Thyrel Trh Drug Description: Detailed Datasheet
- Generic Name: protirelin
- Brand Name: Thyrel Trh
side effects drug center thyrel trh (protirelin) drug
Drug Description
THYREL® TRH
(protirelin)
Injection
FOR INTRAVENOUS ADMINISTRATION
DESCRIPTION
Chemically, Thyrel® TRH (protirelin) is identified as 5-oxo-L-prolyl-L-histidyl-L-proline amide. It is a synthetic tripeptide that is believed to be structurally identical to the naturally-occurring thyrotropin-releasing hormone produced by the hypothalamus. The CAS Registry Number is 24305-27-9. The structual formula is:
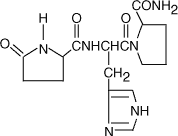
Thyrel TRH (protirelin) is supplied as 1 mL ampuls. Each ampul contains 500 µg protirelin in a sterile nonpyrogenic isotonic saline solution having a pH of approximately 6.5. In addition, each ampul contains sodium chloride 9.0 mg, Water for Injection, hydrochloric acid and sodium hydroxide as needed to adjust pH. Thyrel TRH (protirelin) is intended for intravenous administration.
Indications
Thyrel TRH (protirelin) is indicated as an adjunctive agent in the diagnostic assessment of thyroid function. As an adjunct to other diagnostic procedures, testing with Thyrel® TRH (protirelin) may yield useful information in patients with pituitary or hypothalamic dysfunction.
Thyrel TRH (protirelin) is indicated as an adjunct to evaluate the effectiveness of thyrotropin suppression with a particular dose of T4 in patients with nodular or diffuse goiter. A normal TSH baseline value and a minimal difference between the 30 minute and baseline response to Thyrel TRH (protirelin) injection would indicate adequate suppression of the pituitary secretion of TSH.
Thyrel TRH (protirelin) may be used, adjunctively, for adjustment of thyroid hormone dosage given to patients with primary hypothyroidism. A normal or slightly blunted TSH response, thirty minutes following Thyrel TRH (protirelin) injection, would indicate adequate replacement therapy.
Dosage
DOSAGE AND ADMINISTRATION
Thyrel TRH (protirelin) is intended for intravenous administration with the patient in the supine position. The drug is administered as a bolus over a period of 15 to 30 seconds, with the patient remaining supine until all scheduled postinjection blood samples have been taken. Blood pressure should be measured before Thyrel TRH (protirelin) is administered and at frequent intervals during the first 15 minutes thereafter (see WARNINGS). Have the patient urinate before injecting Thyrel TRH (protirelin) .
Dosage:
Adults: 500 µg. Doses between 200 and 500 µg have been used. 500 µg is considered the optimum dose to give the maximum response in the greatest number of patients. Doses greater than 500 µg are unlikely to elicit a greater TSH response.
Children age 6 to 16 years; 7 µg/kg body weight up to dose of 500 µg.
Infants and children up to 6 years: Experience is limited in this age group; doses of 7 µg/kg have been administered.
One blood sample for TSH assay should be drawn immediately prior to the injection of Thyrel® TRH, and a second sample should be obtained 30 minutes after injection.
The TSH response to Thyrel TRH (protirelin) is reduced by repetitive administration of the drug. Accordingly, if the Thyrel TRH (protirelin) test is repeated, an interval of seven days before testing is recommended.
Elevated serum lipids may interfere with the TSH assay. Thus, fasting (except in patients with hypopituitarism) or a low-fat meal is recommended prior to the test.
INTERPRETATION OF TEST RESULTS
Interpretation of the TSH response to Thyrel TRH (protirelin) requires an understanding of thyroid-pituitary-hypothalamic physiology and knowledge of the clinical status of the individual patient.
Because the TSH test results may vary with the laboratory, the physician should be familiar with the TSH assay method used and the normal range for the laboratory performing the assay. TSH response 30 minutes after Thyrel TRH (protirelin) administration in normal subjects and in patients with hyperthyroidism and hypothyroidism are presented in Figure 1. The diagnoses were established prior to the administration of Thyrel TRH (protirelin) on the basis of the clinical history, physical examination, and the results of other thyroid and/or pituitary function tests.

Among the normal euthyroid subjects, women and children were found to have higher levels of TSH at 30 minutes than men.
