Zejula Drug Description: Detailed Datasheet
- Generic Name: niraparib capsules
- Brand Name: Zejula
- Drug Class: How Do PARP Inhibitors Work?
side effects drug center zejula (niraparib capsules) drug
Drug Description
What is Zejula and how is it used?
Zejula is a prescription medicine used for the:
- maintenance treatment of adults with advanced ovarian cancer, fallopian tube cancer, or primary peritoneal cancer. Zejula is used after the cancer has responded (complete or partial response) to treatment with platinum-based chemotherapy.
- maintenance treatment of adults with ovarian cancer, fallopian tube cancer, or primary peritoneal cancer that comes back. Zejula is used after the cancer has responded (complete or partial response) to treatment with platinum-based chemotherapy.
- treatment of adults with advanced ovarian cancer, fallopian tube cancer, or primary peritoneal cancer who have been treated with 3 or more prior types of chemotherapy and who have tumors with:
- a certain “BRCA” gene mutation, or
- gene mutation problems and who have progressed more than 6 months after their last treatment with platinum-based chemotherapy.
Your healthcare provider will perform a test to make sure that Zejula is right for you.
It is not known if Zejula is safe and effective in children.
What are the possible side effects of Zejula?
Zejula can cause serious side effects, including:
- See “What is the most important information I should know about Zejula?”
The most common side effects of Zejula include:
- heart not beating regularly
- changes in liver function or other blood tests
- nausea
- pain in your joints, muscles, and back
- constipation
- headache
- vomiting
- dizziness
- pain in the stomach area
- change in the way food tastes
- mouth sores
- trouble sleeping
- diarrhea
- anxiety
- indigestion or heartburn
- sore throat
- dry mouth
- shortness of breath
- tiredness
- cough
- loss of appetite
- rash
- urinary tract infection
- changes in the amount or color of your urine
Your healthcare provider may change your dose, temporarily stop, or permanently stop treatment with Zejula, if you have certain side effects.
These are not all the possible side effects of Zejula. Call your healthcare provider for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
DESCRIPTION
Niraparib is an orally available poly(ADP-ribose) polymerase (PARP) inhibitor.
The chemical name for niraparib tosylate monohydrate is 2-{4-[(3S)-piperidin-3-yl]phenyl}-2Hindazole 7-carboxamide 4-methylbenzenesulfonate hydrate (1:1:1). The molecular formula is C26H30N4O5S and it has a molecular weight of 510.61 amu. The molecular structure is shown below:
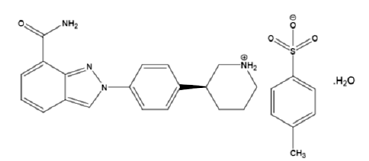 |
Niraparib tosylate monohydrate is a white to off-white, non-hygroscopic crystalline solid. Niraparib solubility is pH independent below the pKa of 9.95, with an aqueous free base solubility of 0.7 mg/mL to 1.1 mg/mL across the physiological pH range.
Each ZEJULA capsule contains 159.4 mg niraparib tosylate monohydrate equivalent to 100 mg niraparib free base as the active ingredient. The inactive ingredients in the capsule fill are magnesium stearate and lactose monohydrate. The capsule shell consists of titanium dioxide, gelatin in the white capsule body; and FD&C Blue #1, FD&C Red #3, FD&C Yellow #5 and gelatin in the purple capsule cap. The black printing ink consists of shellac, dehydrated alcohol, isopropyl alcohol, butyl alcohol, propylene glycol, purified water, strong ammonia solution, potassium hydroxide and black iron oxide. The white printing ink consists of shellac, dehydrated alcohol, isopropyl alcohol, butyl alcohol, propylene glycol, sodium hydroxide, povidone and titanium oxide.
Indications
First-Line Maintenance Treatment Of Advanced Ovarian Cancer
ZEJULA is indicated for the maintenance treatment of adult patients with advanced epithelial ovarian, fallopian tube, or primary peritoneal cancer who are in a complete or partial response to first-line platinum-based chemotherapy.
Maintenance Treatment Of Recurrent Ovarian Cancer
ZEJULA is indicated for the maintenance treatment of adult patients with recurrent epithelial ovarian, fallopian tube, or primary peritoneal cancer who are in a complete or partial response to platinum-based chemotherapy.
Treatment Of Advanced Ovarian Cancer After 3 Or More Chemotherapies
ZEJULA is indicated for the treatment of adult patients with advanced ovarian, fallopian tube, or primary peritoneal cancer who have been treated with 3 or more prior chemotherapy regimens and whose cancer is associated with homologous recombination deficiency (HRD) positive status defined by either:
- a deleterious or suspected deleterious BRCA mutation, or
- genomic instability and who have progressed more than 6 months after response to the last platinum-based chemotherapy [see Clinical Studies].
Select patients for therapy based on an FDA-approved companion diagnostic for ZEJULA.
Dosage
DOSAGE AND ADMINISTRATION
Patient Selection For Treatment Of Advanced Ovarian Cancer After 3 Or More Chemotherapies
Select patients for treatment of advanced ovarian cancer after 3 or more chemotherapy regimens associated with HRD positive status based on either deleterious or suspected deleterious BRCA mutation and/or genomic instability score (GIS) [see Clinical Studies].
Information on FDA-approved tests for the detection of either deleterious or suspected deleterious BRCA mutation or genomic instability for this indication is available at https://www.fda.gov/companiondiagnostics.
Recommended Dosage
Continue treatment with ZEJULA until disease progression or unacceptable toxicity.
Instruct patients to take their dose of ZEJULA at approximately the same time each day. Advise patients to swallow each capsule whole and not to chew, crush, or split ZEJULA prior to swallowing. ZEJULA may be taken with or without food. Bedtime administration may be a potential method for managing nausea.
In the case of a missed dose of ZEJULA, instruct patients to take their next dose at its regularly scheduled time. If a patient vomits or misses a dose of ZEJULA, an additional dose should not be taken.
First-Line Maintenance Treatment Of Advanced Ovarian Cancer
- For patients weighing <77 kg (<170 lbs) OR with a platelet count of <150,000/mcL, the recommended dosage is 200 mg (two 100-mg capsules) taken orally once daily.
- For patients weighing ≥77 kg (≥170 lbs) AND who have a platelet count ≥150,000/mcL, the recommended dosage is 300 mg (three 100-mg capsules) taken orally once daily.
For the maintenance treatment of advanced ovarian cancer, patients should start treatment with ZEJULA no later than 12 weeks after their most recent platinum-containing regimen.
Maintenance Treatment Of Recurrent Ovarian Cancer
The recommended dosage of ZEJULA is 300 mg (three 100-mg capsules) taken orally once daily.
For the maintenance treatment of recurrent ovarian cancer, patients should start treatment with ZEJULA no later than 8 weeks after their most recent platinum-containing regimen.
Treatment Of Advanced Ovarian Cancer After 3 Or More Chemotherapies
The recommended dosage of ZEJULA is 300 mg (three 100-mg capsules) taken orally once daily.
