EryPed Drug Description: Detailed Datasheet
- Generic Name: erythromycin ethylsuccinate
- Brand Name: EryPed
- Drug Class: Macrolides
side effects drug center eryped (erythromycin ethylsuccinate) drug
Drug Description
What is EryPed and how is it used?
EryPed (erythromycin ethylsuccinate) is a macrolide antibiotic used to treat many different types of infections caused by bacteria. EryPed is available in generic form.
What are side effects of EryPed?
Common side effects of EryPed include:
- nausea,
- vomiting,
- diarrhea,
- stomach pain or cramping,
- loss of appetite,
- mild heartburn,
- dizziness,
- headache,
- feeling tired,
- vaginal itching or discharge, or
- itching or skin rash
To reduce the development of drug-resistant bacteria and maintain the effectiveness of Ery-Ped and other antibacterial drugs, Ery-Ped should be used only to treat or prevent infections that are proven or strongly suspected to be caused by bacteria.
DESCRIPTION
Erythromycin is produced by a strain of Saccharopolyspora erythraea (formerly Streptomyces erythraeus) and belongs to the macrolide group of antibiotics. It is basic and readily forms salts with acids. The base, the stearate salt, and the esters are poorly soluble in water. Erythromycin ethylsuccinate is an ester of erythromycin suitable for oral administration. Erythromycin ethylsuccinate is known chemically as erythromycin 2'-(ethyl succinate). The molecular formula is C43H75NO16 and the molecular weight is 862.06. The structural formula is:
 |
Ery-Ped 200 (erythromycin ethylsuccinate for oral suspension) when reconstituted with water, forms a suspension containing erythromycin ethylsuccinate equivalent to 200 mg erythromycin per 5 mL (teaspoonful) or 100 mg per 2.5 mL (dropperful) with an appealing fruit flavor. Ery-Ped 400 when reconstituted with water, forms a suspension containing erythromycin ethylsuccinate equivalent to 400 mg of erythromycin per 5 mL (teaspoonful) with an appealing banana flavor.
These products are intended primarily for pediatric use but can also be used in adults.
Inactive Ingredients
Ery-Ped 200, Ery-Ped 400: Caramel, polysorbate, sodium citrate, sucrose, xanthan gum and artificial flavors.
Indications & Dosage
INDICATIONS
ENTEREG is indicated to accelerate the time to upper and lower gastrointestinal recovery following surgeries that include partial bowel resection with primary anastomosis.
DOSAGE AND ADMINISTRATION
For hospital use only. The recommended adult dosage of ENTEREG is 12 mg administered 30 minutes to 5 hours prior to surgery followed by 12 mg twice daily beginning the day after surgery until discharge for a maximum of 7 days. Patients should not receive more than 15 doses of ENTEREG.
ENTEREG can be taken with or without food [see CLINICAL PHARMACOLOGY].
HOW SUPPLIED
Dosage Forms And Strengths
12 mg blue, hard-gelatin capsules with “ADL2698” printed on both the body and the cap of the capsule.
Storage And Handling
ENTEREG capsules, 12 mg, are blue, hard-gelatin capsules printed with “ADL2698” on both the body and the cap of the capsule. ENTEREG capsules are available in unit-dose packs of 30 capsules (30 doses) (NDC 67919-020-10) for hospital use only.
Store at 25°C (77°F); excursions permitted to 15–30°C (59–86°F) [see USP Controlled Room Temperature].
Manufactured by: Pharmaceutical Manufacturing Research Services, Inc., Horsham, PA 19044, USA. Revised: Nov 2020
Side Effects & Drug Interactions
SIDE EFFECTS
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Potential Risk of Myocardial Infarction with Long-Term Use [see WARNINGS AND PRECAUTIONS]
- Gastrointestinal-Related Adverse Reactions in Opioid-Tolerant Patients [see WARNINGS AND PRECAUTIONS]
- Risk of Serious Adverse Reactions in Patients with Severe Hepatic Impairment [see WARNINGS AND PRECAUTIONS]
- Risk of Serious Adverse Reactions in Patients with Complete Gastrointestinal Obstruction [see WARNINGS AND PRECAUTIONS]
- Risk of Serious Adverse Reactions in Pancreatic and Gastric Anastomoses [see WARNINGS AND PRECAUTIONS]
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be compared directly with rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice. The adverse event information from clinical trials does, however, provide a basis for identifying the adverse events that appear to be related to drug use and for approximating rates.
