Trianal Drug Description: Detailed Datasheet
- Generic Name:
- Brand Name:
side effects drug center () drug
Drug Description
TRIANAL
(butalbital, acetylsalicylic acid and caffeine) Tablets and Capsules
DESCRIPTION
Drug Substance
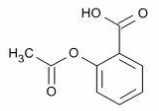
Acetylsalicylic acid (ASA)
Common name: Acetylsalicylic acid (ASA)
Chemical name: benzoic acid, 2-(acetyloxy)-
Molecular formula: C9H8O4
Molecular mass: 180.16 g/mol
Structural formula:
 |
Caffeine
Common name: Caffeine
Chemical name: 1,3,7-trimethylxanthine
Molecular formula: C8H10N4O2
Molecular mass: 194.19 g/mol
Structural formula :
 |
Butalbital
Common name: Butalbital
Chemical name: 5-allyl-5-isobutylbarbituric acid
Molecular formula: C11H16N2O3
Molecular mass: molecular weight 224.26 g/mol
Structural formula:
 |
Indications
TRIANAL TABLETS/TRIANAL CAPSULES (ASA-caffeine-butalbital) are indicated for the relief of tension-type headache.
Evidence supporting the efficacy and safety of TRIANAL TABLETS/TRIANAL CAPSULES in the treatment of multiple recurrent headaches is unavailable. Caution in this regard is required because repeated use of TRIANAL TABLETS/TRIANAL CAPSULES may cause medication overuse headaches and butalbital is habit-forming and potentially abusable (see WARNINGS and PRECAUTIONS, Drug Abuse And Dependence).
The clinical effectiveness of ASA-caffeine-butalbital in tension-type headache has been established in double-blind, placebo-controlled, multi-clinic trials. A factorial design study compared ASA-caffeine-butalbital with each of its major components. This study demonstrated that each component contributes to the efficacy of ASA-caffeine-butalbital in the treatment of the target symptoms of tension type headache (headache pain, psychic tension, and muscle contraction in the head, neck, and shoulder region). For each symptom and the symptom complex as a whole, ASA-caffeine-butalbital were shown to have significantly superior clinical effects to either component alone.
ASA-caffeine-butalbital has not been studied in pediatrics and should not be administered to children < 18 years of age.
Dosage
DOSAGE AND ADMINISTRATION
Dosage
Adult
1 or 2 tablets/capsules followed by 1 tablet/capsule every 3 to 4 hours, if needed.
Maximum Daily Dose
6 tablets/capsules daily, or as prescribed by the physician.
TRIANAL TABLETS/TRIANAL CAPSULES should not be administered to children.
Extended and repeated use of this product is not recommended because of the potential for physical dependence.
HOW SUPPLIED
Storage And Stability
Store between 15 and 30°C.
Keep out of reach and sight of children.
Dosage Forms, Packaging And Composition
Trianal Tablet
White, round, flat-faced, beveled edge tablet, engraved “T” in a triangle on one side and “TRIANAL” on the other. Available in bottles of 100 and 500.
Trianal Capsule
Light blue opaque cap and purple opaque body hard gelatin capsule, printed in white “TRIANAL” on cap and with 3 “T”s in their own triangle on body. Available in bottles of 100.
Composition
TRIANAL TABLET contains butalbital 50 mg, caffeine 40 mg and ASA 330 mg per tablet. Non-medicinal ingredients: Croscarmellose sodium, Microcrystalline cellulose, Sodium lauryl sulphate and Stearic acid.
TRIANAL CAPSULE contains butalbital 50 mg, caffeine 40 mg and ASA 330 mg per capsule. Non-medicinal ingredients: Brilliant Blue FCF sodium salt (FD&C Blue No. 1), Croscarmellose sodium, Erythrosine (FD&C Red No. 3), Gelatin, Microcrystalline cellulose, Stearic acid and Titanium dioxide. The imprinting ink contains: Ammonium hydroxide, Butyl alcohol, Ethanol, Isopropyl alcohol, Propylene glycol, Shellac, Simethicone and Titanium dioxide.
Prepared by: Laboratoire Riva Inc. Revised: July 2018
Side Effects
The most frequent adverse reactions are drowsiness and dizziness. Less frequent adverse reactions are constipation, rash, miosis, lightheadedness and gastrointestinal disturbances including nausea, vomiting and flatulence. A single incidence of bone marrow suppression has been reported with the use of ASA-caffeine-butalbital. Several cases of dermatological reactions including toxic epidermal necrolysis, Stevens-Johnson syndrome, lichenoid eruption and erythema multiforme have been reported.
The following adverse drug events may be borne in mind as potential effects of the components of TRIANAL TABLETS/TRIANAL CAPSULES. Potential effects of high dosage are listed in the SYMPTOMS and TREATMENT of OVERDOSAGE section below.
