Rescula Drug Description: Detailed Datasheet
- Generic Name: unoprostone isopropyl
- Brand Name: Rescula
- Drug Class: Antiglaucoma, Prostaglandin Agonists
side effects drug center rescula (unoprostone isopropyl) drug
Drug Description
Rescula
(unoprostone isopropyl) Ophthalmic Solution
DESCRIPTION
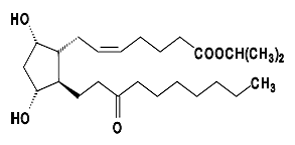
Rescula (unoprostone isopropyl) Ophthalmic Solution 0.15% is a synthetic docosanoid. Unoprostone isopropyl has the chemical name isopropyl (+)-(Z)-7-[(1R,2R,3R,5S)-3,5dihydroxy-2-(3-oxodecyl)cyclopentyl]-5-heptenoate. Its molecular formula is C25H44O5 and its chemical structure is:
 |
Unoprostone isopropyl is a clear, colorless, viscous liquid that is very soluble in acetonitrile, ethanol, ethyl acetate, isopropanol, dioxane, ether, and hexane. It is practically insoluble in water. Rescula (unoprostone isopropyl ophthalmic solution) 0.15% is supplied as a sterile, isotonic, buffered, aqueous solution of unoprostone isopropyl with a pH of 5.0–6.5 and an osmolality of 235–300 mOsmol/kg.
Each mL of Rescula contains 1.5 mg of unoprostone isopropyl. Benzalkonium chloride 0.015% is added as a preservative. Inactive ingredients are mannitol, polysorbate 80, edetate disodium, sodium hydroxide or hydrochloric acid (to adjust pH), and water for injection.
Indications & Dosage
INDICATIONS
Rescula (unoprostone isopropyl) Ophthalmic Solution 0.15% is indicated for the lowering of intraocular pressure in patients with open-angle glaucoma or ocular hypertension.
DOSAGE AND ADMINISTRATION
The recommended dosage is one drop in the affected eye(s) twice daily.
Rescula may be used concomitantly with other topical ophthalmic drug products to lower intraocular pressure. If two drugs are used, they should be administered at least five (5) minutes apart [see PATIENT INFORMATION].
HOW SUPPLIED
Dosage Forms And Strengths
Unoprostone isopropyl ophthalmic solution 1.5 mg/mL.
Storage And Handling
Rescula (unoprostone isopropyl) Ophthalmic Solution 0.15% is supplied sterile in a low-density polyethylene bottle with a low-density polyethylene dropper tip, a turquoise polypropylene closure, and a clear tamper-evident shrinkband.
5 mL in a 7.5 mL bottle NDC 17350-015-05
Storage
Store between 2° - 25°C (36° - 77°F).
Marketed by: Sucampo Pharma Americas, LLC, Bethesda, MD 20814
Side Effects & Drug Interactions
SIDE EFFECTS
Clinical Studies Experience
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly compared to rates in the clinical studies of another drug and may not reflect the rates observed in practice.
In clinical studies, the most common ocular adverse reactions with use of Rescula were burning/stinging, burning/stinging upon drug instillation, dry eyes, itching, increased length of eyelashes, and injection. These were reported in approximately 10–25% of patients. Approximately 10–14% of patients were observed to have an increase in the length of eyelashes ( ≥ 1 mm) at 12 months, while 7% of patients were observed to have a decrease in the length of eyelashes.
Ocular adverse reactions occurring in approximately 5–10% of patients were abnormal vision, eyelid disorder, foreign body sensation, and lacrimation disorder.
Ocular adverse reactions occurring in approximately 1–5% of patients were blepharitis, cataract, conjunctivitis, corneal lesion, discharge from the eye, eye hemorrhage, eye pain, keratitis, irritation, photophobia, and vitreous disorder.
Other ocular adverse reactions reported in less than 1% of patients were acute elevated intraocular pressure, color blindness, corneal deposits, corneal edema, corneal opacity, diplopia, hyperpigmentation of the eyelid, increased number of eyelashes, iris hyperpigmentation, iritis, optic atrophy, ptosis, retinal hemorrhage, and visual field defect.
