topicort® (desoximetasone) Ointment USP, 0.05% contains the active synthetic corticosteroid desoximetasone. The topical corticosteroids constitute a class of primarily synthetic steroids used as anti-inflammatory and antipruritic agents.
Each gram of Topicort® (desoximetasone) Ointment USP, 0.05% contains 0.5 mg of desoximetasone in an ointment base consisting of mineral oil and white petrolatum.
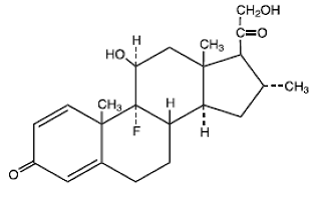
The chemical name of desoximetasone is Pregna-1, 4-diene-3, 20-dione, 9-fluoro-11, 21dihydroxy- 16-methyl-,(11ß,16α)-.
Desoximetasone has the molecular formula C22H29FO4 and a molecular weight of 376.47. The CAS Registry Number is 382-67-2.
The structural formula is:
 |
Indications & Dosage
INDICATIONS
Topicort® (desoximetasone cream USP) 0.05%, Topicort® (desoximetasone cream USP) 0.25%, and Topicort® (desoximetasone gel USP) 0.05% are indicated for the relief of the inflammatory and pruritic manifestations of corticosteroid-responsive dermatoses.
DOSAGE AND ADMINISTRATION
Apply a thin film of Topicort® (desoximetasone cream USP) 0.05%, Topicort® (desoximetasone cream USP) 0.25%, or Topicort® (desoximetasone gel USP) 0.05% to the affected skin areas twice daily. Rub in gently.
HOW SUPPLIED
Topicort® (desoximetasone cream USP) 0.05% is supplied in:
5 gram tubes for physician samples, 15 gram (NDC 51672-5205-1), 30 gram (NDC 51672-5205-2), 60 gram (NDC 51672-5205-3) and 100 gram (NDC 51672-5205-7) tubes.
Topicort® (desoximetasone cream USP) 0.25% is supplied in:
5 gram tubes for physician samples, 15 gram (NDC 51672-5204-1), 30 gram (NDC 51672-5204-2), 60 gram (NDC 51672-5204-3) and 100 gram (NDC 51672-5204-7) tubes.
Topicort® (desoximetasone gel USP) 0.05% is supplied in:
5 gram tubes for physician samples, 15 gram (NDC 51672-5202-1), 30 gram (NDC 51672-5202-2) and 60 gram (NDC 51672-5202-3) tubes.
Store at controlled room temperature between 20° to 25°C (68° to 77°F), excursions permitted to 15° to 30°C (59° to 86°F). [See USP Controlled Room Temperature]
Manufactured by: Taro Pharmaceuticals Inc., Brampton, Ontario, Canada L6T 1C1. Revised: Sep 2015
Side Effects & Drug Interactions
SIDE EFFECTS
The following local adverse reactions are reported infrequently with topical corticosteroids, but may occur more frequently with the use of occlusive dressings. These reactions are listed in an approximate decreasing order of occurrence:
Burning, itching, irritation, dryness, folliculitis, hypertrichosis, acneiform eruptions, hypopigmentation, perioral dermatitis, allergic contact dermatitis, maceration of the skin, secondary infection, skin atrophy, striae, and miliaria.
In controlled clinical studies the incidence of adverse reactions were low (0.8%) for Topicort® (desoximetasone cream USP) 0.25%, and included burning, folliculitis, and folliculo-pustular lesions. The incidence of adverse reactions were also 0.8% for Topicort® (desoximetasone cream USP) 0.05% and included pruritus, erythema, vesiculation, and burning sensation. The incidence of adverse reactions for Topicort® (desoximetasone gel USP), 0.05% was 0.3% with one subject reporting stinging and burning at the site of application.
DRUG INTERACTIONS
No Information Provided
Warnings & Precautions
WARNINGS
Keep out of reach of children.