ASA: occult blood, hemolytic anemia, iron deficiency anemia, dyspepsia, nausea, peptic ulcer, prolonged bleeding time, nephropathy toxic when taken in high doses for prolonged periods, urine uric acid decreased, hepatitis.
Caffeine: tachycardia, irritability, tremor, dependence, hyperglycemia.
Butalbital: incoordination, difficulty thinking, poor memory, faulty judgment, decreased attention, emotional lability, exaggeration of personality traits.
Drug Interactions
The concomitant use of alcohol or other CNS depressants may have an additive effect, and patients should be warned accordingly.
The CNS effects of butalbital may be enhanced by monoamine oxidase (MAO) inhibitors.
In patients receiving concomitant corticosteroids and chronic use of ASA, withdrawal of corticosteroids may result in salicylism because corticosteroids enhance renal clearance of salicylates and their withdrawal is followed by return to normal rates of renal clearance.
The prolonged ingestion of barbiturates gives rise to enzyme induction. This increases the rate of metabolism of certain drugs, including oral anticoagulants and oral contraceptives, thus reducing their effectiveness.
TRIANAL TABLETS/TRIANAL CAPSULES may enhance the effects of:
- Oral antidiabetic agents and insulin, causing hypoglycemia by contributing an additive effect if dosage of TRIANAL TABLETS/TRIANAL CAPSULES exceeds maximum recommended dosage.
- Oral anticoagulants, causing bleeding by inhibiting prothrombin formation in the liver and displacing anticoagulants from plasma protein binding sites.
- 6-mercaptopurine and methotrexate, causing bone marrow toxicity and blood dyscrasias by displacing these drugs from secondary binding sites, and, in the case of methotrexate, also reducing its excretion.
- Non-steroidal anti-inflammatory agents, increasing the risk of peptic ulceration and bleeding by contributing additive effects.
- Other narcotic analgesics, alcohol, general anesthetics, tranquilizers such as chlordiazepoxide, sedative-hypnotics, or other CNS depressants, causing increased CNS depression.
TRIANAL TABLETS/TRIANAL CAPSULES may diminish the effects of:
Uricosuric agents such as probenecid and sulfinpyrazone, reducing their effectiveness in the treatment of gout. ASA competes with these agents for protein binding sites.
Drug/Laboratory Test Interactions
ASA may interfere with the following laboratory determinations in blood: serum amylase, fasting blood glucose, cholesterol, protein, aspartate aminotransferase (AST), uric acid, prothrombin time and bleeding time. ASA may interfere with the following laboratory determinations in urine: glucose, 5-hydroxyindoleactic acid, Gerhardt ketone, vanillylmandelic acid (VMA), uric acid, diacetic acid, and spectrophotometric detection of barbiturates.
Drug Abuse And Dependence
TRIANAL TABLETS/TRIANAL CAPSULES products have the potential for being abused and thus, continuous daily use should be avoided.
Butalbital
Barbiturates may be habit-forming
Tolerance, psychological dependence, and physical dependence may occur especially following prolonged use of high doses of barbiturates. The average daily dose for the barbiturate addict is usually about 1,500 mg. As tolerance to barbiturates develops, the amount needed to maintain the same level of intoxication increases; tolerance to a fatal dosage, however, does not increase more than two-fold. As this occurs, the margin between an intoxication dosage and fatal dosage becomes smaller. The lethal dose of a barbiturate is far less if alcohol is also ingested. Major withdrawal symptoms (convulsions and delirium) may occur within 16 hours and last up to 5 days after abrupt cessation of these drugs. Intensity of withdrawal symptoms gradually declines over a period of approximately 15 days. Treatment of barbiturate dependence consists of cautious and gradual withdrawal of the drug. Barbiturate dependent patients can be withdrawn by using a number of different withdrawal regimens. One method involves initiating treatment at the patient’s regular dosage level and gradually decreasing the daily dosage as tolerated by the patient.
Warnings
Therapeutic doses of ASA can cause anaphylactic shock and other severe allergic reactions. It should be ascertained if the patient is allergic to ASA, although a specific history of allergy may be lacking.
Significant bleeding can result from ASA therapy in patients with peptic ulcer or other gastrointestinal lesions, and in patients with bleeding disorders.
Thrombocytopenia has been reported in association with the use of ASA, and may be the underlying cause of the increased risk of bleeding, intracerebral hemorrhage and hemorrhagic stroke observed in patients treated with ASA as an antiplatelet therapy.
ASA administered pre-operatively may prolong the bleeding time.
A possible association between Reye's syndrome and the use of salicylates has been suggested but not established. Reye's syndrome has also occurred in many patients not exposed to salicylates. However, caution is advised when prescribing salicylate-containing medications for young adults with influenza or chickenpox.
