Inmazeb Drug Description: Detailed Datasheet
- Generic Name: atoltivimab, maftivimab, and odesivimab-ebgn for injection
- Brand Name: Inmazeb
side effects drug center inmazeb (atoltivimab, maftivimab, and odesivimab-ebgn for injection) drug
Drug Description
What is Inmazeb and how is it used?
Inmazeb (atoltivimab, maftivimab, and odesivimab-ebgn) is a combination of Zaire ebolavirus glycoprotein-directed human monoclonal antibodies used to treat infection caused by Zaire ebolavirus in adult and pediatric patients, including neonates born to a mother who is RT-PCR positive for Zaire ebolavirus infection.
What are side effects of Inmazeb?
Side effects of Inmazeb include:
- fever,
- chills,
- fast heart rate,
- fast, shallow breathing,
- vomiting,
- low blood pressure (hypotension),
- diarrhea, and
- low blood oxygen (hypoxia)
DESCRIPTION
Atoltivimab, maftivimab, and odesivimab-ebgn is a combination of Zaire ebolavirus glycoprotein (GP) directed recombinant human IgG1 human monoclonal antibodies of similar structure. The human monoclonal antibodies, atoltivimab, maftivimab, and odesivimab are produced by recombinant DNA technology in Chinese hamster ovary (CHO) cell suspension culture and have an approximate molecular weight of 145 kDa, 146 kDa and 144 kDa, respectively.
INMAZEB (atoltivimab, maftivimab, and odesivimab-ebgn) injection for intravenous use is a sterile, preservative-free, clear to slightly opalescent, colorless to pale yellow solution, that is free from visible particulates. Each vial contains 241.7 mg of atoltivimab, 241.7 mg of maftivimab, and 241.7 mg of odesivimab in 14.5 mL. Each mL contains 16.67 mg of atoltivimab, 16.67 mg of maftivimab, 16.67 mg of odesivimab, and L-histidine (0.74 mg), Lhistidine monohydrochloride monohydrate (1.09 mg), polysorbate 80 (1 mg), sucrose (100 mg), and Water for Injection, USP with a pH of 6.0.
Indications & Dosage
INDICATIONS
INMAZEB is indicated for the treatment of infection caused by Zaire ebolavirus in adult and pediatric patients, including neonates born to a mother who is RT-PCR positive for Zaire ebolavirus infection [see DOSAGE AND ADMINISTRATION, and Clinical Studies].
Limitations Of Use
The efficacy of INMAZEB has not been established for other species of the Ebolavirus and Marburgvirus genera.
Zaire ebolavirus can change over time, and factors such as emergence of resistance, or changes in viral virulence could diminish the clinical benefit of antiviral drugs. Consider available information on drug susceptibility patterns for circulating Zaire ebolavirus strains when deciding whether to use INMAZEB.
DOSAGE AND ADMINISTRATION
Recommended Dosage
INMAZEB is a combination of three human monoclonal antibodies co-formulated in a 1:1:1 ratio of atoltivimab, maftivimab, and odesivimab. The recommended dosage of INMAZEB is 50 mg of atoltivimab, 50 mg of maftivimab, and 50 mg of odesivimab per kg diluted and administered as a single intravenous infusion [see DOSAGE AND ADMINISTRATION].
Preparation And Administration
INMAZEB must be prepared and administered under the supervision of a healthcare provider. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. INMAZEB should be clear to slightly opalescent, colorless to pale yellow solution that is free from visible particulates. Discard the vial if the solution is cloudy, discolored or contains particulate matter.
Preparation For Intravenous Infusion
- The recommended dosage is based on 50 mg of atoltivimab, 50 mg of maftivimab, and 50 mg of odesivimab per kg. For example, a patient weighing 50 kg the recommended dosage is 2500 mg of atoltivimab, 2500 mg of maftivimab, and 2500 mg of odesivimab.
- Determine the number of vials needed based on the calculated dose in volume (mL). Refer to Table 1 to determine the calculated dose based on volume (mL) of INMAZEB per patient’s weight (kg). Multiple INMAZEB vials may be needed. Each vial contains 14.5 mL of INMAZEB solution. For example, for a 50 kg patient, the volume of INMAZEB needed is 150 mL (11 vials).
- Do not shake the vial.