Dosage Adjustments For Adverse Reactions
To manage adverse reactions, consider interruption of treatment, dose reduction, or dose discontinuation. The recommended dose modifications for adverse reactions are listed in Tables 1, 2, and 3.
Table 1: Recommended Dose Modifications for Adverse Reactions
| Starting Dose Level | 200 mg | 300 mg |
| First dose reduction | 100 mg/daya (one 100-mg capsule) | 200 mg/day (two 100-mg capsules) |
| Second dose reduction | Discontinue ZEJULA. | 100 mg/daya (one 100-mg capsule) |
| a If further dose reduction below 100 mg/day is required, discontinue ZEJULA. | ||
Table 2: Dose Modifications for Non-Hematologic Adverse Reactions
| Non-hematologic CTCAE ≥Grade 3 adverse reaction that persists despite medical management |
|
| CTCAE ≥Grade 3 treatment-related adverse reaction lasting more than 28 days while patient is administered ZEJULA 100 mg/day | Discontinue ZEJULA. |
| CTCAE = Common Terminology Criteria for Adverse Events. | |
Table 3: Dose Modifications for Hematologic Adverse Reactions
| Monitor complete blood counts weekly for the first month, monthly for the next 11 months of treatment, and periodically after this time [see WARNINGS AND PRECAUTIONS]. | |
| Platelet count <100,000/mcL | First occurrence:
|
| Neutrophil <1,000/mcL or hemoglobin <8 g/dL |
|
| Hematologic adverse reaction requiring transfusion |
|
| a If myelodysplastic syndrome or acute myeloid leukemia (MDS/AML) is confirmed, discontinue ZEJULA [see WARNINGS AND PRECAUTIONS]. | |
Dosage Adjustment For Hepatic Impairment
Moderate Hepatic Impairment
For patients with moderate hepatic impairment, reduce the starting dosage of ZEJULA to 200 mg once daily. Monitor patients for hematologic toxicity and reduce the dose further, if needed [see DOSAGE AND ADMINISTRATION, Use In Specific Populations, CLINICAL PHARMACOLOGY].
HOW SUPPLIED
Dosage Forms And Strengths
100-mg capsule having a white body with “100 mg” printed in black ink, and a purple cap with “Niraparib” printed in white ink.
Storage And Handling
ZEJULA is available as capsules having a white body printed with “100 mg” in black ink and a purple cap printed with “Niraparib” in white ink.
Each capsule contains 100 mg of niraparib free base.
ZEJULA capsules are packaged as
90-count bottles NDC 69656-103-90
30-count bottles NDC 69656-103-30
Store at 20°C to 25°C (68°F to 77°F); excursions are permitted between 15°C to 30°C (59°F to 86°F) [see USP Controlled Room Temperature].
Manufactured for :GlaxoSmithKline Research Triangle Park, NC 27709. Revised: Mar 2021
Side Effects & Drug Interactions
SIDE EFFECTS
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Myelodysplastic Syndrome/Acute Myeloid Leukemia [see WARNINGS AND PRECAUTIONS]
- Bone Marrow Suppression [see WARNINGS AND PRECAUTIONS]
- Cardiovascular Effects [see WARNINGS AND PRECAUTIONS]
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of ZEJULA monotherapy 300 mg once daily has been studied in 367 patients with platinum-sensitive recurrent ovarian, fallopian tube, and primary peritoneal cancer in Trial 1 (NOVA). Adverse reactions in Trial 1 led to dose reduction or interruption in 69% of patients, most frequently from thrombocytopenia (41%) and anemia (20%). The permanent discontinuation rate due to adverse reactions in Trial 1 was 15%. The median exposure to ZEJULA in these patients was 250 days.
Table 4 and Table 5 summarize the common adverse reactions and abnormal laboratory findings, respectively, observed in patients treated with ZEJULA.
Table 4: Adverse Reactions Reported in ≥10% of Patients Receiving ZEJULA
| Grades 1-4* | Grades 3-4* | |||
| ZEJULA N=367 % | Placebo N=179 % | ZEJULA N=367 % | Placebo N=179 % | |
| Blood and Lymphatic System Disorders | ||||
| Thrombocytopenia | 61 | 5 | 29 | 0.6 |
| Anemia | 50 | 7 | 25 | 0 |
| Neutropenia† | 30 | 6 | 20 | 2 |
| Leukopenia | 17 | 8 | 5 | 0 |
| Cardiac Disorders | ||||
| Palpitations | 10 | 2 | 0 | 0 |
| Gastrointestinal Disorders | ||||
| Nausea | 74 | 35 | 3 | 1 |
| Constipation | 40 | 20 | 0.8 | 2 |
| Vomiting | 34 | 16 | 2 | 0.6 |
| Abdominal pain/distention | 33 | 39 | 2 | 2 |
| Mucositis/stomatitis | 20 | 6 | 0.5 | 0 |
| Diarrhea | 20 | 21 | 0.3 | 1 |
| Dyspepsia | 18 | 12 | 0 | 0 |
| Dry mouth | 10 | 4 | 0.3 | 0 |
| General Disorders and Administration Site Conditions | ||||
| Fatigue/Asthenia | 57 | 41 | 8 | 0.6 |
| Metabolism and Nutrition Disorders | ||||
| Decreased appetite | 25 | 15 | 0.3 | 0.6 |
| Infections and Infestations | ||||
| Urinary tract infection | 13 | 8 | 0.8 | 1 |
| Investigations | ||||
| AST/ ALT elevation | 10 | 5 | 4 | 2 |
| Musculoskeletal and Connective Tissue Disorders | ||||
| Myalgia | 19 | 20 | 0.8 | 0.6 |
| Back pain | 18 | 12 | 0.8 | 0 |
| Arthralgia | 13 | 15 | 0.3 | 0.6 |
| Nervous System Disorders | ||||
| Headache | 26 | 11 | 0.3 | 0 |
| Dizziness | 18 | 8 | 0 | 0 |
| Dysgeusia | 10 | 4 | 0 | 0 |
| Psychiatric Disorders | ||||
| Insomnia | 27 | 8 | 0.3 | 0 |
| Anxiety | 11 | 7 | 0.3 | 0.6 |
| Respiratory, Thoracic, and Mediastinal Disorders | ||||
| Nasopharyngitis | 23 | 14 | 0 | 0 |
| Dyspnea | 20 | 8 | 1 | 1 |
| Cough | 16 | 5 | 0 | 0 |
| Skin and Subcutaneous Tissue Disorders | ||||
| Rash | 21 | 9 | 0.5 | 0 |
| Vascular Disorders | ||||
| Hypertension | 20 | 5 | 9 | 2 |
| *CTCAE=Common Terminology Criteria for Adverse Events version 4.02 †Neutropenia includes preferred terms of neutropenic infection and neutropenic sepsis | ||||
Table 5: Abnormal Laboratory Findings in ≥25% of Patients Receiving ZEJULA
| Grades 1-4 | Grades 3-4 | |||
| ZEJULA N=367 (%) | Placebo N= 179 (%) | ZEJULA N= 367 (%) | Placebo N= 179 (%) | |
| Decrease in hemoglobin | 85 | 56 | 25 | 0.5 |
| Decrease in platelet count | 72 | 21 | 35 | 0.5 |
| Decrease in WBC count | 66 | 37 | 7 | 0.7 |
| Decrease in absolute neutrophil count | 53 | 25 | 21 | 2 |
| Increase in AST | 36 | 23 | 1 | 0 |
| Increase in ALT | 28 | 15 | 1 | 2 |
| N=number of patients; WBC=white blood cells; ALT=Alanine aminotransferase; AST=Aspartate aminotransferase | ||||
The following adverse reactions and laboratory abnormalities have been identified in ≥1 to <10% of the 367 patients receiving ZEJULA in the NOVA trial and not included in the table: tachycardia, peripheral edema, hypokalemia, bronchitis, conjunctivitis, gamma-glutamyl transferase increased, blood creatinine increased, blood alkaline phosphatase increased, weight decreased, depression, epistaxis.