The data described below reflect exposure to ENTEREG 12 mg in 1,793 patients in 10 placebo-controlled studies. The population was 19 to 97 years old, 64% were female, and 84% were Caucasian; 64% were undergoing a surgery that included bowel resection. The first dose of ENTEREG was administered 30 minutes to 5 hours before the scheduled start of surgery and then twice daily until hospital discharge (or for a maximum of 7 days of postoperative treatment).
Among ENTEREG-treated patients undergoing surgeries that included a bowel resection, the most common adverse reaction (incidence ≥1.5%) occurring with a higher frequency than placebo was dyspepsia (ENTEREG, 1.5%; placebo, 0.8%). Adverse reactions are events that occurred after the first dose of study medication treatment and within 7 days of the last dose of study medication or events present at baseline that increased in severity after the start of study medication treatment.
DRUG INTERACTIONS
Effects Of Alvimopan On Intravenous Morphine
Coadministration of alvimopan does not appear to alter the pharmacokinetics of morphine and its metabolite, morphine-6-glucuronide, to a clinically significant degree when morphine is administered intravenously. Dosage adjustment for intravenously administered morphine is not necessary when it is coadministered with ENTEREG.
Effects Of Concomitant Acid Blockers Or Antibiotics
A population pharmacokinetic analysis suggests that the pharmacokinetics of alvimopan were not affected by concomitant administration of acid blockers (proton pump inhibitors (PPIs), histamine-2 (H2) receptor antagonists) or antibiotics. No dosage adjustments are necessary in patients taking acid blockers or antibiotics with ENTEREG.
Warnings
Hepatotoxicity
There have been reports of hepatic dysfunction, including increased liver enzymes, and hepatocellular and/or cholestatic hepatitis, with or without jaundice, occurring in patients receiving oral erythromycin products.
QT Prolongation
Erythromycin has been associated with prolongation of the QT interval and infrequent cases of arrhythmia. Cases of torsades de pointes have been spontaneously reported during postmarketing surveillance in patients receiving erythromycin. Fatalities have been reported. Erythromycin should be avoided in patients with known prolongation of the QT interval, patients with ongoing proarrhythmic conditions such as uncorrected hypokalemia or hypomagnesemia, clinically significant bradycardia, and in patients receiving Class IA (quinidine, procainamide) or Class III (dofetilide, amiodarone, sotalol) antiarrhythmic agents. Elderly patients may be more susceptible to drug-associated effects on the QT interval.
Syphilis In Pregnancy
There have been reports suggesting that erythromycin does not reach the fetus in adequate concentration to prevent congenital syphilis. Infants born to women treated during pregnancy with oral erythromycin for early syphilis should be treated with an appropriate penicillin regimen.
Clostridium Difficile Associated Diarrhea
Clostridium difficile associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including Ery-Ped, and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon leading to overgrowth of C. difficile.
C. difficile produces toxins A and B which contribute to the development of CDAD. Hypertoxin producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibiotic use. Careful medical history is necessary since CDAD has been reported to occur over two months after the administration of antibacterial agents.
If CDAD is suspected or confirmed, ongoing antibiotic use not directed against C. difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibiotic treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated.
Drug Interactions
Serious adverse reactions have been reported in patients taking erythromycin concomitantly with CYP3A4 substrates. These include colchicine toxicity with colchicine; rhabdomyolysis with simvastatin, lovastatin, and atorvastatin; and hypotension with calcium channel blockers metabolized by CYP3A4 (e.g. verapamil, amlodipine, diltiazem) (see DRUG INTERACTIONS).
There have been post-marketing reports of colchicine toxicity with concomitant use of erythromycin and colchicine. This interaction is potentially life-threatening, and may occur while using both drugs at their recommended doses (see DRUG INTERACTIONS).