Among the patients with hyperthyroidism or primary (thyroidal), secondary pituitary), or tertiary (hypothalamic) hypothyroidism, no significant differences in TSH levels by age or sex were found.
Normal: Baseline TSH levels of less than 10 microunits/mL (µU/mL) were observed in 97% of euthyroid normal subjects tested. Thirty minutes after Thyrel TRH (protirelin) , the serum TSH increased by 2.0 mµ/mL or more in 95% of euthyroid subjects.
Hyperthyroidism: All hyperthyroid patients tested had baseline TSH levels of less than 10 µU/mL and a rise of less than 2 µU/mL 30 minutes after Thyrel® TRH.
Primary (thyroidal) hypothyroidism: The diagnosis of primary hypothyroidism is frequently supported by finding clearly elevated baseline TSH levels; 93% of patients tested had levels above 10 µU/mL, Thyrel TRH (protirelin) administration to these patients generally would not be expected to yield additional useful information. Ninety-four percent of patients with primary hypothyroidism given Thyrel® TRH in clinical trials responded with a rise in TSH of 2.0 µU/mL or greater. Since this response is also found in normal subjects, Thyrel TRH (protirelin) testing does not differentiate primary hypothyroidism from normal.
| Baseline Serum TSH (µU/mL) |
Change of Serum TSH (µU/mL) at 30 minutes |
|
| Euthyroidism (normal thyroid function) |
10 or less (usually 6 or less; 20% have <1.5 µU/mL) |
2 or more (usually 6 to 30) |
| Hyperthyroidism | 10 or less (usually 4 or less) |
less than 2 |
| Primary Hypothyroidism (thyroidal) |
more than 10 (usually 15 to 100) |
2 or more (usually 20 or more) |
| Secondary Hypothyroidism (pituitary) |
10 or less (usually 6 or less) |
less than 2 (59%) 2 to 50 (41%) |
| Tertiary Hypothyroidism (hypothalamic) |
10 or less (often less than 2) |
2 or more |
Secondary (pituitary) and tertiary (hypothalamic) hypothyroidism: In the presence of clinical and other laboratory evidence of hypothyroidism, the finding of a baseline TSH level less than 10 µU/mL should suggest secondary or tertiary hypothyroidism. In this situation, a response to Thyrel TRH (protirelin) of less than 2 µU/mL suggests secondary hypothyroidism since this response was observed in about 60% of patients with secondary hypothyroidism and only approximately 5% of patients with tertiary hypothyroidism. A TSH response to Thyrel TRH (protirelin) greater than 2 µU/mL is not helpful in differentiating between secondary and tertiary hypothyroidism since this response was noted in about 40% of the former and about 95% of the latter.
Establishing the diagnosis of secondary or tertiary hypothyroidism requires a careful history and physical examination along with appropriate tests of anterior pituitary and/or target gland function. The Thyrel TRH (protirelin) test should not be used as the only laboratory determinant for establishing these diagnoses.
HOW SUPPLIED
As 1 mL ampuls boxes of 5 (NDC 55566-0081-5). Each mL contains Thyrel TRH (protirelin) 0.50 mg (500 µg), sodium chloride 9.0 mg for isotonicity, hydrochloric acid and sodium hydroxide as needed to adjust pH.
Store at controlled room temperature 15° to 30°C (59° to 86°F).
Rx only
Manufactured for:
FERRING PHARMACEUTICALS INC.
Tarrytown, NY 10591
By: Taylor Pharmaceuticals
Decatur, IL 6252
Side Effects & Drug Interactions
SIDE EFFECTS
Side effects have been reported in about 50% of the patients tested with Thyrel TRH (protirelin) . Generally, the side effects are minor, have occurred promptly, and have persisted for only a few minutes following injection.
Cardiovascular reactions:
Marked changes in blood pressure, including both hypertension and hypotension with or without syncope, have been reported in a small number of patients.
Endocrine reaction:
Breast enlargement and leakage in lactating women for up to two or three days.
Other reactions:
Headaches, sometimes severe, and transient amaurosis in patients with pituitary tumors. Rarely, convulsions may occur in patients with predisposing conditions, e.g., epilepsy, brain damage. Nausea; urge to urinate; flushed sensation; lightheadedness; bad taste in mouth; abdominal discomfort; and dry mouth. Less frequently reported were: Anxiety; sweating; tightness in the throat; pressure in the chest; tingling sensation; drowsiness; and allergic reactions.