Butalbital is habit-forming and potentially abusable. Consequently, the extended use of TRIANAL TABLETS/TRIANAL CAPSULES (ASA-caffeine-butalbital) is not recommended (see Drug Abuse And Dependence).
TRIANAL TABLETS/TRIANAL CAPSULES are associated with exacerbation of headache (medication overuse headaches) in susceptible patients. Repeated use of TRIANAL TABLETS/TRIANAL CAPSULES can lead to “rebound” headaches as each dose wears off. With repeated doses, physical and psychological dependence can develop. In addition to dependence, butalbital-containing products can lead to tolerance, and at higher doses can produce withdrawal symptoms after discontinuation (see Drug Abuse And Dependence).
Precautions
General
Because of its ASA content, TRIANAL TABLETS/TRIANAL CAPSULES (ASA-caffeine-butalbital) should be used with caution in patients with a history of bleeding tendencies, in patients on anticoagulant therapy and in patients with underlying hemostatic defects and with extreme caution in patients with peptic ulceration.
Precautions should be taken when administering salicylates to persons with known allergies. Hypersensitivity to ASA is particularly likely in patients with nasal polyps and relatively common in those with asthma.
Long-term use of preparations containing barbiturates may lead to habituation and physical dependence. TRIANAL TABLETS/TRIANAL CAPSULES, because of their butalbital content, should be avoided in patients with head injury, in whom a depressed CNS is suspected. Similarly, it should not be used in patients with actual or a predisposition towards respiratory depression.
TRIANAL TABLETS/TRIANAL CAPSULES should be prescribed with caution for certain special-risk patients, such as the elderly or debilitated, and those with severe impairment of renal or hepatic function, coagulation disorders, head injuries, elevated intracranial pressure, acute abdominal conditions, hypothyroidism, urethral stricture, Addison's disease, prostatic hypertrophy, peptic ulcer, or in osteomalacia and osteoporosis.
Occupational Hazards
Barbiturate-containing preparations may impair the mental and/or physical abilities needed for certain potentially hazardous activities such as driving a vehicle or operating machinery. Patients should be cautioned accordingly. Patients should also be cautioned about the combined effects of TRIANAL TABLETS/TRIANAL CAPSULES with other CNS depressants, including other opioids, phenothiazine, sedative/hypnotics and alcohol.
Carcinogenesis, Mutagenesis, Impairment Of Fertility
Long-term studies have been conducted in mice and rats with ASA, alone or in combination with other drugs, in which no evidence of carcinogenesis was seen. No adequate studies have been conducted in animals to determine whether ASA has a potential for mutagenesis or impairment of fertility. No adequate studies have been conducted in animals to determine whether butalbital has a potential for carcinogenesis, mutagenesis, or impairment of fertility.
Use In Pregnancy
TRIANAL TABLETS/TRIANAL CAPSULES should not be given to a pregnant woman unless the potential benefit justifies the potential risk to the fetus.
Teratogenicity
Animal reproduction studies have not been conducted with TRIANAL TABLETS/TRIANAL CAPSULES. It is also not known whether TRIANAL TABLETS/TRIANAL CAPSULES can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity.
Withdrawal seizures were reported in a two-day-old male infant whose mother had taken a butalbital containing drug during the last 2 months of pregnancy. Butalbital was found in the infant’s serum. The infant was given phenobarbital 5 mg/kg, which was tapered without further seizure or other withdrawal symptoms.
In controlled studies involving 41,337 pregnant women and their offspring, there was no evidence that ASA taken during pregnancy caused stillbirth, neonatal death or reduced birth weight. In controlled studies of 50,282 pregnant women and their offspring, ASA administration in moderate and heavy doses during the first four lunar months of pregnancy showed no teratogenic effect.
Therapeutic doses of ASA in pregnant women close to term may cause bleeding in mother, fetus, or neonate. During the last 6 months of pregnancy, regular use of ASA in high doses may prolong pregnancy and delivery.
Labour And Delivery
Ingestion of ASA prior to delivery may prolong delivery or lead to bleeding in the mother or neonate.
Nursing Women
ASA, caffeine and barbiturates are excreted into breast milk, but the significance of their effects on nursing infants is not known. Because of potential for serious adverse reactions in nursing infants, the use of TRIANAL TABLETS/TRIANAL CAPSULES by nursing mothers is not recommended unless the expected benefit to the mother is greater than any possible risk to the infant.
During pregnancy and lactation, TRIANAL TABLETS/TRIANAL CAPSULES should be taken only as prescribed.
Pediatric Use
Safety and efficacy in patients younger than 18 years of age have not been studied.