Rhabdomyolysis with or without renal impairment has been reported in seriously ill patients receiving erythromycin concomitantly with lovastatin. Therefore, patients receiving concomitant lovastatin and erythromycin should be carefully monitored for creatine kinase (CK) and serum transaminase levels. (See package insert for lovastatin)
Precautions
General
Prescribing Ery-Ped in the absence of a proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria.
Since erythromycin is principally excreted by the liver, caution should be exercised when erythromycin is administered to patients with impaired hepatic function. (See CLINICAL PHARMACOLOGY and WARNINGS sections.)
Exacerbation of symptoms of myasthenia gravis and new onset of symptoms of myasthenic syndrome has been reported in patients receiving erythromycin therapy.
There have been reports of infantile hypertrophic pyloric stenosis (IHPS) occurring in infants following erythromycin therapy. In one cohort of 157 newborns who were given erythromycin for pertussis prophylaxis, seven neonates (5%) developed symptoms of non-bilious vomiting or irritability with feeding and were subsequently diagnosed as having IHPS requiring surgical pyloromyotomy. A possible dose-response effect was described with an absolute risk of IHPS of 5.1% for infants who took erythromycin for 8-14 days and 10% for infants who took erythromycin for 15-21 days.2 Since erythromycin may be used in the treatment of conditions in infants which are associated with significant mortality or morbidity (such as pertussis or neonatal Chlamydia trachomatis infections), the benefit of erythromycin therapy needs to be weighed against the potential risk of developing IHPS. Parents should be informed to contact their physician if vomiting or irritability with feeding occurs.
Prolonged or repeated use of erythromycin may result in an overgrowth of nonsusceptible bacteria or fungi. If superinfection occurs, erythromycin should be discontinued and appropriate therapy instituted.
When indicated, incision and drainage or other surgical procedures should be performed in conjunction with antibiotic therapy. Observational studies in humans have reported cardiovascular malformations after exposure to drug products containing erythromycin during early pregnancy.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment Of Fertility
Long-term oral dietary studies conducted with erythromycin stearate in rats up to 400 mg/kg/day and in mice up to 500 mg/kg/day (approximately 1-2 fold of the maximum human dose on a body surface area basis) did not provide evidence of tumorigenicity. Erythromycin stearate did not show genotoxic potential in the Ames, and mouse lymphoma assays or induce chromosomal aberrations in CHO cells. There was no apparent effect on male or female fertility in rats treated with erythromycin base by oral gavage at 700 mg/kg/day (approximately 3 times the maximum human dose on a body surface area basis).
Pregnancy
Teratogenic Effects
There is no evidence of teratogenicity or any other adverse effect on reproduction in female rats fed erythromycin base by oral gavage at 350 mg/kg/day (approximately twice the maximum recommended human dose on a body surface area) prior to and during mating, during gestation, and through weaning. No evidence of teratogenicity or embryotoxicity was observed when erythromycin base was given by oral gavage to pregnant rats and mice at 700 mg/kg/day and to pregnant rabbits at 125 mg/kg/day (approximately 1-3 times the maximum recommended human dose).
Use In Specific Populations
Labor And Delivery
The effect of erythromycin on labor and delivery is unknown.
Nursing Mothers
Erythromycin is excreted in human milk. Caution should be exercised when erythromycin is administered to a nursing woman.
Pediatric Use
See INDICATIONS AND USAGE and DOSAGE AND ADMINISTRATION sections.
Geriatric Use
Elderly patients, particularly those with reduced renal or hepatic function, may be at increased risk for developing erythromycin-induced hearing loss. (See ADVERSE REACTIONS and DOSAGE AND ADMINISTRATION).
Elderly patients may be more susceptible to development of torsades de pointes arrhythmias than younger patients. (See WARNINGS).
Elderly patients may experience increased effects of oral anticoagulant therapy while undergoing treatment with erythromycin. (See PRECAUTIONS - DRUG INTERACTIONS).
Ery-Ped 200 contains 117.5 mg (5.1 mEq) of sodium per individual dose.