Pituitary apoplexy requiring acute neurosurgical intervention has been reported infrequently for patients with pituitary macroadenomas following the acute administration of protirelin injection in the setting of combined anterior pituitary function testing in conjunction with LHRH and insulin.
DRUG INTERACTIONS
No Information Provided.
Warnings
Transient changes in blood pressure, either increases or decreases, frequently occur immediately following administration of Thyrel TRH (protirelin) . Blood pressure should therefore be measured before Thyrel TRH (protirelin) is administered and at frequent intervals during the first 15 minutes after its administration.
Increases in systolic pressure (usually less than 30 mm Hg) and/or increases in diastolic pressure (usually less than 20 mm Hg) have been observed more frequently than decreases in pressure. These changes have not ordinarily persisted for more than 15 minutes nor have they required therapy. More severe degrees of hypertension or hypotension with or without syncope have been reported in a few patients. To minimize the incidence and/or severity of hypotension, the patient should be supine before, during, and after Thyrel TRH (protirelin) administration. If a clinically important change in blood pressure occurs, monitoring of blood pressure should be continued until it returns to baseline levels.
Thyrel TRH (protirelin) should not be administered to patients in whom marked, rapid changes in blood pressure would be dangerous unless the potential benefit clearly outweighs the potential risk.
Precautions
Thyroid hormones reduce the TSH response to Thyrel® TRH (protirelin) . Accordingly, patients in whom Thyrel TRH (protirelin) is to be used diagnostically should be taken off liothyronine (T3) approximately seven days prior to testing and should be taken off thyroid medications containing levothyroxine (T4), e.g., desiccated thyroid, thyroglobulin, or liotrix, at least 14 days before testing. Hormone therapy is NOT to be discontinued when the test is used to evaluate the effectiveness of thyroid suppression with a particular dose of T4 in patients with nodular or diffuse goiter, or for adjustment of thyroid hormone dosage given to patients with primary hypothyroidism.
Chronic administration of levodopa has been reported to inhibit the TSH response to Thyrel TRH (protirelin) .
It is not advisable to withdraw maintenance doses of adrenocortical drugs used in the therapy of known hypopituitarism. Several published reports have shown that prolonged treatment with glucocorticoids at physiologic doses has no significant effect on the TSH response to thyrotropin releasing hormone, but that the administration of pharmacologic doses of steroids reduces the TSH response. Therapeutic doses of acetylsalicylic acid (2 to 3.6 g/day) have been reported to inhibit the TSH response to protirelin. The ingestion of acetylsalicylic acid caused the peak level of TSH to decrease approximately 30% as compared to values obtained without acetylsalicylic acid administration. In both cases, the TSH peak occurred 30 minutes post-administration of protirelin.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term animal studies have not been performed to evaluate the carcinogenic potential of protirelin. Studies to determine potential effects concerning mutagenesis or impairment of fertility have also not been performed.
Pregnancy (Category C)
Protirelin has been shown to increase the number of resorptions in rabbits, but not in rats, when given in doses 1 ½ and 6 times the human dose. There are no adequate and well-controlled studies in pregnant women. Thyrel TRH (protirelin) should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when Thyrel® TRH (protirelin) is administered to a nursing woman.
Overdosage & Contraindications
OVERDOSE
No Information Provided.
CONTRAINDICATIONS
Thyrel TRH (protirelin) is contraindicated in patients with a known hypersensitivity to the drug.
Clinical Pharmacology
Pharmacologically, Thyrel TRH (protirelin) increases the release of the thyroid stimulating hormone (TSH) from the anterior pituitary. Prolactin release is also increased. It has recently been observed that approximately 65% of acromegalic patients tested respond with a rise in circulating growth hormone levels; the clinical significance is as yet not clear. Following intravenous administration, the mean plasma half-life of protirelin in normal subjects is approximately five minutes. TSH levels rise rapidly and reach a peak at 20 to 30 minutes. The decline in TSH levels takes place more slowly, approaching baseline levels after approximately three hours.
Medication Guide
PATIENT INFORMATION
No Information Provided.