DRUG INTERACTIONS
No Information Provided
Warnings & Precautions
WARNINGS
Included as part of the PRECAUTIONS section.
PRECAUTIONS
Myelodysplastic Syndrome/Acute Myeloid Leukemia
Myelodysplastic syndrome/acute myeloid leukemia (MDS/AML), including cases with fatal outcome, have been reported in patients who received monotherapy with ZEJULA in clinical trials. In 1,785 patients treated with ZEJULA in clinical trials, MDS/AML occurred in 15 patients (0.8%).
The duration of therapy with ZEJULA in patients who developed secondary MDS/cancer therapy-related AML varied from 0.5 months to 4.9 years. All of these patients had received previous chemotherapy with platinum agents and/or other DNA-damaging agents, including radiotherapy. Discontinue ZEJULA if MDS/AML is confirmed.
Bone Marrow Suppression
Hematologic adverse reactions, including thrombocytopenia, anemia, neutropenia, and/or pancytopenia have been reported in patients treated with ZEJULA [see ADVERSE REACTIONS].
In PRIMA, the overall incidences of ≥Grade 3 thrombocytopenia, anemia, and neutropenia were reported in 39%, 31%, and 21%, respectively, of patients receiving ZEJULA. Discontinuation due to thrombocytopenia, anemia, and neutropenia occurred in 4%, 2%, and 2%, respectively, of patients. In patients who were administered a starting dose of ZEJULA based on baseline weight or platelet count, ≥Grade 3 thrombocytopenia, anemia, and neutropenia were reported in 22%, 23%, and 15%, respectively, of patients receiving ZEJULA. Discontinuation due to thrombocytopenia, anemia, and neutropenia occurred in 3%, 3%, and 2%, respectively, of patients.
In NOVA, ≥Grade 3 thrombocytopenia, anemia, and neutropenia were reported in 29%, 25%, and 20%, respectively, of patients receiving ZEJULA. Discontinuation due to thrombocytopenia, anemia, and neutropenia occurred in 3%, 1%, and 2%, respectively, of patients.
In QUADRA, ≥Grade 3 thrombocytopenia, anemia, and neutropenia were reported in 28%, 27%, and 13%, respectively, of patients receiving ZEJULA. Discontinuation due to thrombocytopenia, anemia, and neutropenia occurred in 4%, 2%, and 1%, respectively, of patients.
Do not start ZEJULA until patients have recovered from hematological toxicity caused by previous chemotherapy (≤Grade 1). Monitor complete blood counts weekly for the first month, monthly for the next 11 months of treatment, and periodically after this time. If hematological toxicities do not resolve within 28 days following interruption, discontinue ZEJULA and refer the patient to a hematologist for further investigations, including bone marrow analysis and blood sample for cytogenetics [see DOSAGE AND ADMINISTRATION].
Hypertension And Cardiovascular Effects
Hypertension and hypertensive crisis have been reported in patients treated with ZEJULA.
In PRIMA, Grade 3 to 4 hypertension occurred in 6% of patients treated with ZEJULA compared with 1% of placebo-treated patients with a median time from first dose to first onset of 43 days (range: 1 to 531 days) and with a median duration of 12 days (range: 1 to 61 days). There were no discontinuations due to hypertension.
In NOVA, Grade 3 to 4 hypertension occurred in 9% of patients treated with ZEJULA compared with 2% of placebo-treated patients with a median time from first dose to first onset of 77 days (range: 4 to 504 days) and with a median duration of 15 days (range: 1 to 86 days). Discontinuation due to hypertension occurred in <1% of patients.
In QUADRA, Grade 3 to 4 hypertension occurred in 5% of patients treated with ZEJULA with a median time from first dose to first onset of 15 days (range: 1 to 316 days) and with a median duration of 7 days (range: 1 to 118 days). Discontinuation due to hypertension occurred in <0.2% of patients.
Monitor blood pressure and heart rate at least weekly for the first 2 months, then monthly for the first year and periodically thereafter during treatment with ZEJULA. Closely monitor patients with cardiovascular disorders, especially coronary insufficiency, cardiac arrhythmias, and hypertension. Medically manage hypertension with antihypertensive medications and adjustment of the dose of ZEJULA, if necessary [see DOSAGE AND ADMINISTRATION, Nonclinical Toxicology].
Posterior Reversible Encephalopathy Syndrome
Posterior reversible encephalopathy syndrome (PRES) occurred in 0.1% of 2,165 patients treated with ZEJULA in clinical trials and has also been described in postmarketing reports [see ADVERSE REACTIONS]. Signs and symptoms of PRES include seizure, headache, altered mental status, visual disturbance, or cortical blindness, with or without associated hypertension. A diagnosis of PRES requires confirmation by brain imaging, preferably magnetic resonance imaging.
Monitor all patients treated with ZEJULA for signs and symptoms of PRES. If PRES is suspected, promptly discontinue ZEJULA and administer appropriate treatment. The safety of reinitiating ZEJULA in patients previously experiencing PRES is not known.
Embryo-Fetal Toxicity
Based on its mechanism of action, ZEJULA can cause fetal harm when administered to a pregnant woman [see CLINICAL PHARMACOLOGY]. ZEJULA has the potential to cause teratogenicity and/or embryo-fetal death since niraparib is genotoxic and targets actively dividing cells in animals and patients (e.g., bone marrow) [see WARNINGS AND PRECAUTIONS, Nonclinical Toxicology]. Due to the potential risk to a fetus based on its mechanism of action, animal developmental and reproductive toxicology studies were not conducted with niraparib.
Apprise pregnant women of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment and for 6 months after the last dose of ZEJULA [see Use In Specific Populations].
Allergic Reactions To FD&C Yellow No. 5 (Tartrazine)
ZEJULA capsules contain FD&C Yellow No. 5 (tartrazine), which may cause allergic-type reactions (including bronchial asthma) in certain susceptible persons. Although the overall incidence of FD&C Yellow No. 5 (tartrazine) sensitivity in the general population is low, it is frequently seen in patients who also have aspirin hypersensitivity.
Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (PATIENT INFORMATION).
Myelodysplastic Syndrome/Acute Myeloid Leukemia
Advise patients to contact their healthcare provider if they experience weakness, feeling tired, fever, weight loss, frequent infections, bruising, bleeding easily, breathlessness, blood in urine or stool, and/or laboratory findings of low blood cell counts or a need for blood transfusions. This may be a sign of hematological toxicity or MDS or AML, which has been reported in patients treated with ZEJULA [see WARNINGS AND PRECAUTIONS].
Bone Marrow Suppression
Advise patients that periodic monitoring of their blood counts is required. Advise patients to contact their healthcare provider for new onset of bleeding, fever, or symptoms of infection [see WARNINGS AND PRECAUTIONS].
Hypertension And Cardiovascular Effects
Advise patients to undergo blood pressure and heart rate monitoring at least weekly for the first 2 months, then monthly for the first year of treatment and periodically thereafter. Advise patients to contact their healthcare provider if blood pressure is elevated [see WARNINGS AND PRECAUTIONS].
Posterior Reversible Encephalopathy Syndrome
Inform patients that they are at risk of developing posterior reversible encephalopathy syndrome (PRES) that can present with signs and symptoms including seizure, headaches, altered mental status, or vision changes. Advise patients to contact their healthcare provider if they develop any of these signs or symptoms [see WARNINGS AND PRECAUTIONS].
Dosing Instructions
Inform patients on how to take ZEJULA [see DOSAGE AND ADMINISTRATION]. ZEJULA should be taken once daily. Instruct patients that if they miss a dose of ZEJULA not to take an extra dose to make up for the one that they missed. They should take their next dose at the regularly scheduled time. Each capsule should be swallowed whole. ZEJULA may be taken with or without food. Bedtime administration may be a potential method for managing nausea.
Embryo-Fetal Toxicity
Advise females to inform their healthcare provider if they are pregnant or become pregnant. Inform female patients of the risk to a fetus and potential loss of the pregnancy [see WARNINGS AND PRECAUTIONS, Use In Specific Populations].
Contraception
Advise females of reproductive potential to use effective contraception during treatment with ZEJULA and for at least 6 months after receiving the last dose [see Use In Specific Populations].
Lactation
Advise patients not to breastfeed while taking ZEJULA and for 1 month after the last dose [see Use In Specific Populations].
Allergic Reactions To FD&C Yellow No. 5 (Tartrazine)
Advise patients that ZEJULA capsules contain FD&C Yellow No. 5 (tartrazine), which may cause allergic-type reactions (including bronchial asthma) in certain susceptible persons or in patients who also have aspirin hypersensitivity [see WARNINGS AND PRECAUTIONS].
Trademarks are owned by or licensed to the GSK group of companies.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment Of Fertility
Carcinogenicity studies have not been conducted with niraparib.
Niraparib was clastogenic in an in vitro mammalian chromosomal aberration assay and in an in vivo rat bone marrow micronucleus assay. This clastogenicity is consistent with genomic instability resulting from the primary pharmacology of niraparib and indicates potential for genotoxicity in humans. Niraparib was not mutagenic in a bacterial reverse mutation assay (Ames) test.
Fertility studies in animals have not been conducted with niraparib. In repeat-dose oral toxicity studies, niraparib was administered daily for up to 3 months’ duration in rats and dogs. Reduced sperm, spermatids, and germ cells in epididymides and testes were observed at doses ≥10 mg/kg and ≥1.5 mg/kg in rats and dogs, respectively. These dose levels resulted in systemic exposures approximately 0.3 and 0.012 times, respectively, the human exposure (AUC0-24h) at the recommended dose of 300 mg daily. There was a trend toward reversibility of these findings 4 weeks after dosing was stopped.
Use In Specific Populations
Pregnancy
Risk Summary
Based on its mechanism of action, ZEJULA can cause fetal harm when administered to pregnant women [see CLINICAL PHARMACOLOGY]. There are no data regarding the use of ZEJULA in pregnant women to inform the drug-associated risk. ZEJULA has the potential to cause teratogenicity and/or embryo-fetal death since niraparib is genotoxic and targets actively dividing cells in animals and patients (e.g., bone marrow) [see WARNINGS AND PRECAUTIONS, Nonclinical Toxicology]. Due to the potential risk to a fetus based on its mechanism of action, animal developmental and reproductive toxicology studies were not conducted with niraparib. Apprise pregnant women of the potential risk to a fetus.
The background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Lactation
Risk Summary
No data are available regarding the presence of niraparib or its metabolites in human milk, or on its effects on the breastfed child or milk production. Because of the potential for serious adverse reactions in a breastfed child, advise a lactating woman not to breastfeed during treatment with ZEJULA and for 1 month after receiving the final dose.
Females And Males Of Reproductive Potential
ZEJULA can cause fetal harm when administered to a pregnant woman [see Use In Specific Populations].
Pregnancy Testing
Verify the pregnancy status of females of reproductive potential prior to initiating treatment with ZEJULA.
Contraception
Females
Advise females of reproductive potential to use effective contraception during treatment with ZEJULA and for at least for 6 months following the last dose.
Infertility
Males
Based on animal studies, ZEJULA may impair fertility in males of reproductive potential [see Nonclinical Toxicology].
Pediatric Use
The safety and effectiveness of ZEJULA have not been established in pediatric patients.
Geriatric Use
In PRIMA, 39% of patients were aged 65 years or older and 10% were aged 75 years or older. In NOVA, 35% of patients were aged 65 years or older and 8% were aged 75 years or older. No overall differences in safety and effectiveness of ZEJULA were observed between these patients and younger patients but greater sensitivity of some older individuals cannot be ruled out.
Renal Impairment
No dose adjustment is necessary for patients with mild (CLcr: 60 to 89 mL/min) to moderate (CLcr: 30 to 59 mL/min) renal impairment. The degree of renal impairment was determined by creatinine clearance as estimated by the Cockcroft-Gault equation. The safety of ZEJULA in patients with severe renal impairment or end-stage renal disease undergoing hemodialysis is unknown.
Hepatic Impairment
For patients with moderate hepatic impairment, reduce the starting dosage of niraparib to 200 mg once daily [see DOSAGE AND ADMINISTRATION]. Niraparib exposure increased in patients with moderate hepatic impairment [total bilirubin ≥1.5 x upper level of normal (ULN) to 3.0 x ULN and any aspartate transaminase (AST) level]. Monitor patients for hematologic toxicity and reduce the dose further, if needed [see DOSAGE AND ADMINISTRATION].
For patients with mild hepatic impairment (total bilirubin <1.5 x ULN and any AST level or bilirubin ≤ ULN and AST > ULN), no dose adjustment is needed.
The recommended dose of ZEJULA has not been established for patients with severe hepatic impairment (total bilirubin >3.0 x ULN and any AST level) [see CLINICAL PHARMACOLOGY].