Geriatric Use
Clinical studies of ASA-caffeine-butalbital did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
Butalbital is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
Laboratory Tests
In patients with severe hepatic or renal disease, effects of therapy should be monitored with serial liver and/or renal function tests.
Information For Patients
- Patients should be informed that TRIANAL TABLETS/TRIANAL CAPSULES contain ASA and should not be taken by patients with an ASA allergy.
- TRIANAL TABLETS/TRIANAL CAPSULES may impair the mental and/or physical abilities required for performance of potentially hazardous tasks such as driving a car or operating machinery. Such tasks should be avoided while taking TRIANAL TABLETS/TRIANAL CAPSULES.
- Alcohol and other CNS depressants may produce an additive CNS depression when taken with TRIANAL TABLETS/TRIANAL CAPSULES and should be avoided.
- Butalbital may be habit-forming. Patients should take the drug only for as long as it is prescribed, in the amounts prescribed, and no more frequently than prescribed.
- For information on use in geriatric patients, see PRECAUTIONS, Geriatric Use.
Overdosage & Contraindications
OVERDOSE
For management of a suspected drug overdose, contact your regional Poison Control Centre immediately.
The toxic effects of acute overdosage of TRIANAL TABLETS/TRIANAL CAPSULES (ASA-caffeine-butalbital) are attributable mainly to its barbiturate component, and, to a lesser extent, ASA. Because toxic effects of caffeine occur in very high dosages only, the possibility of significant caffeine toxicity from TRIANAL TABLETS/TRIANAL CAPSULES overdosage is unlikely.
Symptoms
- Acute barbiturate poisoning: drowsiness, confusion and coma, with reduced or absent reflexes; prominent, persistent respiratory depression; hypotension, followed by circulatory collapse and a typical shock-like state in severe intoxication; respiratory complications, renal failure, and possibly death.
- Acute ASA poisoning: principal toxic effects include hyperpnea, hypercapnia; acid-base disturbances with the development of metabolic acidosis, especially in children; and gastrointestinal irritation with vomiting and abdominal pain. Also, acetone odour in breath, tinnitus, sweating, hyperthermia, dehydration, hypoprothrombinemia with spontaneous bleeding, restlessness, delirium, convulsions and coma may occur.
- Acute caffeine poisoning: insomnia, restlessness, tinnitus and flashes of light; tachycardia and extrasystoles; tremor, delirium and coma, following high doses in the region of 10g. Death has not been reported with caffeine overdosage.
Note
Because large doses of barbiturates alone may cause marked respiratory and CNS depression, an even more profound depressant effect may be expected after an overdosage of TRIANAL CAPSULES/TRIANAL TABLETS.
The dangers of TRIANAL TABLETS/TRIANAL CAPSULES overdosage are increased when the drug is ingested in the presence of alcohol, phenothiazines, minor tranquilizers and/or narcotics.
Treatment
The management of acute TRIANAL TABLETS/TRIANAL CAPSULES overdosage may involve the treatment of the toxic effects of all its constituents, with the possible exception of caffeine, which is toxic in very high doses only. Generally, it is the management of the barbiturate intoxication and the correction of the acid-base imbalance due to salicylism which demand most attention. The therapeutic procedures most commonly employed are:
Elimination Of The Offending Drug
1) Emesis: if the patient is conscious, induce vomiting mechanically or with syrup of ipecac (15 to 30 mL). 2) Perform gastric lavage followed by the administration of activated charcoal if the pharyngeal and laryngeal reflexes are present and if less than 4 hours has elapsed since ingestion. Do not attempt gastric lavage on the unconscious patient unless cuffed endotracheal intubation has been performed to prevent aspiration and pulmonary complications. 3) Catharsis: Following gastric lavage, a saline cathartic (sodium or magnesium sulfate 30g in 250 mL of water) may be introduced and left in the stomach. 4) Encourage diuresis by administration of i.v. fluids assisted, if necessary, by 100 to 150 mL 25% mannitol solution given slowly i.v. Note: Mannitol should not be mixed with blood in a transfusion set, as red cell crenation and agglutination may occur. 5) Alkalinization of the urine (see caution): i.v. isotonic sodium bicarbonate solution accelerates urinary excretion of barbiturates. Maximum alkalinization may be more successfully attained if the sodium bicarbonate infusion is accompanied by acetazolamide 250 mg given as a single i.v. injection every 6 hours. (Caution perform urinary alkalinization with care in children). 6) Peritoneal dialysis and hemodialysis have been used with success in acute barbiturate intoxication and may be lifesaving. However, before embarking on either method, weigh the risks inherent to these procedures against the risk of not using them at all.