The most frequently reported nonocular adverse reaction associated with the use of Rescula in the clinical trials was flu-like syndrome that was observed in approximately 6% of patients. Nonocular adverse reactions reported in the 1–5% of patients were accidental injury, allergic reaction, back pain, bronchitis, increased cough, diabetes mellitus, dizziness, headache, hypertension, insomnia, pharyngitis, pain, rhinitis, and sinusitis.
Postmarketing Experience
The following adverse reactions have been identified during post-approval use of Rescula. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Voluntary reports of adverse reactions occurring with the use of Rescula include corneal erosion.
There have been rare spontaneous reports with a different formulation of unoprostone isopropyl (0.12%) of chemosis, dry mouth, nausea, vomiting and palpitations.
DRUG INTERACTIONS
No information provided.
Warnings & Precautions
WARNINGS
Included as part of the PRECAUTIONS section.
PRECAUTIONS
Iris Pigmentation
Unoprostone isopropyl ophthalmic solution may gradually increase the pigmentation of the iris. The pigmentation change is believed to be due to increased melanin content in the melanocytes rather than to an increase in the number of melanocytes. The long term effects of increased pigmentation are not known. Iris color changes seen with administration of unoprostone isopropyl ophthalmic solution may not be noticeable for several months to years. Typically, the brown pigmentation around the pupil spreads concentrically towards the periphery of the iris and the entire iris or parts of the iris become more brownish. Neither nevi nor freckles of the iris appear to be affected by treatment. Treatment with Rescula solution can be continued in patients who develop noticeably increased iris pigmentation.
Patients who receive treatment with Rescula should be informed of the possibility of increased pigmentation [see PATIENT INFORMATION].
Lid Pigmentation
Unoprostone isopropyl has been reported to cause pigment changes (darkening) to periorbital pigmented tissues and eyelashes. The pigmentation is expected to increase as long as unoprostone isopropyl is administered, but has been reported to be reversible upon discontinuation of unoprostone isopropyl ophthalmic solution in most patients.
Intraocular Inflammation
Rescula should be used with caution in patients with active intraocular inflammation (e.g., uveitis) because the inflammation may be exacerbated.
Macular Edema
Macular edema, including cystoid macular edema, has been reported. Rescula should be used with caution in aphakic patients, in pseudophakic patients with a torn posterior lens capsule, or in patients with known risk factors for macular edema.
Contamination of Tip And Solution
To minimize contaminating the dropper tip and solution, care should be taken not to touch the eyelids or surrounding areas with the dropper tip of the bottle. Keep bottle tightly closed when not in use. There have been reports of bacterial keratitis associated with the use of multiple-dose containers of topical ophthalmic products [see PATIENT INFORMATION].
Use with Contact Lenses
Rescula contains benzalkonium chloride, which may be absorbed by soft contact lenses. Contact lenses should be removed prior to application of solution and may be reinserted 15 minutes following its administration [see PATIENT INFORMATION].
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
Unoprostone isopropyl was not carcinogenic in rats administered oral doses up to 12 mg/kg/day for up to 2 years (approximately 580 and 240 fold the recommended human dose of 0.005 mg/kg/day based on AUC0–24 in male and female rats, respectively). Under the conditions tested, unoprostone isopropyl and unoprostone free acid were neither mutagenic in an Ames assay nor clastogenic in a chromosome aberration assay in Chinese hamster lung–derived fibroblast cells. Under the conditions tested, unoprostone isopropyl was not genotoxic in a mouse lymphoma mutation assay or clastogenic in an in vivo chromosomal aberration test in mouse bone marrow. Unoprostone isopropyl did not impair male or female fertility in rats at subcutaneous doses up to 50 mg/kg (approximately 10,000 fold the recommended human dose of 0.005 mg/kg/day).
Use In Specific Populations
Pregnancy
Pregnancy Category C - Teratogenic effects
There were no teratogenic effects observed in rats and rabbits up to 5 and 0.3 mg/kg/day (approximately 1,000 and 60 fold the recommended human dose of 0.005 mg/kg/day in the rat and rabbit, respectively). There was an increase in the incidence of miscarriages and a decrease in live birth index in rats administered unoprostone isopropyl during organogenesis at subcutaneous doses of 5 mg/kg. There was an increase in incidence of miscarriages and resorptions and a decrease in the number of live fetuses in rabbits administered unoprostone isopropyl during organogenesis at subcutaneous doses of 0.3 mg/kg. The no observable adverse effect level (NOAEL) for embryofetal toxicity in rats and rabbits was 2 and 0.1 mg/kg (approximately 400 and 20 fold the recommended human dose of 0.005 mg/kg/day in the rat and rabbit, respectively).