PRECAUTIONS
General
Systemic absorption of topical corticosteroids can produce reversible hypothalamic-pituitary-adrenal (HPA) axis suppression with the potential for clinical glucocorticosteroid insufficiency. This may occur during treatment or upon withdrawal of the topical corticosteroid.
Because of the potential for systemic absorption, use of topical corticosteroids may require that patients be periodically evaluated for HPA axis suppression. Factors that predispose a patient using a topical corticosteroid to HPA axis suppression include the use of more potent steroids, use over large surface areas, use over prolonged periods, use under occlusion, use on an altered skin barrier, and use in patients with liver failure.
An ACTH stimulation test may be helpful in evaluating patients for HPA axis suppression. If HPA axis suppression is documented, an attempt should be made to gradually withdraw the drug, to reduce the frequency of application, or to substitute a less potent steroid. Manifestations of adrenal insufficiency may require supplemental systemic corticosteroids. Recovery of HPA axis function is generally prompt and complete upon discontinuation of topical corticosteroids.
Cushing's syndrome, hyperglycemia, and unmasking of latent diabetes mellitus can also result from systemic absorption of topical corticosteroids.
Use of more than one corticosteroid-containing product at the same time may increase the total systemic corticosteroid exposure.
Pediatric patients may be more susceptible to systemic toxicity from use of topical corticosteroids.
Local Adverse Reactions With Topical Corticosteroids
Local adverse reactions may be more likely to occur with occlusive use, prolonged use or use of higher potency corticosteroids. Reactions may include atrophy, striae, telangiectasias, burning, itching, irritation, dryness, folliculitis, acneiform eruptions, hypopigmentation, perioral dermatitis, allergic contact dermatitis, secondary infection, and miliaria. Some local adverse reactions may be irreversible.
Allergic Contact Dermatitis With Topical Corticosteroids
Allergic contact dermatitis to any component of topical corticosteroids is usually diagnosed by a failure to heal rather than a clinical exacerbation. Clinical diagnosis of allergic contact dermatitis can be confirmed by patch testing.
Concomitant Skin Infections
Concomitant skin infections should be treated with an appropriate antimicrobial agent. If the infection persists, Topicort® (desoximetasone cream USP) 0.05%, Topicort® (desoximetasone cream USP) 0.25%, or Topicort® (desoximetasone gel USP) 0.05% should be discontinued until the infection has been adequately treated.
Laboratory Tests
The following tests may be helpful in evaluating the hypothalamic-pituitary-adrenal (HPA) axis suppression:
Urinary free cortisol test
ACTH stimulation test
Carcinogenesis, Mutagenesis, And Impairment Of Fertility
Long-term animal studies have not been performed to evaluate the carcinogenic potential or the effect on fertility of topical corticosteroids.
Desoximetasone was nonmutagenic in the Ames test.
Pregnancy
Teratogenic Effects
Pregnancy Category C
Corticosteroids have been shown to be teratogenic in laboratory animals when administered systemically at relatively low dosage levels. Some corticosteroids have been shown to be teratogenic after dermal application in laboratory animals.
Desoximetasone has been shown to be teratogenic and embryotoxic in mice, rats, and rabbits when given by subcutaneous or dermal routes of administration in doses 3 to 30 times the human dose of Topicort® (desoximetasone cream USP) 0.25% and 15 to 150 times the human dose of Topicort® (desoximetasone cream USP) 0.05%, or Topicort® (desoximetasone gel USP) 0.05%.
There are no adequate and well-controlled studies in pregnant women on teratogenic effects from topically applied corticosteroids. Therefore, Topicort® (desoximetasone cream USP) 0.05%, Topicort® (desoximetasone cream USP) 0.25%, or Topicort® (desoximetasone gel USP) 0.05%, should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. Drugs of this class should not be used extensively on pregnant patients, in large amounts, or for prolonged periods of time.
Nursing Mothers
It is not known whether topical administration of corticosteroids could result in sufficient systemic absorption to produce detectable quantities in breast milk. Systemically administered corticosteroids are secreted into breast milk in quantities not likely to have a deleterious effect on the infant.