Ery-Ped 400 contains 117.5 mg (5.1 mEq) of sodium per individual dose.
Based on the 200 mg/5 mL strength, at the usual recommended doses, adult patients would receive a total of 940 mg/day (40.8 mEq) of sodium. Based on the 400 mg/5 mL strength, at the usual recommended doses, adult patients would receive a total of 470 mg/day (20.4 mEq) of sodium. The geriatric population may respond with a blunted natriuresis to salt loading. This may be clinically important with regard to such diseases as congestive heart failure.
REFERENCES
2. Honein, M.A., et al.: Infantile hypertrophic pyloric stenosis after pertussis prophylaxis with erythromycin: a case review and cohort study. The Lancet 1999;354 (9196): 2101-5
Overdosage & Contraindications
OVERDOSE
No Information Provided
CONTRAINDICATIONS
ENTEREG is contraindicated in patients who have taken therapeutic doses of opioids for more than 7 consecutive days immediately prior to taking ENTEREG [see WARNINGS AND PRECAUTIONS].
Clinical Pharmacology
Mechanism Of Action
Alvimopan is a selective antagonist of the cloned human μ-opioid receptor with a Ki of 0.4 nM (0.2 ng/mL) and no measurable opioid-agonist effects in standard pharmacologic assays. The dissociation of [3H]-alvimopan from the human μ-opioid receptor is slower than that of other opioid ligands, consistent with its higher affinity for the receptor. At concentrations of 1 to 10 μM, alvimopan demonstrated no activity at any of over 70 non-opioid receptors, enzymes, and ion channels.
Postoperative ileus is the impairment of gastrointestinal motility after intra-abdominal surgery or other, non-abdominal surgeries. Postoperative ileus affects all segments of the gastrointestinal tract and may last from 5 to 6 days, or even longer. This may potentially delay gastrointestinal recovery and hospital discharge until its resolution. It is characterized by abdominal distention and bloating, nausea, vomiting, pain, accumulation of gas and fluids in the bowel, and delayed passage of flatus and defecation. Postoperative ileus is the result of a multifactorial process that includes inhibitory sympathetic input and release of hormones, neurotransmitters, and other mediators (e.g., endogenous opioids). A component of postoperative ileus also results from an inflammatory reaction and the effects of opioid analgesics. Morphine and other μ-opioid receptor agonists are universally used for the treatment of acute postsurgical pain; however, they are known to have an inhibitory effect on gastrointestinal motility and may prolong the duration of postoperative ileus.
Following oral administration, alvimopan antagonizes the peripheral effects of opioids on gastrointestinal motility and secretion by competitively binding to gastrointestinal tract μ-opioid receptors. The antagonism produced by alvimopan at opioid receptors is evident in isolated guinea pig ileum preparations in which alvimopan competitively antagonizes the effects of morphine on contractility. Alvimopan achieves this selective gastrointestinal opioid antagonism without reversing the central analgesic effects of μ-opioid agonists.
Pharmacodynamics
In an exploratory study in healthy subjects, alvimopan 12 mg administered twice a day reduced the delay in small and large bowel transit induced by codeine 30 mg administered 4 times a day, as measured by gastrointestinal scintigraphy. In the same study, concomitant alvimopan did not reduce the delay in gastric emptying induced by codeine.
Cardiac Electrophysiology
At a dosage of 24 mg twice daily (two times the approved recommended dosage) for 7 days, ENTEREG does not prolong the QT interval to any clinically relevant extent. The potential for QTc effects at higher doses has not been studied.
Pharmacokinetics
Following oral administration of alvimopan, an amide hydrolysis compound is present in the systemic circulation, which is considered a product exclusively of intestinal flora metabolism. This compound is referred to as the ‘metabolite’. It is also a mu-opioid receptor antagonist with a Ki of 0.8 nM (0.3 ng/mL).