Prior to intravenous infusion, INMAZEB must be further diluted in an intravenous PVC infusion bag containing either 0.9% Sodium Chloride Injection, USP, 5% Dextrose Injection, USP, or Lactated Ringer’s Injection, USP. For neonates, the INMAZEB solution should be diluted in 5% Dextrose Injection, USP (see Table 1). The total volume of the infusion VV-LAB-000979 v0.16 Internal Approved solution to be administered is based on the patient’s body weight and is specified in Table 1.
Select a diluent solution infusion bag of appropriate fill volume based on the patient’s body weight (see Table 1). Withdraw and discard from the bag a volume of diluent solution equal to the calculated dose in volume (mL) of INMAZEB. Then add the calculated volume of INMAZEB to the bag.
For example, for a 50 kg patient withdraw and discard 150 mL of diluent from a 500 mL infusion bag. Then add 150 mL of INMAZEB to obtain a total infusion volume of 500 mL.
Table 1: INMAZEB Infusion Volumes and Times by Body Weight
| Body Weight (kg) | Volume of INMAZEB per kg of Body Weighta | Total Infusion Volume After Dilution (mL)b | Infusion Time |
| 0.5 to less than 1 | 3 mL per kg of body weight | 7 | 4 hours |
| 1 to 1.9 | 15 | ||
| 2 to 3.9 | 25 | 3 hours | |
| 4 to 7 | 50 | ||
| 8 to 15 | 100 | ||
| 16 to 38 | 250 | 2 hours | |
| 39 to 79 | 500 | ||
| 80 to 149 | 1,000 | ||
| 150 and above | 2,000 | 4 hours | |
| a The dose is 50 mg of atolivimab, 50 mg of maftivimab, and 50 mg of odesivimab per kg of body weight (a volume of 3 mL/kg). b The recommended infusion volume ensures the final concentration of the diluted solution is 9.5 mg/mL to 23.7 mg/mL. 5% Dextrose Injection, USP is recommended for neonates. | |||
- Mix the diluted solution by gentle inversion. Do not shake.
- INMAZEB does not contain preservatives. It is always recommended to administer intravenous medication immediately after preparation when possible. Store the diluted INMAZEB solution as specified in Table 2.
- Do not freeze the diluted solution.
- Discard any unused medicinal product or waste material.
Table 2: Diluted INMAZEB Solution Storage Conditions
| Diluent Used to Prepare Solution for Infusion | Diluted INMAZEB Solution Storage Conditions |
| 0.9% Sodium Chloride Injection, USP | Store at room temperature up to 25°C (77°F) for no more than 8 hours or refrigerated at 2°C to 8°C (36°F to 46°F) for no more than 24 hours. |
| 5% Dextrose Injection, USP or Lactated Ringer’s Injection, USP | Store at room temperature up to 25°C (77°F) for no more than 4 hours or refrigerated at 2°C to 8°C (36°F to 46°F) for no more than 4 hours. |
Administration
- INMAZEB must be administered by a healthcare provider.
- Allow the diluted infusion solution to come to room temperature prior to administration.
- Administer the diluted infusion solution intravenously through an intravenous line containing a sterile, in-line or add-on 0.2-micron filter.
- The infusion rate is based on the patient’s body weight and prepared infusion volume. Select an appropriate infusion rate for the diluted infusion solution (see Table 1). It is important to follow the infusion time outlined in Table 1 based on the patient’s weight.
- The rate of infusion of INMAZEB may be slowed or interrupted if the patient develops any signs of infusion-associated events or other adverse events [see WARNINGS AND PRECAUTIONS].
- Do not mix other medications with INMAZEB.
- Compatibility studies of INMAZEB have not been performed when co-administering other drugs simultaneously through the same infusion line.
HOW SUPPLIED
Dosage Forms And Strengths
INMAZEB is a clear to slightly opalescent and colorless to pale yellow solution available as:
Injection: 241.7 mg of atoltivimab, 241.7 mg of maftivimab, and 241.7 mg of odesivimab per 14.5 mL (16.67 mg/16.67 mg/16.67 mg per mL) in a single-dose vial.
Storage And Handling
INMAZEB (atoltivimab, maftivimab, and odesivimab-ebgn) injection is a clear to slightly opalescent and colorless to pale yellow solution. It is supplied in a carton containing one single dose vial of:
241.7 mg of atoltivimab, 241.7 mg of maftivimab, and 241.7 mg of odesivimab per 14.5 mL (16.67 mg/16.67 mg/16.67 mg per mL) (NDC 61755-018-01)
Prior To Dilution
Store INMAZEB vial refrigerated at 2°C to 8°C (36°F to 46°F) in the original carton to protect from light. Do not freeze or shake.