There was an increase in incidence of premature delivery, a decrease in live birth index, and a decrease in weight at birth and through postpartum Day 7 in rats administered unoprostone isopropyl during late gestation through postpartum Day 21 at subcutaneous doses of 1.25 mg/kg. In addition, pups from rats administered 1.25 mg/kg subcutaneously exhibited delayed growth and development characterized by delayed incisor eruption and eye opening. There was an increase in the number of stillborn pups and a decrease in perinatal survival in rats administered unoprostone isopropyl during late gestation through weaning at subcutaneous doses of ≥ 0.5 mg/kg. The NOAEL for pre- and postnatal toxicity in rats was 0.2 mg/kg (approximately 40 fold the recommended human dose of 0.005 mg/kg/day).
There are no adequate and well-controlled studies in pregnant women. Because animal studies are not always predictive of human response, Rescula should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Nursing Mothers
It is not known whether Rescula is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when Rescula is administered to a nursing woman.
Pediatric Use
Safety and effectiveness in pediatric patients have not been established.
Geriatric Use
No overall differences in safety or effectiveness have been observed between elderly and other adult patients.
Overdosage & Contraindications
OVERDOSE
No information provided.
CONTRAINDICATIONS
Rescula is contraindicated in patients with hypersensitivity to unoprostone isopropyl or any other ingredient in this product.
Clinical Pharmacology
Mechanism of Action
Rescula is believed to reduce elevated intraocular pressure (IOP) by increasing the outflow of aqueous humor through the trabecular meshwork. Unoprostone isopropyl (UI) may have a local effect on BK (Big Potassium) channels and ClC-2 chloride channels, but the exact mechanism is unknown at this time.
Pharmacokinetics
Absorption
After application to the eye, unoprostone isopropyl is absorbed through the cornea and conjunctival epithelium where it is hydrolyzed by esterases to unoprostone free acid.
A study conducted with 18 healthy volunteers dosed bilaterally with unoprostone isopropyl ophthalmic solution twice daily for 14 days demonstrated little systemic absorption of unoprostone isopropyl. The systemic exposure of its metabolite unoprostone free acid was minimal following the ocular administration. Mean peak unoprostone free acid concentration was less than 1.5 ng/mL. Little or no accumulation of unoprostone free acid was observed.
Metabolism
Following ocular application, unoprostone isopropyl is hydrolyzed by esterases in the cornea to its biological active metabolite, unoprostone free acid. Unoprostone free acid is further metabolized to several inactive metabolites with lower molecular weight and increased polarity via ε- or β-oxidation. No secondary conjugation is found and no significant effect on hepatic microsomal enzyme activity has been observed.
Elimination
Elimination of unoprostone free acid from human plasma is rapid, with a half-life of 14 minutes. Plasma levels of unoprostone free acid dropped below the lower limit of quantitation ( < 0.25 ng/mL) 1 hour following ocular instillation. The metabolites are excreted predominately in urine.
Clinical Studies
In six (6) month randomized controlled clinical studies in patients with a mean baseline intraocular pressure of 23 mmHg, Rescula lowered intraocular pressure by approximately 3– 4 mmHg throughout the day. Rescula appeared to lower intraocular pressure without affecting cardiovascular or pulmonary function.
Medication Guide
PATIENT INFORMATION
Handling the Bottle
Patients should be instructed that the Rescula bottle must be maintained intact and to avoid allowing the tip of the bottle to contact surrounding structures, fingers, or any other unintended surface in order to avoid contamination of the bottle or applicator by common bacteria known to cause ocular infections. Serious infections may result from using contaminated solutions.
Potential for Iris Darkening
Patients should be advised about the potential for increased brown iris pigmentation which is likely to be permanent.
Potential For Eyelid Skin Darkening
Patients should be informed about the possibility of eyelid skin darkening, which may be reversible after discontinuation of Rescula.
Use with Contact Lenses
Patients should be advised that Rescula contains benzalkonium chloride, which may be absorbed by soft contact lenses. Contact lenses should be removed prior to application of Rescula and may be reinserted 15 minutes following its administration.
Multiple Therapies
If more than one topical ophthalmic therapy is being used patients should be instructed to administer the drugs at least 5 minutes apart.