Absorption
Following oral administration of ENTEREG capsules in healthy plasma subjects, the alvimopan concentration peaked at approximately 2 hours post-dose. No significant accumulation in the concentration of alvimopan was observed following twice daily dosing. The mean peak plasma concentration was 10.98 (±6.43) ng/mL and mean AUC0–12h was 40.2 (±22.5) ng•h/mL after dosing of alvimopan at 12 mg twice daily for 5 days. The absolute bioavailability was estimated to be 6% (range, 1% to 19%). There was a delay in the appearance of the ‘metabolite’, which had a median Tmax of 36 hours following administration of a single dose of alvimopan. Concentrations of the ‘metabolite’ were highly variable between subjects and within a subject. The ‘metabolite’ accumulated after multiple doses of ENTEREG. The mean Cmax for the ‘metabolite’ after alvimopan 12 mg twice daily for 5 days was 35.73 ± 35.29 ng/mL.
Concentrations of alvimopan and its ‘metabolite’ are higher (approximately 1.9-fold and 1.4-fold, respectively) in postoperative ileus patients than in healthy subjects.
Effect of Food
A high-fat meal decreased the extent and rate of alvimopan absorption. The Cmax and AUC were decreased by approximately 38% and 21%, respectively, and the Tmax was prolonged by approximately 1 hour. The clinical significance of this decreased bioavailability is unknown. In postoperative ileus clinical trials, the preoperative dose of ENTEREG was administered in a fasting state. Subsequent doses were given without regard to meals.
Distribution
The steady-state volume of distribution of alvimopan was estimated to be 30±10 L. Plasma protein binding of alvimopan and its ‘metabolite’ was independent of concentration over ranges observed clinically and averaged 80% and 94%, respectively. Both alvimopan and the ‘metabolite’ were bound to albumin and not to alpha-1 acid glycoprotein.
Elimination
Metabolism and Excretion
In vitro data suggest that alvimopan is not a substrate of CYP enzymes. The average plasma clearance for alvimopan was 402 (±89) mL/min. Renal excretion accounted for approximately 35% of total clearance. There was no evidence that hepatic metabolism was a significant route for alvimopan elimination. Biliary secretion was considered the primary pathway for alvimopan elimination. Unabsorbed drug and unchanged alvimopan resulting from biliary excretion were then hydrolyzed to its ‘metabolite’ by gut microflora. The ‘metabolite’ was eliminated in the feces and in the urine as unchanged ‘metabolite’, the glucuronide conjugate of the ‘metabolite’, and other minor metabolites. The mean terminal phase half-life of alvimopan after multiple oral doses of ENTEREG ranged from 10 to 17 hours. The terminal half-life of the ‘metabolite’ ranged from 10 to 18 hours.
Specific Populations
Geriatric Patients
The pharmacokinetics of alvimopan, but not its ‘metabolite’, were related to age, but this effect was not clinically significant and does not warrant dosage adjustment based on increased age.
Racial or Ethnic Groups
The pharmacokinetic characteristics of alvimopan were not affected by Hispanic or Black race. Plasma ‘metabolite’ concentrations were lower in Black and Hispanic patients (by 43% and 82%, respectively) than in Caucasian patients following alvimopan administration. These changes are not considered to be clinically significant in surgical patients. Japanese healthy male subjects had an approximately 2-fold increase in plasma alvimopan concentrations, but no change in ‘metabolite’ pharmacokinetics. The pharmacokinetics of alvimopan have not been studied in subjects of other East Asian ancestry. Dosage adjustment in Japanese patients is not required [see Use In Specific Populations].
Male And Female Patients
There was no effect of sex on the pharmacokinetics of alvimopan or the ‘metabolite’.
Patients With Hepatic Impairment
Exposure to alvimopan following a single 12 mg dose tended to be higher (1.5-to 2-fold, on average) in patients with mild or moderate hepatic impairment (as defined by Child-Pugh Class A and B, n = 8 each) compared with healthy controls (n = 4). There were no consistent effects on the Cmax or half-life of alvimopan in patients with hepatic impairment. However, 2 of 16 patients with mild-to-moderate hepatic impairment had longer than expected half-lives of alvimopan, indicating that some accumulation may occur upon multiple dosing. The Cmax of the ‘metabolite’ tended to be more variable in patients with mild or moderate hepatic impairment than in matched normal subjects. A study of 3 patients with severe hepatic impairment (Child-Pugh Class C), indicated similar alvimopan exposure in 2 patients and an approximately 10-fold increase in Cmax and exposure in 1 patient with severe hepatic impairment when compared with healthy controls [see WARNINGS AND PRECAUTIONS, Use In Specific Populations].