Maintenance Of Adequate Pulmonary Ventilation
Respiratory depression is an early and often profound manifestation of acute barbiturate poisoning. Meticulous attention to this aspect of treatment is essential. Perform pharyngeal and tracheal suction diligently to remove excess mucous secretions. Judicious administration of oxygen is also indicated. However, oxygen without assisted respiration must be used with caution, as its use in hypoventilation hypoxia may result in further respiratory depression and hypercapnia. In more critical cases, endotracheal intubation or tracheotomy, with or without assisted respiration, may be necessary.
Correction Of Hypotension
Vigorous treatment is essential, as circulatory collapse and renal failure are frequent causes of death. 1) Mild cases: The usual head-down position and other supportive measures may be adequate. 2) Severe cases: Vasopressors (dopamine, levarterenol) may be given i.v. with the usual precautions and serial blood pressure monitoring.
Special Features Due To Salicylate Overdosage
1) The prominent features of salicylate intoxication are metabolic acidosis and electrolyte disturbance, and these require evaluation and correction. Sodium bicarbonate 400 mg (5 mEq)/kg as a 1% solution in 5% dextrose water is not only effective in correcting acidosis, but effectively and rapidly accelerates salicylate excretion by the kidneys. The administration of sodium bicarbonate must be carefully monitored with frequent blood pH and plasma CO2 content determinations, as large amounts of sodium bicarbonate may result in severe alkalosis, particularly in children. THAM, an osmotic alkalinizing diuretic, also greatly increases the excretion of salicylate. This is given as a 0.3 molar solution at a rate not exceeding 5 mL/kg/hour. Potassium deficiency may occur and should be corrected. 2) Treat hyperthermia and dehydration with ice packs and i.v. fluids. 3) Treat hypoprothrombinemia with vitamin K1, 50 mg given daily i.v. 4) Hemodialysis, peritoneal dialysis or exchange transfusion are indicated in very severe salicylate intoxication. However, in TRIANAL TABLETS/TRIANAL CAPSULES overdosage, these measures are indicated mainly for barbiturate intoxication but would be effective for both.
Methemoglobinemia over 30% should be treated with methylene blue by slow intravenous administration.
General Supportive Measures
1) Good nursing care is of prime importance, particularly in the comatose patient, and should include regular observation and accurate recording of the vital signs and depth of coma, maintenance of a free airway, frequent turning and other routine measures usually adopted with unconscious patients. 2) Careful supervision and recording of fluid intake and output is essential. 3) Take blood samples to determine barbiturate blood concentrations and for electrolyte and other pertinent blood studies.
Toxic And Lethal Doses (For Adults)
ASA: toxic blood level greater than 30 mg/100 mL; lethal dose 10-30 g
Caffeine: toxic dose greater than 1 g (25 tablets/capsules); lethal dose 6.5-10g
Butalbital: toxic dose 1 g (20 tablets/capsules); lethal dose 2-5 g
CONTRAINDICATIONS
TRIANAL TABLETS/TRIANAL CAPSULES (ASA-caffeine-butalbital) are contraindicated under the following conditions:
- Hypersensitivity or intolerance to ASA, caffeine, or butalbital or to any of the components.
- Patients with a hemorrhagic diathesis (e.g., hemophilia, hypoprothrombinemia, von Willebrand’s disease, thrombocytopenia, thrombasthenia and other ill-defined hereditary platelet dysfunctions, severe vitamin K deficiency and severe liver damage).
- Patients with the syndrome of nasal polyps, angioedema and bronchospastic reactivity to ASA or other nonsteroidal anti-inflammatory drugs. Anaphylactoid reactions have occurred in such patients.
- Peptic ulcer or other serious gastrointestinal lesions.
- Patients with porphyria.
- In patients with a history of abuse or overdosage due to alcohol, hypnotics, analgesics and psychotropic drugs.
Clinical Pharmacology
Pharmacologically, TRANAL TABLETS/TRIANAL CAPSULES (ASA-caffeine-butalbital) combines the analgesic properties of acetylsalicylic acid (ASA) with the anxiolytic and muscle relaxant properties of butalbital.
Pharmacokinetics
The behaviour of the individual components is described below.
Acetylsalicylic Acid (ASA)
ASA is a salicylate that binds to the cyclooxygenase enzyme leading to a reduction in prostaglandin activity. The systemic availability of ASA after an oral dose is highly dependent on the dosage form, the presence of food, the gastric emptying time, gastric pH, antacids, buffering agents, and particle size. These factors affect not necessarily the extent of absorption of total salicylates but more the stability of ASA prior to absorption.
During the absorption process and after absorption, ASA is mainly hydrolyzed to salicylic acid and distributed to all body tissues and fluids, including fetal tissues, breast milk, and the central nervous system (CNS). Highest concentrations are found in plasma, liver, renal cortex, heart, and lung. In plasma, about 50% - 80% of the salicylic acid and its metabolites are loosely bound to plasma proteins.