Overdosage & Contraindications
OVERDOSE
No Information provided
CONTRAINDICATIONS
None.
Clinical Pharmacology
Mechanism Of Action
Niraparib is an inhibitor of PARP enzymes, including PARP-1 and PARP-2, that play a role in DNA repair. In vitro studies have shown that niraparib-induced cytotoxicity may involve inhibition of PARP enzymatic activity and increased formation of PARP-DNA complexes resulting in DNA damage, apoptosis, and cell death. Increased niraparib-induced cytotoxicity was observed in tumor cell lines with or without deficiencies in BRCA½. Niraparib decreased tumor growth in mouse xenograft models of human cancer cell lines with deficiencies in BRCA½ and in human patient-derived xenograft tumor models with HRD that had either mutated or wild-type BRCA½.
Pharmacodynamics
The pharmacodynamic response of niraparib has not been characterized.
Hypertension And Cardiovascular Effects
Niraparib has the potential to cause effects on pulse rate and blood pressure in patients receiving the recommended dose, which may be related to pharmacological inhibition of the dopamine transporter (DAT), norepinephrine transporter (NET), and serotonin transporter (SERT) [see Nonclinical Toxicology].
In the PRIMA study, mean pulse rate and blood pressure increased over baseline in the niraparib arm relative to the placebo arm at most on-study assessments. Mean greatest increases from baseline in pulse rate on treatment were 22.4 and 14.0 beats/min in the niraparib and placebo arms, respectively. Mean greatest increases from baseline in systolic blood pressure on treatment were 24.4 and 19.6 mmHg in the niraparib and placebo arms, respectively. Mean greatest increases from baseline in diastolic blood pressure on treatment were 15.9 and 13.9 mmHg in the niraparib and placebo arms, respectively.
In the NOVA study, mean pulse rate and blood pressure increased over baseline in the niraparib arm relative to the placebo arm at all on-study assessments. Mean greatest increases from baseline in pulse rate on treatment were 24.1 and 15.8 beats/min in the niraparib and placebo arms, respectively. Mean greatest increases from baseline in systolic blood pressure on treatment were 24.5 and 18.3 mmHg in the niraparib and placebo arms, respectively. Mean greatest increases from baseline in diastolic blood pressure on treatment were 16.5 and 11.6 mmHg in the niraparib and placebo arms, respectively.
Cardiac Electrophysiology
The potential for QTc prolongation with niraparib was evaluated in a randomized, placebo-controlled trial in patients with cancer (367 patients on niraparib and 179 patients on placebo). No large changes in the mean QTc interval (>20 ms) were detected in the trial following the treatment of niraparib 300 mg once daily.
Pharmacokinetics
Following a single-dose administration of 300 mg niraparib, the mean (±SD) peak plasma concentration (Cmax) was 804 (±403) ng/mL. The exposure (Cmax and AUC) of niraparib increased in a dose-proportional manner with daily doses ranging from 30 mg (0.1 times the approved recommended dose) to 400 mg (1.3 times the approved recommended dose). The accumulation ratio of niraparib exposure following 21 days of repeated daily doses was approximately 2-fold for doses ranging from 30 to 400 mg.
Absorption
The absolute bioavailability of niraparib is approximately 73%. Following oral administration of niraparib, peak plasma concentration, Cmax, is reached within 3 hours.
Concomitant administration of a high-fat meal (800 to 1,000 calories with approximately 50% of total caloric content of the meal from fat) did not significantly affect the pharmacokinetics of niraparib.
Distribution
Niraparib is 83.0% bound to human plasma proteins. The average (±SD) apparent volume of distribution (Vd/F) was 1,220 (±1,114) L. In a population pharmacokinetic analysis, the Vd/F of niraparib was 1,074 L in patients with cancer .
Elimination
Following multiple daily doses of 300 mg of niraparib, the mean half-life (t½) is 36 hours. In a population pharmacokinetic analysis, the apparent total clearance (CL/F) of niraparib was 16.2 L/h in patients with cancer.
Metabolism
Niraparib is metabolized by carboxylesterases (CEs) to form a major inactive metabolite, which subsequently undergoes glucuronidation.
Excretion
Following administration of a single oral 300-mg dose of radio-labeled niraparib, the average percent recovery of the administered dose over 21 days was 47.5% (range: 33.4% to 60.2%) in urine and 38.8% (range: 28.3% to 47.0%) in feces. In pooled samples collected over 6 days, unchanged niraparib accounted for 11% and 19% of the administered dose recovered in urine and feces, respectively.
Specific Populations
Age (18 to 65 years), race/ethnicity, and mild to moderate renal impairment (CLcr ≥30 to 90 mL/min) had no clinically significant effect on the pharmacokinetics of niraparib.
The effect of severe renal impairment (CLcr <30 mL/min) or end-stage renal disease undergoing hemodialysis on the pharmacokinetics of niraparib is unknown.
Patients With Hepatic Impairment
Mild hepatic impairment (total bilirubin <1.5 x ULN and any AST level or bilirubin ≤ ULN and AST > ULN) had no clinically significant effect on the pharmacokinetics of niraparib.
In a trial of patients with moderate hepatic impairment (total bilirubin ≥1.5 x ULN to 3.0 x ULN and any AST level) (n = 8), niraparib AUCinf was 1.56 (90% CI: 1.06 to 2.30) times higher compared with patients with normal hepatic function (n = 9) following administration of a single 300-mg dose. Niraparib dosage reduction is recommended for patients with moderate hepatic impairment [see DOSAGE AND ADMINISTRATION]. Moderate hepatic impairment did not have an effect on niraparib Cmax or on niraparib protein binding.
The effect of severe hepatic impairment (total bilirubin >3.0 x ULN and any AST level) on the pharmacokinetics of niraparib is unknown. Drug Interaction Studies
No clinical drug interaction studies have been performed with ZEJULA.
In Vitro Studies
Inhibition Of Cytochrome P450 (CYP) Enzymes
Neither niraparib nor the major primary metabolite M1 is an inhibitor of CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, and CYP3A4.
Induction Of CYP Enzymes
Neither niraparib nor M1 is a CYP3A4 inducer. Niraparib weakly induces CYP1A2 in vitro.
Substrate Of CYP Enzymes
Niraparib is a substrate of CEs and the resulting M1 is further metabolized through the formation of glucuronides in vivo.
Inhibition Of Uridine 5'-Diphospho-Glucuronosyltransferases (UGTs)
Niraparib did not exhibit inhibitory effect against the UGT isoforms (UGT1A1, UGT1A4, UGT1A9, and UGT2B7) up to 200 microM in vitro. Therefore, the potential for a clinically relevant inhibition of UGTs by niraparib is minimal.
Inhibition Of Transporter Systems
Niraparib is a weak inhibitor of breast cancer resistance protein (BCRP), but does not inhibit P-glycoprotein (P-gp), bile salt export pump (BSEP), or multidrug resistance-associated protein 2 (MRP2).