Patients With Renal Impairment
There was no relationship between renal function (i.e., creatinine clearance [CrCl]) and plasma alvimopan pharmacokinetics (Cmax, AUC, or half-life) in patients with mild (CrCl 51–80 mL/min), moderate (CrCl 31–50 mL/min), or severe (CrCl less than 30 mL/min) renal impairment (n = 6 each). Renal clearance of alvimopan was related to renal function; however, because renal clearance was only a small fraction (35%) of the total clearance, renal impairment had a small effect on the apparent oral clearance of alvimopan. The half-lives of alvimopan were comparable in the mild, moderate, and control renal impairment groups but longer in the severe renal impairment group. Exposure to the ‘metabolite’ tended to be 2-to 5-fold higher in patients with moderate or severe renal impairment compared with patients with mild renal impairment or control subjects. Thus, there may be accumulation of alvimopan and ‘metabolite’ in patients with severe renal impairment receiving multiple doses of ENTEREG. Patients with end-stage renal disease were not studied [see WARNINGS AND PRECAUTIONS, Use In Specific Populations].
Patients With Crohn’s Disease
There was no relationship between disease activity in patients with Crohn’s disease (measured as Crohn’s Disease Activity Index or bowel movement frequency) and alvimopan pharmacokinetics (AUC or Cmax). Patients with active or quiescent Crohn’s disease had increased variability in alvimopan pharmacokinetics, and exposure tended to be 2-fold higher in patients with quiescent disease than in those with active disease or in normal subjects. Concentrations of the ‘metabolite’ were lower in patients with Crohn’s disease.
Drug Interaction Studies
Potential For Drugs To Affect Alvimopan Pharmacokinetics
Concomitant administration of ENTEREG with inducers or inhibitors of CYP enzymes is unlikely to alter the metabolism of alvimopan because ENTEREG is metabolized mainly by non-CYP enzyme pathway. No clinical studies have been performed to assess the effect of concomitant administration of inducers or inhibitors of cytochrome P450 enzymes on alvimopan pharmacokinetics.
In vitro studies suggest that alvimopan and its ‘metabolite’ are substrates for p-glycoprotein. A population pharmacokinetic analysis did not reveal any evidence that alvimopan or ‘metabolite’ pharmacokinetics were influenced by concomitant medications that are mild-to-moderate p-glycoprotein inhibitors. No clinical studies of concomitant administration of alvimopan and strong inhibitors of pglycoprotein (e.g., verapamil, cyclosporine, amiodarone, itraconazole, quinine, spironolactone, quinidine, diltiazem, bepridil) have been conducted.
A population pharmacokinetic analysis suggests that the pharmacokinetics of alvimopan were not affected by concomitant administration of acid blockers or antibiotics. However, plasma concentrations of the ‘metabolite’ were lower in patients receiving acid blockers or preoperative oral antibiotics (49% and 81%, respectively). No dosage adjustments are necessary in these patients.
Potential For Alvimopan To Affect The Pharmacokinetics Of Other Drugs
Alvimopan and its ‘metabolite’ are not inhibitors of CYP 1A2, 2C9, 2C19, 3A4, 2D6, and 2E1 in vitro at concentrations far in excess of those observed clinically.
Alvimopan and its ‘metabolite’ are not inducers of CYP 1A2, 2B6, 2C9, 2C19, and 3A4.
In vitro studies also suggest that alvimopan and its ‘metabolite’ are not inhibitors of pglycoprotein.
These in vitro findings suggest that ENTEREG is unlikely to alter the pharmacokinetics of coadministered drugs through inhibition or induction of CYP enzymes or inhibition of p-glycoprotein.