The clearance of total salicylates is subject to saturable kinetics; however, first-order elimination kinetics are still a good approximation for doses up to 650 mg. The plasma half-life for ASA is about 12 minutes and for salicylic acid and/or total salicylates is about 3 hours.
The elimination of therapeutic doses is through the kidneys either as salicylic acid or other biotransformation products. The renal clearance is greatly augmented by an alkaline urine as is produced by concurrent administration of sodium bicarbonate or potassium citrate.
The biotransformation of ASA occurs primarily in the hepatocytes. The major metabolites are salicyluric acid (75%), the phenolic and acyl glucuronides of salicylate (15%), and gentisic and gentisuric acid (1%).
See OVERDOSE for toxicity information.
Caffeine
Caffeine is a CNS stimulant with primary effects on adenosine receptors. Like most xanthines, caffeine is rapidly absorbed and distributed in all body tissues and fluids, including the CNS, fetal tissues, and breast milk.
Caffeine is cleared rapidly through metabolism and excretion in the urine. The plasma half-life is about 3 hours. Hepatic biotransformation prior to excretion results in about equal amounts of 1 methylxanthine and 1-methyluric acid. Of the 70% of the dose that has been recovered in the urine, only 3% was unchanged drug.
See OVERDOSE for toxicity information.
Butalbital
Butalbital is a short to intermediate-acting barbiturate which is thought to act on the CNS through enhanced gamma-aminobutyric acid (GABA) binding to GABA A receptors. Butalbital is well absorbed from the gastrointestinal tract and is expected to distribute to most of the tissues in the body. Barbiturates, in general, may appear in breast milk and readily cross the placental barrier. They are bound to plasma and tissue proteins to a varying degree and binding increases directly as a function of lipid solubility.
Elimination of butalbital is primarily via the kidney (59% - 88% of the dose) as unchanged drug or metabolites. The plasma half-life is about 35 hours. The elimination half-life of butalbital is about 61 hours (range: 35 to 88 hours). Urinary excretion products included parent drug (about 3.6% of the dose), 5-isobutyl-5-(2,3-dihydroxypropyl) barbituric acid (about 24% of the dose), 5 allyl-5(3-hydroxy-2-methyl-1-propyl) barbituric acid (about 4.8% of the dose), products with the barbituric acid ring hydrolyzed with excretion of urea (about 14% of the dose), as well as unidentified materials. Of the material excreted in the urine, 32% was conjugated.
The in vitro plasma protein binding of butalbital is 45% over the concentration range of 0.5 – 20 mcg/mL. This falls within the range of plasma protein binding (20% - 45%) reported with other barbiturates such as phenobarbital, pentobarbital, and secobarbital sodium. The plasma-to-blood concentration ratio was almost unity indicating that there is no preferential distribution of butalbital into either plasma or blood cells.
See OVERDOSE section for toxicity information.
Medication Guide
PATIENT INFORMATION
TRIANAL
(acetylsalicylic acid-caffeine-butalbital)
This leaflet is part III of a three-part "Product Monograph" published when TRIANAL TABLETS/TRIANAL CAPSULES was approved for sale in Canada and is designed specifically for Consumers. This leaflet is a summary and will not tell you everything about TRIANAL TABLETS/TRIANAL CAPSULES. Contact your doctor or pharmacist if you have any questions about the drug.
ABOUT THIS MEDICATION
What the medication is used for:
TRIANAL TABLETS/TRIANAL CAPSULES are used for the relief of tension-type headaches.
What it does:
TRIANAL TABLETS/TRIANAL CAPSULES consist of acetylsalicylic acid (ASA), caffeine and butalbital. ASA reduces pain, fever and inflammation. Caffeine is a mild stimulant which may enhance pain-relieving effects. Butalbital is a sedative that causes relaxation. This combination is used to relieve tension-type headaches.
When it should not be used:
You should NOT take TRIANAL TABLETS/TRIANAL CAPSULES if you:
- have a history of allergic reactions to ASA, caffeine, butalbital or any other components of the TRIANAL TABLETS/TRIANAL CAPSULES (see “What the nonmedicinal ingredients are”)
- have a condition that predisposes to bleeding such as hemophilia, hypoprothrombinemia, von Willebrand’s disease, thrombocytopenia, thrombasthenia and other ill-defined hereditary platelet dysfunctions, severe vitamin K deficiency and severe liver damage
- have nasal polyps, allergic reaction or bronchospastic reactivity to ASA or other nonsteroidal anti-inflammatory drugs (NSAIDs)
- have stomach ulcers or other serious stomach or bowel sores
- have a disease called porphyria
- have a history of drug abuse or drug overdose due to alcohol, sleeping pills, drugs to treat pain or any other prescription or illegal drugs
- are pregnant, in labour or breast-feeding.