Niraparib is an inhibitor of multidrug and toxin extrusion (MATE) 1 and 2 with IC50 of 0.18 microM and ≤0.14 microM, respectively. Increased plasma concentrations of coadministered drugs that are substrates of these transporters (e.g., metformin) cannot be excluded.
The M1 metabolite is not an inhibitor of P-gp, BCRP, BSEP, MRP2, or MATE1 or 2. Neither niraparib nor M1 is an inhibitor of organic anion transporting polypeptide (OATP)1B1, OATP1B3, organic cation transporter (OCT1)1, organic anion transporter (OAT)1, OAT3, or OCT2.
Substrate Of Transporter Systems:
Niraparib is a substrate of P-gp and BCRP. Niraparib is not a substrate of BSEP, MRP2, or MATE1 or 2. The M1 metabolite is not a substrate of P-gp, BCRP, BSEP, or MRP2. However, M1 is a substrate of MATE1 and 2. Neither niraparib nor M1 is a substrate of OATP1B1, OATP1B3, OCT1, OAT1, OAT3, or OCT2.
Animal Toxicology And/Or Pharmacology
In vitro, niraparib bound to DAT, NET, and SERT and inhibited uptake of norepinephrine and dopamine in cells with IC50 values that were lower than the Cmin at steady-state in patients receiving the recommended dose. Niraparib has the potential to cause effects in patients related to inhibition of these transporters (e.g., cardiovascular, central nervous system).
Intravenous administration of niraparib to vagotomized dogs over 30 minutes at 1, 3, and 10 mg/kg resulted in an increased range of arterial pressures of 13% to 20%, 18% to 27%, and 19% to 25%, respectively, and increased range of heart rates of 2% to 11%, 4% to 17%, and 12% to 21%, respectively, above pre-dose levels. The unbound plasma concentrations of niraparib in dogs at these dose levels were approximately 0.5, 1.5, and 5.8 times the unbound Cmax at steady-state in patients receiving the recommended dose.
In addition, niraparib crossed the blood-brain barrier in rats and monkeys following oral administration. The cerebrospinal fluid:plasma Cmax ratios of niraparib administered at 10 mg/kg orally to 2 rhesus monkeys were 0.10 and 0.52.
Clinical Studies
First-Line Maintenance Treatment Of Advanced Ovarian Cancer
PRIMA (NCT02655016) was a double-blind, placebo-controlled trial in which patients (N = 733) in complete or partial response to first-line platinum-based chemotherapy were randomized 2:1 to ZEJULA or matched placebo. Initially, the patients received a starting dosage of 300 mg once daily regardless of body weight or platelet count. The study was amended to include a starting dose of 200 mg for patients weighing <77 kg (<170 lbs) OR with a platelet count of <150,000/mcL or 300 mg for patients weighing ≥77 kg (≥170 lbs) AND who had a platelet count ≥150,000/mcL.
Patients were randomized post-completion of first-line platinum-based chemotherapy plus surgery. Randomization was stratified by best response during the front-line platinum regimen (complete response vs. partial response), neoadjuvant chemotherapy (NACT) (yes vs. no), and HRD status (positive vs. negative or not determined). HRD status was determined using the FDA-approved Myriad myChoice CDx assay. HRD positive status included either tumor BRCA mutant (tBRCAm) or a genomic instability score (GIS) ≥42.
The major efficacy outcome measure, progression-free survival (PFS), was determined by blinded independent central review (BICR) per Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1. In some cases, criteria other than RECIST, such as clinical signs and symptoms and increasing CA-125, were also applied. Overall survival was an additional efficacy outcome measure. PFS testing was performed hierarchically: first in the homologous recombination (HR)-deficient (HRD positive) population, then in the overall population. The median age of 62 ranged from 32 to 85 years among patients randomized with ZEJULA and 33 to 88 years among patients randomized with placebo. Eighty-nine percent of all patients were White. Sixty-nine percent of patients randomized with ZEJULA and 71% of patients randomized with placebo had an Eastern Cooperative Oncology Group Performance Status (ECOG PS) of 0 at study baseline. Approximately 45% of patients were enrolled in the U.S. or Canada. In the overall population, 65% of patients had stage III disease and 35% had stage IV disease. Sixty-seven percent of the patients received NACT. Sixty-nine percent of the patients had a complete response to the first-line platinum-based chemotherapy. Approximately 35% (n = 258) of patients received a starting dose of 200 or 300 mg depending on baseline body weight and platelet count. Among those patients, 186 patients received a starting dose of 200 mg.
PRIMA demonstrated a statistically significant improvement in PFS for patients randomized to ZEJULA as compared with placebo in the HR-deficient and overall population (Table 12, Figure 1, and Figure 2).
Table 12: Efficacy Results - PRIMA (determined by BICRa)
| HR-Deficient Population | Overall Population | |||
| ZEJULA (n = 247) |
Placebo (n = 126) |
ZEJULA (n = 487) |
Placebo (n = 246) |
|
| Progression-free | 81 | 73 | 232 | 155 |
| survival events, n (%) | (33) | (58) | (48) | (63) |
| Progression-free | 21.9 | 10.4 | 13.8 | 8.2 |
| survival median in months (95% CI) | (19.3, NE) | (8.1, 12.1) | (11.5, 14.9) | (7.3, 8.5) |
| Hazard ratiob | 0.43 | 0.62 | ||
| (95% CI) | (0.31, 0.59) | (0.50, 0.76) | ||
| P valuec | <0.0001 | <0.0001 | ||
| HR = Homologous Recombination, NE = not estimable. a Efficacy analysis was based on blinded independent central review. b Based on a stratified Cox proportional hazards model. c Based on a stratified log-rank test. |
||||
In exploratory subgroup analyses of patients who were administered a starting dose of ZEJULA or matched placebo based on baseline weight or platelet count, the hazard ratio for PFS was 0.39 (95% CI [0.22, 0.72]) in the HR-deficient subgroup (n = 130) and 0.68 (95% CI [0.48, 0.97]) in the overall population (n = 258).
Figure 1: Progression-Free Survival in Patients with HR-Deficient Tumors (Intent-to-Treat Population, n = 373)
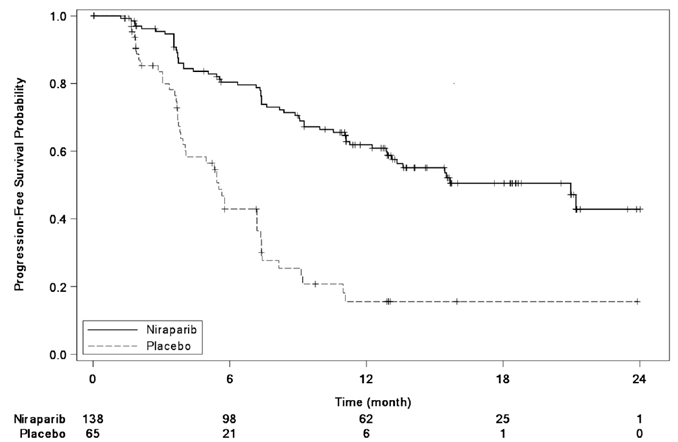 |
Figure 2: Progression-Free Survival in the Overall Population (Intent-to-Treat Population, n = 733)
 |
At the time of the PFS analysis, overall survival data were immature, with 11% deaths in the overall population.