Clinical Studies
The efficacy of ENTEREG in the management of postoperative ileus was evaluated in 6 multicenter, randomized, double-blind, parallel-group, placebo-controlled studies: 5 US studies (Studies 1-4 and 6) and 1 non–US study (Study 5). Patients 18 years of age or older undergoing partial large or small bowel resection surgery with primary anastomosis for colorectal or small bowel disease, total abdominal hysterectomy, or radical cystectomy for bladder cancer (in this procedure, resected segments of bowel are used for reconstruction of the urinary tract) under general anesthesia were randomly assigned to receive oral doses of ENTEREG 12 mg or matching placebo. The initial dose was administered at least 30 minutes and up to 5 hours prior to the scheduled start of surgery for most patients, and subsequent doses were administered twice daily beginning on the first postoperative day and continued until hospital discharge or a maximum of 7 days. There were no limitations on the type of general anesthesia used, but intrathecal or epidural opioids or anesthetics were prohibited.
All patients in the US studies were scheduled to receive intravenous patient-controlled opioid analgesia. In the non–US study, patients were scheduled to receive opioids either by intravenous patient-controlled opioid analgesia or bolus parenteral administration (intravenous or intramuscular). In all studies, there was no restriction on the type of opioid used or the duration of intravenous patient-controlled opioid analgesia. A standardized accelerated postoperative care pathway was implemented: early nasogastric tube removal (before the first postoperative dose); early ambulation (day following surgery); early diet advancement (liquids offered the day following surgery for patients undergoing bowel resection and by the third day following surgery for patients undergoing radical cystectomy; solids by the second day following surgery for patients undergoing bowel resection and by the fourth day following surgery for patients undergoing radical cystectomy), as tolerated.
Patients who received more than 3 doses of an opioid (regardless of route) during the 7 days prior to surgery and patients with complete bowel obstruction or who were scheduled for a total colectomy, colostomy, or ileostomy were excluded.
The primary endpoint for all studies was time to achieve resolution of postoperative ileus, a clinically defined composite measure of both upper and lower gastrointestinal recovery. Although both 2component (GI2: toleration of solid food and first bowel movement) and 3-component (GI3: toleration of solid food and either first flatus or bowel movement) endpoints were used in all studies, GI2 is presented as it represents the most objective and clinically relevant measure of treatment response in patients undergoing surgeries that include a bowel resection. The time from the end of surgery to when the discharge order was written represented the length of hospital stay. In the 6 studies, 1,058 patients who underwent a surgery that included a bowel resection received placebo (not including 157 for total abdominal hysterectomy) and 1,096 patients received ENTEREG 12 mg (not including 143 for total abdominal hysterectomy).
The efficacy of ENTEREG following total abdominal hysterectomy has not been established. Therefore, the following data are presented only for surgeries that included a bowel resection (i.e., bowel resection or radical cystectomy).
Bowel Resection Or Radical Cystectomy
A total of 2,154 patients underwent a surgery that included a bowel resection. The average age was 62 years, 54% were males, and 89% were Caucasian. The most common indications for surgery were colon or rectal cancer/malignancy, bladder cancer, and diverticular disease. In the non–US bowel resection study (Study 5), average daily postoperative opioid consumption was approximately 50% lower and the use of non-opioid analgesics substantially higher, as compared with the US bowel resection studies (Studies 1-4) for both treatment groups. During the first 48 hours postoperatively, the use of non-opioid analgesics was 69% compared with 4% for the non–US and US bowel resection studies, respectively. In each of the 6 studies, ENTEREG accelerated the time to recovery of gastrointestinal function, as measured by the composite endpoint GI2, and time to discharge order written as compared with placebo. Hazard ratios greater than 1 indicate a higher probability of achieving the event during the study period with treatment with ENTEREG than with placebo. Table 1 provides the Hazard Ratios, Kaplan Meier means, medians, and mean and median treatment differences (hours) in gastrointestinal recovery between ENTEREG and placebo.