What the medicinal ingredients are:
ASA (acetylsalicylic acid), caffeine and butalbital.
What the nonmedicinal ingredients are:
TRIANAL TABLETS contain following non-medicinal ingredients: Croscarmellose sodium, Microcrystalline cellulose, Sodium lauryl sulphate and Stearic acid.
TRIANAL CAPSULES contain following non-medicinal ingredients: Brilliant Blue FCF sodium salt (FD&C Blue No. 1), Croscarmellose sodium, Erythrosine (FD&C Red No. 3), Gelatin, Microcrystalline cellulose, Stearic acid and Titanium dioxide. The imprinting ink contains: Ammonium hydroxide, Butyl alcohol, Ethanol, Isopropyl alcohol, Propylene glycol, Shellac, Simethicone and Titanium dioxide.
What dosage forms it comes in:
TRIANAL TABLETS/TRIANAL CAPSULES contain 330 mg ASA, 40 mg caffeine, 50 mg butalbital.
WARNINGS and PRECAUTIONS
Keep TRIANAL TABLETS/TRIANAL CAPSULES out of the reach of children. You should not give TRIANAL TABLETS/TRIANAL CAPSULES to anyone as inappropriate use may have severe medical consequences.
BEFORE you use TRIANAL TABLETS/TRIANAL CAPSULES talk to your doctor or pharmacist if you:
- are allergic to ASA as it can cause anaphylactic shock and other severe allergic reactions
- have nasal polyps or asthma
- have a history of stomach ulcers, sores in your stomach or bowel or any other serious stomach problems
- have a history of bleeding
- will be having surgery
- have severe liver or kidney disease
- have a blood clotting disorder or are taking blood thinners
- have recently suffered a head injury or elevated pressure in your brain
- have problems with your thyroid gland
- have narrowing of the urethra caused by injury or disease
- have Addison’s disease
- have an enlarged prostate gland
- have softening or weakening of bones or osteoporosis
- have any allergies to any medicines, food, dyes or preservatives
- have the flu or chickenpox
- are pregnant, may become pregnant, in labour or breastfeeding.
IMPORTANT : PLEASED READ
TRIANAL TABLETS/TRIANAL CAPSULES are controlled medications. Butalbital is habit forming (tolerance, mental and physical dependence) and potentially abusable. Some patients, particularly those who have abused drugs in the past, may have a higher risk of abusing or developing an addiction while taking a barbiturate-containing product such as TRIANAL TABLETS/TRIANAL CAPSULES. Physical dependence may lead to withdrawal side effects when you stop taking this medicine. Continuous daily use of TRIANAL TABLETS/TRIANAL CAPSULES should be avoided as medication overuse (rebound) headaches may result in addition to the tolerance and dependence risks. Patients should take TRIANAL TABLETS/TRIANAL CAPSULES for as long as it is prescribed, in the amounts prescribed, and no more frequently than prescribed.
While there are important differences between physical dependence and addiction, each is a reason for close medical supervision and honest discussions with your doctor. If you have questions or concerns about abuse, addiction or physical dependence, please tell your doctor.
ASA may increase the risk of Reye's syndrome, a rare but often fatal condition. Caution should be used in administering ASA containing medications to young adults who have fever, flu or chicken pox. TRIANAL TABLETS/TRIANAL CAPSULES should not be administered to children. Before you have any medical tests done, tell the person in charge that you are taking TRIANAL TABLETS/TRIANAL CAPSULES. ASA may interfere with the results of certain tests done in blood and urine.
Driving and operating machinery
TRIANAL TABLETS/TRIANAL CAPSULES may impair the mental and/or physical abilities required for performance of potentially hazardous task such as driving a car or operating machinery. If you experience drowsiness or dizziness, such tasks should be avoided. Avoid alcohol as it can increase drowsiness and dizziness.
Pregnancy, labour and breastfeeding
TRIANAL TABLETS/TRIANAL CAPSULES are not recommended during pregnancy because they may cause withdrawal symptoms in the newborn baby. Taking TRIANAL TABLETS/TRIANAL CAPSULES close to delivery may make delivery longer or lead to bleeding in the mother or in the newborn baby. TRIANAL TABLETS/TRIANAL CAPSULES pass into breast milk and may harm a nursing infant.
INTERACTIONS WITH THIS MEDICATION
Tell your doctor or pharmacist if you are taking or have recently taken any other prescription or over-the-counter medicines, vitamins or natural health products during your treatment with TRIANAL TABLETS/TRIANAL CAPSULES.
Check with your doctor or pharmacist before taking any other medication with TRIANAL TABLETS/TRIANAL CAPSULES.