Maintenance Treatment Of Recurrent Ovarian Cancer
NOVA (NCT01847274) was a double-blind, placebo-controlled trial in which patients (N = 553) with platinum-sensitive recurrent epithelial ovarian, fallopian tube, or primary peritoneal cancer were randomized 2:1 to ZEJULA 300 mg orally daily or matched placebo within 8 weeks of the last therapy. Treatment was continued until disease progression or unacceptable toxicity. All patients had received at least 2 prior platinum-containing regimens and were in response (complete or partial) to their most recent platinum-based regimen.
Randomization was stratified by time to progression after the penultimate platinum therapy (6 to <12 months and ≥12 months), use of bevacizumab in conjunction with the penultimate or last platinum regimen (yes/no), and best response during the most recent platinum regimen (complete response and partial response). Eligible patients were assigned to 1 of 2 cohorts based on the results of the BRACAnalysis CDx. Patients with deleterious or suspected deleterious germline BRCA mutations (gBRCAm) were assigned to the germline BRCA-mutated (gBRCAmut) cohort (n = 203), and those without germline BRCA mutations were assigned to the non-gBRCAmut cohort (n = 350).
The major efficacy outcome measure, PFS, was determined primarily by central independent assessment per RECIST version 1.1. In some cases, criteria other than RECIST, such as clinical signs and symptoms and increasing CA-125, were also applied.
The median age of patients ranged from 57 to 64 years among patients treated with ZEJULA and 58 to 67 years among patients treated with placebo. Eighty-six percent of all patients were White. Sixty-seven percent of patients receiving ZEJULA and 69% of patients receiving placebo had an ECOG PS of 0 at study baseline. Approximately 40% of patients were enrolled in the U.S. or Canada, and 51% of all patients were in complete response to most recent platinum-based regimen, with 39% on both arms with an interval of 6 to 12 months since the penultimate platinum regimen. Twenty-six percent of those treated with ZEJULA and 31% treated with placebo had received prior bevacizumab therapy. Approximately 40% of patients had 3 or more lines of treatment.
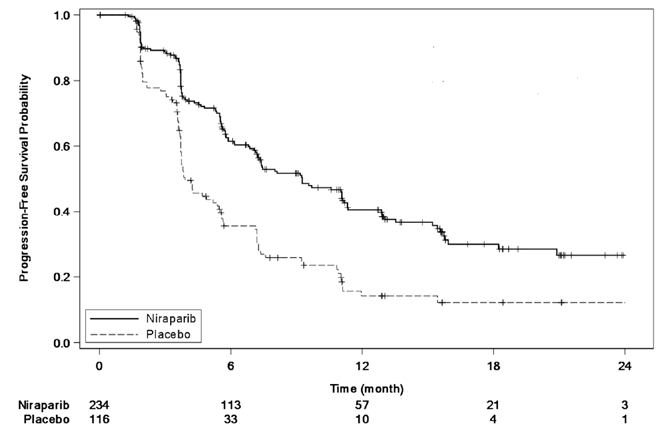
The trial demonstrated a statistically significant improvement in PFS for patients randomized to ZEJULA as compared with placebo in the gBRCAmut cohort and the non-gBRCAmut cohort (Table 13, Figure 3, and Figure 4).
Table 13: Efficacy Results - NOVA (IRC Assessment,a Intent-to-Treat Population)
| gBRCAmut Cohort | non-gBRCAmut Cohort | |||
| ZEJULA (n = 138) |
Placebo (n = 65) |
ZEJULA (n = 234) |
Placebo (n = 116) |
|
| Progression-free | 21.0 | 5.5 | 9.3 | 3.9 |
| survival median in months (95% CI) | (12.9, NR) | (3.8, 7.2) | (7.2, 11.2) | (3.7, 5.5) |
| Hazard ratiob | 0.26 | 0.45 | ||
| (95% CI) | (0.17, 0.41) | (0.34, 0.61) | ||
| P valuec | <0.0001 | <0.0001 | ||
| IRC = Independent Review Committee, gBRCAmut = germline BRCA-mutated, NR = not reached. a Efficacy analysis was based on blinded central independent radiologic and clinical oncology review committee. b Based on a stratified Cox proportional hazards model. c Based on a stratified log-rank test. |
||||
Figure 3: Progression-Free Survival in the gBRCAmut Cohort Based on IRC Assessment (Intent-to-Treat Population, n = 203)
 |
Figure 4: Progression-Free Survival in the Non-gBRCAmut Cohort Overall Based on IRC Assessment (Intent-to-Treat Population, n = 350)
 |
At the time of the PFS analysis, limited overall survival data were available with 17% deaths across the 2 cohorts.
Treatment Of Advanced Ovarian Cancer After 3 Or More Chemotherapies
The efficacy of ZEJULA was studied in 98 patients with advanced ovarian cancer with HRD positive tumors in the single-arm QUADRA (NCT02354586) trial. Patients were required to have been treated with 3 or more prior lines of chemotherapy and those with prior exposure to PARP inhibitors were excluded. Patients were selected using a clinical trial assay. Those without BRCA mutations must have progressed at least 6 months after their last dose of platinum therapy. All patients received ZEJULA capsules at a starting dosage of 300 mg once daily as monotherapy until disease progression or unacceptable toxicity.
HRD positive status was determined using the Myriad myChoice CDx as either tBRCAm (n = 63) and/or a GIS ≥42 (n = 35). GIS is an algorithmic measurement of loss of heterozygosity, telomeric allelic imbalance, and large-scale state transitions.
The major efficacy outcome measures were objective response rate (ORR) and duration of response as assessed by the investigator according to RECIST v. 1.1.
The median age of the patients was 63 years (range: 39 to 91 years), the majority were White (82%), and all had an ECOG PS of 0 (59%) or 1 (41%).
The efficacy results for QUADRA are summarized in Table 14.
Table 14: Efficacy Results - QUADRA (Investigator Assessment)
| Efficacy Results | HRD Positive Cohorta (N = 98) |
| Objective response rate (95% CI)b | 24% (16, 34) |
| Complete responses | 0% |
| Partial responses | 24% |
| Median duration of response in months (95% CI) | 8.3 (6.5, NE) |
| NE = not estimable. a Homologous recombination deficiency (HRD) positive status is defined as tBRCA-mutated and/or genomic instability score >42. b Confirmed response rate. The objective response rate as assessed by blinded independent central review was consistent. |
|
For patients with tBRCAm ovarian cancer, investigator-assessed ORR was 39% (7/18; 95% CI: [17, 64]) in patients with platinum-sensitive disease, 29% (6/21; 95% CI: [11, 52]) in patients with platinum-resistant disease, and 19% (3/16; 95% CI: [4, 46]) in patients with platinum-refractory disease.