Table 1: GI2 Recovery (Hours) in Bowel Resection Patients
| Study No.* | ENTEREG 12 mg | Placebo | Treatment Difference | Hazard Ratio (95% CI) | |||
| Mean† | Median | Mean† | Median | Means† | Medians | ||
| 1 | 92.0 | 80.0 | 111.8 | 96.6 | 19.8 | 16.6 | 1.533 (1.293, 1.816) |
| 2 | 105.9 | 98.0 | 132.0 | 115.2 | 26.1 | 17.2 | 1.625 (1.256, 2.102) |
| 3 | 116.4 | 101.8 | 130.3 | 116.8 | 14.0 | 15.0 | 1.365 (1.057, 1.764) |
| 4 | 106.7 | 101.4 | 119.9 | 113.3 | 13.2 | 11.9 | 1.400 (1.035, 1.894) |
| 5 | 98.2 | 92.8 | 108.8 | 95.9 | 10.6 | 3.1 | 1.299 (1.070, 1.575) |
| 6 | 132.7 | 117.0 | 164.2 | 145.6 | 31.5 | 28.5 | 1.773 (1.359, 2.311) |
| * Study 1 = 14CL314; Study 2 = 14CL313; Study 3 = 14CL308; Study 4 = 14CL302; Study 5 = SB-767905/001; Study 6 = 14CL403 † The estimates of the means and differences of treatment means are biased because of the censoring of events not achieved prior to the end of the observation period (10 days). The estimates of the differences of treatment means are likely to be underestimates. | |||||||
The Kaplan Meier estimate probabilities of patients receiving ENTEREG who achieved GI2 were numerically higher at all times throughout the study observation period compared with those of patients receiving placebo (see Figures 1 and 2).
Figure 1: Time to GI2 Based on Results from Studies 1 through 5
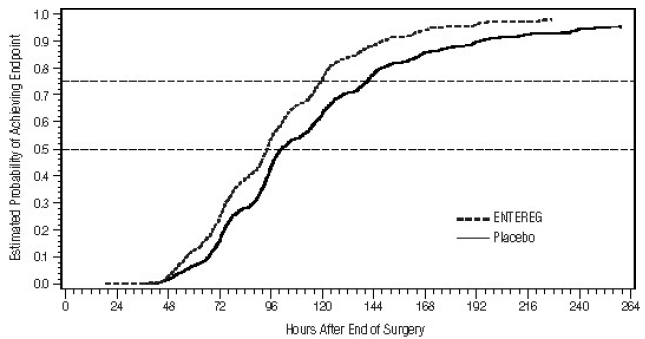 |
Figure 2: Time to GI2 Based on Results from Study 6
 |
In Studies 1–4, the differences between ENTEREG and placebo patient groups in median time to ‘discharge order written’ ranged from 6 to 22 hours, in favor of ENTEREG patients. The group differences in mean time to ‘discharge order written’ ranged from 13 to 21 hours. In Study 6, the median time difference was 19 hours in favor of ENTEREG patients (mean time difference 22 hours).
ENTEREG did not reverse opioid analgesia as measured by visual analog scale pain intensity scores and/or amount of postoperative opioids administered across all 6 studies.
There were no sex-, age-, or race-related differences in treatment effect.
The incidence of anastomotic leak was low and comparable in patients receiving either ENTEREG or placebo (0.7% and 1.0%, respectively).
Medication Guide
PATIENT INFORMATION
Recent Use Of Opioids
Inform patients that they must disclose long-term or intermittent opioid pain therapy to their healthcare provider, including any use of opioids in the week prior to receiving ENTEREG. Inform patients that recent use of opioids may make them more susceptible to adverse reactions to ENTEREG, primarily those limited to the gastrointestinal tract (e.g., abdominal pain, nausea and vomiting, diarrhea).
Hospital Use Only
Inform patients that ENTEREG is available only through a program called the Alvimopan REMS Program under a REMS that restricts use to enrolled hospitals because of the potential risk of myocardial infarction with long-term use of ENTEREG. ENTEREG is for hospital use only for no more than 7 days after their bowel resection surgery.
Most Common Adverse Reaction
Inform patients that the most common adverse reaction with ENTEREG in patients undergoing surgeries that include bowel resection is dyspepsia.