After Dilution
INMAZEB does not contain preservatives. It is always recommended to administer intravenous medication immediately after preparation when possible. Store the diluted INMAZEB solution as specified according to Table 8 below. Do not freeze the diluted solution [see DOSAGE AND ADMINISTRATION].
Table 8: Diluted INMAZEB Solution Storage Conditions
| Diluent Used to Prepare Solution for Infusion | Diluted INMAZEB Solution Storage Conditions |
| 0.9% Sodium Chloride Injection, USP | Store at room temperature up to 25°C (77°F) for no more than 8 hours or refrigerated at 2°C to 8°C (36°F to 46°F) for no more than 24 hours. |
| 5% Dextrose Injection, USP or Lactated Ringer's Injection, USP | Store at room temperature up to 25°C (77°F) for no more than 4 hours or refrigerated or at 2°C to 8°C (36°F to 46°F) for no more than 4 hours. |
Manufactured by: Regeneron Pharmaceuticals, Inc., 777 Old Saw Mill River Road, Tarrytown, NY 10591-6707, U.S. License No. 1760. Revised: Oct 2020
Side Effects & Drug Interactions
SIDE EFFECTS
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Hypersensitivity Reactions Including Infusion-Associated Events [see WARNINGS AND PRECAUTIONS]
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates may not reflect the rates observed in practice.
Overall, 382 adult and pediatric subjects with Zaire ebolavirus infection received INMAZEB in one clinical trial (the PALM trial) and as part of an expanded access program conducted in the Democratic Republic of Congo during a Zaire ebolavirus outbreak in 2018-2019. In the PALM trial, the safety of INMAZEB was evaluated in a multi-center, open-label, randomized controlled trial, in which 154 subjects (115 adult subjects and 39 pediatric subjects) received INMAZEB [50 mg of atoltivimab, 50 mg of maftivimab, and 50 mg of odesivimab per kg (3 mL/kg)] intravenously as a single infusion and 168 subjects received an investigational control [see Clinical Studies]. All subjects received optimized standard of care treatment. During the same outbreak, INMAZEB [50 mg of atoltivimab, 50 mg of maftivimab, and 50 mg of odesivimab per kg (3 mL/kg)] was given to 228 subjects (190 adult subjects and 38 pediatric subjects) in the expanded access program.
The safety data described below is derived from the PALM trial.
Table 3 summarizes adverse events that were reported during INMAZEB infusion. The evaluation of adverse events in subjects who received INMAZEB may have been confounded by the signs and symptoms of the underlying Zaire ebolavirus infection. The most common adverse events reported in at least 20% of subjects who received INMAZEB were pyrexia (or elevation in fever), chills, tachycardia, tachypnea, and vomiting. The adverse event profile in adult and pediatric subjects treated with INMAZEB was similar.
Table 3: Adverse Events That Occurred during INMAZEB Infusion in ≥10% of Adult and Pediatric Subjects in the PALM Trial
| Adverse Eventa | INMAZEB (N=154) % | Controlc (N=168) % |
| Pyrexia (Elevation in fever) | 54 | 58 |
| Chills | 39 | 33 |
| Tachycardia | 20 | 32 |
| Tachypnea | 19 | 28 |
| Vomitingb | 19 | 23 |
| Hypotension | 15 | 31 |
| Diarrheab | 11 | 18 |
| Hypoxiab | 10 | 11 |
| a Adverse events in this table were reported as preferred terms from a list of pre-defined or other adverse events that occurred on the day of infusion, and included signs and symptoms that occurred during or immediately after infusion b Adverse events that were not pre-specified c Investigational therapy administered as three separate infusions | ||
The following pre-specified symptoms, which were assessed on a daily basis while admitted to the treatment unit, were reported in 40% or more of subjects who received INMAZEB: diarrhea, pyrexia, and vomiting. Evaluation of these symptoms may have been confounded by the underlying Zaire ebolavirus infection.
Discontinuation And Infusion Rate Adjustments In The PALM Trial
Approximately 99% of subjects who received INMAZEB in the PALM trial were able to complete their dose within three hours. Two subjects who received INMAZEB (1%) did not receive their complete infusion. One of the two subjects did not complete their INMAZEB infusion because of fever elevation [see WARNINGS AND PRECAUTIONS].