For patients with platinum-sensitive GIS-positive disease (without BRCAmut) (n = 35), investigator-assessed ORR was 20% (95% CI [8, 37]).
Medication Guide
PATIENT INFORMATION
ZEJULA
(zuh-JOO-luh)
(niraparib) Capsules
What is the most important information I should know about ZEJULA?
ZEJULA may cause serious side effects including:
- Bone marrow problems called myelodysplastic syndrome (MDS) or a type of cancer of the blood called acute myeloid leukemia (AML). Some people who have ovarian cancer and who have received previous treatment with chemotherapy or certain other medicines for their cancer have developed MDS or AML during treatment with ZEJULA. MDS or AML may lead to death. If you develop MDS or AML, your healthcare provider will stop treatment with ZEJULA. Symptoms of low blood cell counts (low red blood cells, low white blood cells, and low platelets) are common during treatment with ZEJULA, but can be a sign of serious bone marrow problems, including MDS or AML. Symptoms may include:
- weakness
- fever
- feeling tired
- shortness of breath
- weight loss
- blood in urine or stool
- frequent infections
- bruising or bleeding more easily
Your healthcare provider will do blood tests to check your blood cell counts:
-
- before treatment with ZEJULA.
- weekly for the first month of treatment with ZEJULA.
- every month for the next 11 months, then as needed during treatment with ZEJULA.
- High blood pressure. High blood pressure is common during treatment with ZEJULA and can become serious. Your healthcare provider will check your blood pressure and heart rate at least weekly for the first 2 months, then monthly for the first year and as needed thereafter during your treatment with ZEJULA.
- Posterior reversible encephalopathy syndrome (PRES). PRES is a condition that affects the brain and may happen during treatment with ZEJULA. If you have headache, vision changes, confusion, or seizure with or without high blood pressure, please contact your healthcare provider.
See “What are the possible side effects of ZEJULA?” for more information about side effects.
What is ZEJULA?
ZEJULA is a prescription medicine used for the:
- maintenance treatment of adults with advanced ovarian cancer, fallopian tube cancer, or primary peritoneal cancer. ZEJULA is used after the cancer has responded (complete or partial response) to treatment with platinum-based chemotherapy.
- maintenance treatment of adults with ovarian cancer, fallopian tube cancer, or primary peritoneal cancer that comes back. ZEJULA is used after the cancer has responded (complete or partial response) to treatment with platinum-based chemotherapy.
- treatment of adults with advanced ovarian cancer, fallopian tube cancer, or primary peritoneal cancer who have been treated with 3 or more prior types of chemotherapy and who have tumors with:
- a certain BRCA gene mutation, or
- gene mutation problems and who have progressed more than 6 months after their last treatment with platinum-based chemotherapy.
Your healthcare provider will perform a test to make sure that ZEJULA is right for you.
It is not known if ZEJULA is safe and effective in children.
Before taking ZEJULA, tell your healthcare provider about all of your medical conditions, including if you:
- have heart problems.
- have liver problems.
- have high blood pressure.
- are allergic to FD&C Yellow No. 5 (tartrazine) or aspirin. ZEJULA capsules contain FD&C Yellow No. 5 (tartrazine), which may cause allergic-type reactions (including bronchial asthma) in certain people, especially people who also have an allergy to aspirin.
- are pregnant or plan to become pregnant. ZEJULA can harm your unborn baby and may cause loss of pregnancy (miscarriage).
- If you are able to become pregnant, your healthcare provider may perform a pregnancy test before you start treatment with ZEJULA.
- Females who are able to become pregnant should use effective birth control (contraception) during treatment with ZEJULA and for 6 months after the last dose of ZEJULA. Talk to your healthcare provider about birth control methods that may be right for you.
- Tell your healthcare provider right away if you become pregnant.
- are breastfeeding or plan to breastfeed. It is not known if ZEJULA passes into your breast milk. Do not breastfeed during treatment with ZEJULA and for 1 month after the last dose of ZEJULA. Talk to your healthcare provider about the best way to feed your baby during this time.
Tell your healthcare provider about all the medicines you take, including prescription and over-thecounter medicines, vitamins, and herbal supplements.
How should I take ZEJULA?
- Take ZEJULA exactly as your healthcare provider tells you to.
- Take ZEJULA 1 time each day, at the same time each day.
- ZEJULA may be taken with or without food.
- ZEJULA capsules should be swallowed whole. Do not chew, crush, or split ZEJULA capsules before swallowing.
- Taking ZEJULA at bedtime may help relieve any nausea symptoms you may have.
- Do not stop taking ZEJULA without first talking with your healthcare provider.
- If you miss a dose of ZEJULA, take your next dose at your scheduled time. Do not take an extra dose to make up for a missed dose.
- If you vomit after taking a dose of ZEJULA, do not take an extra dose. Take your next dose at your scheduled time.
- If you take too much ZEJULA, call your healthcare provider or go to the nearest hospital emergency room right away.
What are the possible side effects of ZEJULA?
ZEJULA may cause serious side effects, including:
- See “What is the most important information I should know about ZEJULA?”
The most common side effects of ZEJULA include:
- heart not beating regularly
- changes in liver function or other blood tests
- nausea
- pain in your muscles and back
- constipation
- headache
- vomiting
- dizziness
- pain in the stomach area
- change in the way food tastes
- mouth sores
- trouble sleeping
- diarrhea
- anxiety
- indigestion or heartburn
- sore throat
- dry mouth
- shortness of breath
- tiredness
- cough
- loss of appetite
- rash
- urinary tract infection
- changes in the amount or color of your urine
Your healthcare provider may change your dose, temporarily stop treatment, or permanently stop treatment with ZEJULA if you have certain side effects.
These are not all the possible side effects of ZEJULA.
Call your healthcare provider for medical advice about side effects. You may report side effects to FDA at 1800-FDA-1088.
How should I store ZEJULA?
Store ZEJULA at room temperature between 68° to 77°F (20° to 25°C).
Keep ZEJULA and all medicines out of the reach of children.
General information about the safe and effective use of ZEJULA.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use ZEJULA for a condition for which it was not prescribed. Do not give ZEJULA to other people, even if they have the same symptoms that you have. It may harm them. You can ask your healthcare provider or pharmacist for information about ZEJULA that is written for health professionals.
What are the ingredients in ZEJULA?
Active ingredient: niraparib.
Inactive ingredients:
Capsule fill: magnesium stearate and lactose monohydrate.
Capsule shell: titanium dioxide and gelatin in the white capsule body and FD&C Blue No. 1, FD&C Red No. 3, FD&C Yellow No. 5 (tartrazine), and gelatin in the purple capsule cap.
The black printing ink: shellac, dehydrated alcohol, isopropyl alcohol, butyl alcohol, propylene glycol, purified water, strong ammonia solution, potassium hydroxide, and black iron oxide.
The white printing ink: shellac, dehydrated alcohol, isopropyl alcohol, butyl alcohol, propylene glycol, sodium hydroxide, povidone, and titanium dioxide.
This Patient Information has been approved by the U.S. Food and Drug Administration.