Nevertheless, caution should be exercised when topical corticosteroids are administered to a nursing woman.
Pediatric Use
Pediatric patients may demonstrate greater susceptibility to topical corticosteroid-induced HPA axis suppression and Cushing's syndrome than mature patients because of a larger skin surface area to body weight ratio.
Hypothalamic-pituitary-adrenal (HPA) axis suppression, Cushing's syndrome, and intracranial hypertension have been reported in pediatric patients receiving topical corticosteroids. Manifestations of adrenal suppression in pediatric patients include linear growth retardation, delayed weight gain, low plasma cortisol levels, and absence of response to ACTH stimulation. Manifestations of intracranial hypertension include bulging fontanelles, headaches, and bilateral papilledema.
Administration of topical corticosteroids to pediatric patients should be limited to the least amount compatible with an effective therapeutic regimen. Chronic corticosteroid therapy may interfere with the growth and development of pediatric patients.
Overdosage & Contraindications
OVERDOSE
Topically applied corticosteroids can be absorbed in sufficient amounts to produce systemic effects (see PRECAUTIONS).
CONTRAINDICATIONS
Topicort® (desoximetasone cream USP) 0.05%, Topicort® (desoximetasone cream USP) 0.25%, and Topicort® (desoximetasone gel USP) 0.05% are contraindicated in those patients with a history of hypersensitivity to any of the components of the preparation.
Clinical Pharmacology
Topical corticosteroids share anti-inflammatory, antipruritic and vasoconstrictive actions.
The mechanism of anti-inflammatory activity of the topical corticosteroids is unclear. Various laboratory methods, including vasoconstrictor assays, are used to compare and predict potencies and/or clinical efficacies of the topical corticosteroids. There is some evidence to suggest that a recognizable correlation exists between vasoconstrictor potency and therapeutic efficacy in man.
Pharmacokinetics
The extent of percutaneous absorption of topical corticosteroids is determined by many factors including the vehicle, the integrity of the epidermal barrier, and the use of occlusive dressings.
Topical corticosteroids can be absorbed from normal intact skin. Inflammation and/or other disease processes in the skin increase percutaneous absorption. Occlusive dressings substantially increase the percutaneous absorption of topical corticosteroids. Thus, occlusive dressings may be a valuable therapeutic adjunct for treatment of resistant dermatoses.
Once absorbed through the skin, topical corticosteroids are handled through pharmacokinetic pathways similar to systemically administered corticosteroids. Corticosteroids are bound to plasma proteins in varying degrees. Corticosteroids are metabolized primarily in the liver and are then excreted by the kidneys. Some of the topical corticosteroids and their metabolites are also excreted into the bile.
Pharmacokinetic studies in men with Topicort® (desoximetasone cream USP) 0.25% with tagged desoximetasone showed a total of 5.2% ± 2.9% excretion in urine (4.1% ± 2.3%) and feces (1.1% ± 0.6%) and no detectable level (limit of sensitivity: 0.005 μg/mL) in the blood when it was applied topically on the back followed by occlusion for 24 hours. Seven days after application, no further radioactivity was detected in urine or feces. The halflife of the material was 15 ± 2 hours (for urine) and 17 ± 2 hours (for feces) between the third and fifth trial day. Studies with other similarly structured steroids have shown that predominant metabolite reaction occurs through conjugation to form the glucuronide and sulfate ester.
Medication Guide
PATIENT INFORMATION
Patients using topical corticosteroids should receive the following information and instructions:
- This medication is to be used as directed by the physician. It is for external use only. Avoid contact with the eyes.
- Patients should be advised not to use this medication for any disorder other than for which it was prescribed.
- The treated skin area should not be bandaged or otherwise covered or wrapped as to be occlusive unless directed by the physician.
- Patients should report any signs of local adverse reactions, especially under occlusive dressings.
- Other corticosteroid-containing products should not be used with Topicort® (desoximetasone) Ointment USP, 0.05% without first consulting with the physician. As with other corticosteroids, therapy should be discontinued when control is achieved. If no improvement is seen within 4 weeks, contact the physician.