Selected Laboratory Abnormalities In The PALM Trial
Table 4 presents selected laboratory abnormalities (worsening to Grade 3 or 4 compared to baseline) for adult and pediatric subjects in the PALM trial.
Table 4: Selected Grade 3 and 4 Laboratory Abnormalities, Worsened Grade from Baseline for Adult and Pediatric Subjects in the PALM Trial
| Laboratory Testa | INMAZEB N=154 % | Control N=168 % |
| Sodium, high ≥ 154 mmol/L | 9 | 4 |
| Sodium, low < 125 mmol/L | 7 | 11 |
| Potassium, high ≥ 6.5 mmol/L | 13 | 12 |
| Potassium, low < 2.5 mmol/L | 9 | 8 |
| Creatinine (mg/dL) ≥1.8 x ULNb | 15 | 23 |
| Alanine aminotransferase (U/L) ≥5 x ULN | 10 | 14 |
| Aspartate aminotransferase (U/L) ≥ 5 x ULN | 21 | 18 |
| ULN = upper limit of normal a Graded per Division of AIDS (DAIDS) v2.1 b ULN for creatinine was 1.2 mg/dL. Criterion for increase to ≥ 1.5 x from baseline was applied if the worsening grade was higher. | ||
Immunogenicity
As with all therapeutic proteins, there is potential for immunogenicity. The detection of antibody formation is highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of antibody (including neutralizing antibody) positivity in an assay may be influenced by several factors including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of the incidence of antibodies in the study described below with the incidence of antibodies in other studies or to other atoltivimab, maftivimab, and odesivimab products may be misleading.
The development of anti-atoltivimab, anti-maftivimab, and anti-odesivimab antibodies was evaluated in 24 healthy adults in a single dose, randomized, double-blind, placebo-controlled, dose escalation study. Immunogenic responses against atoltivimab, maftivimab, and odesivimab were not detected at baseline or through 168 days post-dose in any subjects.
DRUG INTERACTIONS
Vaccine Interactions
No vaccine-therapeutic interaction studies have been performed in human subjects using INMAZEB. However, because of the potential for INMAZEB to inhibit replication of a live vaccine virus indicated for prevention of Zaire ebolavirus infection and possibly reduce the efficacy of the vaccine, avoid the concurrent administration of a live vaccine during treatment with INMAZEB. The interval between live vaccination following initiation of INMAZEB therapy should be in accordance with current vaccination guidelines. The efficacy of INMAZEB among subjects who reported receipt of a recombinant live vaccine prior to their enrollment in the PALM clinical trial was similar to subjects who did not receive a vaccine.
Warnings & Precautions
WARNINGS
Included as part of the PRECAUTIONS section.
PRECAUTIONS
Hypersensitivity Reactions Including Infusion-Associated Events
Hypersensitivity reactions including infusion-associated events have been reported during and post-infusion with INMAZEB. These may include acute, life-threatening reactions during and after the infusion. Monitor all patients for signs and symptoms including, but not limited to, hypotension, chills and elevation of fever, during and following INMAZEB infusion. In the case of severe or life-threatening hypersensitivity reactions, discontinue the administration of INMAZEB immediately and administer appropriate emergency care [see ADVERSE REACTIONS].
Infusion could not be completed in 1% of subjects who received INMAZEB due to infusionassociated adverse events. The rate of infusion of INMAZEB may be slowed or interrupted if the patient develops any signs of infusion-associated events or other adverse events [see ADVERSE REACTIONS].
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment Of Fertility
Carcinogenicity, genotoxicity, and fertility studies have not been conducted with INMAZEB.
Use In Specific Populations
Pregnancy
Risk Summary
Zaire ebolavirus infection is life-threatening for both the mother and fetus and treatment should not be withheld due to pregnancy (see Clinical Considerations). Available data from the PALM trial and an expanded access program in which pregnant women with Zaire ebolavirus infection were treated with INMAZEB demonstrate the high rate of maternal and fetal/neonatal morbidity consistent with published literature regarding the risk associated with underlying maternal Zaire ebolavirus infection. These data are insufficient to evaluate for a drug-associated risk of major birth defects, miscarriage, or adverse maternal/fetal outcome. Animal reproduction studies with INMAZEB have not been conducted. Human monoclonal antibodies, such as INMAZEB, are transported across the placenta; therefore, INMAZEB has the potential to be transferred from the mother to the developing fetus.