Tell your doctor if you are taking any of the following medications:
- alcohol and other CNS depressants (such as sleeping pills, muscle relaxants, pain killers, allergy medication (antihistamines), drugs used to treat anxiety, panic attacks and seizures)
- monoamine oxidase (MAO) inhibitors e.g., phenelzine sulphate, tranylcypromine sulphate, moclobemide or selegiline)
- corticosteroids
- oral drugs to treat diabetes and/or insulin
- blood thinners such as warfarin
- drugs used to suppress the immune system such as 6- mercaptopurine and methotrexate
- NSAIDs (non-steroidal anti-inflammatory drugs) to treat pain such as ibuprofen and naproxen
- tranquilizers such as chlordiazepoxide,
- drugs used to treat gout such as probenecid and sulfinpyrazone
- birth control pills.
PROPER USE OF THIS MEDICATION
Take TRIANAL TABLETS/TRIANAL CAPSULES exactly as directed by your doctor.
Usual dose:
TRIANAL TABLETS/TRIANAL CAPSULES comes as a tablet/capsule to take by mouth.
Dosage: Adult: 1 or 2 tablets/capsules followed by 1 tablet/capsule every 3 to 4 hours, if needed.
Maximum daily dose: 6 tablets/capsules daily, or as prescribed by the physician.
IMPORTANT : PLEASED READ
Overdose:
The most important sign of overdose is decreased breathing (abnormally slow or weak breathing), dizziness, confusion or extreme drowsiness. If you accidentally take too much TRIANAL TABLETS/TRIANAL CAPSULES call your doctor and/or your local emergency number and/or a Regional Poison Control Centre immediately, or go to a hospital emergency and take any remaining tablets and the container with you, even though you may not feel sick.
Missed Dose:
If you forget to take a dose of TRIANAL TABLETS/TRIANAL CAPSULES, do not worry. Take the missed dose as soon as you remember it. However, if it is almost time for the next dose, skip the missed dose and continue your regular dosing schedule. Do not take a double dose to make up for the missed one.
SIDE EFFECTS AND WHAT TO DO ABOUT THEM
Like all medicines, TRIANAL TABLETS/TRIANAL CAPSULES may cause unwanted reactions called side effects. Not all of these side effects may occur. Check with your doctor if the unwanted effects do not go away during treatment or become bothersome.
The following side effects may occur during treatment:
- drowsiness, light-headedness and/or dizziness
- constipation
- skin rash
- small pupils
- nausea, vomiting indigestion and/or gas
- increased risk of infection
- fast or irregular heart beat
- irritability
- tremor
- lack of coordination
- difficulty thinking
- poor memory and judgement
- decreased attention
- mood swings
- exaggeration of personality traits.
If any of these side effects affects you severely, tell your doctor.
SERIOUS SIDE EFFECTS, HOW OFTEN THEY HAPPEN AND WHAT TO DO ABOUT THEM
| Symptom / effect | Talk with your doctor or pharmacist right away | Stop taking drug and seek immediate emergency medical attention | ||
| Only if severe | In all cases | |||
| Uncommon | Reye's syndrome: rash on the palms of hands and feet, severe vomiting, high fever, weakness, confusions, headache, fast breathing leading to unresponsiveness and death | |||
| Allergic reactions: itching, rash, hives, difficulty breathing or swallowing not present before using this medicine | ||||
| Unkowon | Serious skin reactions including toxic epidermal necrolysis, Stevens- Johnson syndrome erythema multiforme and exfoliative dermatitis: fever, itching, skin sores | |||
| Anemia: fatigue, breathing difficulties, irregular heart beat or pale skin | ||||
| Stomach ulcer: heartburn, long lasting stomach pain, loss of appetite and weight loss | ||||
| Prolonged bleeding time | ||||
| Hepatitis: loss of appetite, dark urine, yellowing of eyes and skin | ||||
| Blood in the stool | ||||
IMPORTANT : PLEASED READ
This is not a complete list of side effects. For any unexpected effects while taking TRIANAL TABLETS/TRIANAL CAPSULES, contact your doctor or pharmacist.
HOW TO STORE IT
- store your TRIANAL TABLETS/TRIANAL CAPSULES at room temperature (between 15-30°C)
- keep out of reach and sight of children
- discard any expired medicine or medicine no longer needed.
Reporting Side Effects
You can report any suspected side effects associated with the use of health products to Health Canada by:
- Visiting the Web page on Adverse Reaction Reporting (https://www.canada.ca/en/health-canada/services/drugs-health-products/medeffect-canada.html) for information on how to report online, by mail or by fax; or
- Calling toll-free at 1-866-234-2345.
NOTE: Contact your health professional if you need information about how to manage your side effects. The Canada Vigilance Program does not provide medical advice.