Clinical Considerations
Disease-Associated Maternal And/Or Embryo/Fetal Risk
Maternal, fetal and neonatal outcomes are poor among pregnant women infected with Zaire ebolavirus. The majority of such pregnancies result in maternal death with miscarriage, stillbirth, or neonatal death. Treatment should not be withheld due to pregnancy.
Lactation
Risk Summary
The Centers for Disease Control and Prevention recommend that patients with confirmed Zaire ebolavirus not breastfeed their infants to reduce the risk of postnatal transmission of Zaire ebolavirus infection.
There are no data on the presence of atoltivimab, maftivimab, and odesivimab-ebgn in human or animal milk, the effects on the breastfed infant, or the effects on milk production. Maternal IgG is known to be present in human milk. The effects of local gastrointestinal exposure and limited systemic exposure in the breastfed infant to atoltivimab, maftivimab, or odesivimab-ebgn are unknown.
Pediatric Use
The safety and effectiveness of INMAZEB for the treatment of infection caused by Zaire ebolavirus have been established in pediatric patients from birth to less than 18 years of age. Use of INMAZEB for this indication is supported by evidence from a multi-center, open label, randomized controlled trial of INMAZEB in adults and pediatric subjects that included 39 pediatric subjects birth to less than 18 years of age, including neonates born to a mother who is RT-PCR positive for Zaire ebolavirus infection. The 28-day mortality and safety in adult and pediatric subjects treated with INMAZEB were similar [see ADVERSE REACTIONS and Clinical Studies]. An additional 38 pediatric subjects from birth to less than 18 years of age received INMAZEB in an expanded access program.
Geriatric Use
Clinical studies of INMAZEB did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Of the 154 subjects with Zaire ebolavirus infection who received INMAZEB in the randomized controlled trial, 5 (3.2%) were 65 years or older. The limited clinical experience has not identified differences in responses between the elderly and younger subjects.
Overdosage & Contraindications
OVERDOSE
No Information provided
CONTRAINDICATIONS
None.
Clinical Pharmacology
Mechanism Of Action
INMAZEB is an antiviral drug combination of three recombinant human IgG1κ monoclonal antibodies (atoltivimab, maftivimab, and odesivimab) that inhibit Zaire ebolavirus [see Microbiology].
Pharmacodynamics
Atoltivimab, maftivimab, and odesivimab exposure-response relationships and the time course of pharmacodynamic response are unknown.
Pharmacokinetics
No pharmacokinetic data are available in patients with Zaire ebolavirus infection. The pharmacokinetics of atoltivimab, maftivimab, and odesivimab in 18 healthy subjects 21 to 60 years of age are linear and dose-proportional over the range of 1 mg of atoltivimab, 1 mg of maftivimab, and 1 mg of odesivimab per kg to 50 mg of atoltivimab, 50 mg of maftivimab, and 50 mg of odesivimab per kg (0.02 to 1 times the approved recommended dosage) of INMAZEB following a single intravenous (IV) infusion. Pharmacokinetic parameters for the individual antibodies of INMAZEB are provided in Table 5.
Table 5: Pharmacokinetic Parameters of INMAZEB Administered IV in Healthy Subjects
| Atoltivimab 50 mg/kga | Maftivimab 50 mg/kga | Odesivimab 50 mg/kga | |
| Systemic Exposure (n=6) | |||
| Mean (SD) Cmax, mg/L | 1,220 (101) | 1,280 (68.0) | 1,260 (81.2) |
| Mean (SD) AUCmf, mg day/L | 17,100 (4,480) | 18,700 (4,100) | 25,600 (5,040) |
| Distribution | |||
| Mean (SD) Volume of Distribution at Steady State, mL/kg | 58.2 (2.66) | 57.6 (3.89) | 56.0 (3.16) |
| Elimination | |||
| Mean (SD) Elimination Half-Life (days) | 21.2 (3.36) | 22.3 (3.09) | 25.3 (3.86) |
| Mean (SD) Clearance (mL/day/kg) | 3.08 (0.719) | 2.78 (0.558) | 2.02 (0.374) |
| a INMAZEB was administered at a total dose of 50 mg of atoltivimab, 50 mg of maftivimab, and 50 mg of odesivimab per kg in a 1:1:1 ratio. | |||
Specific Populations
The effect of age (< 21 or > 60), renal impairment, or hepatic impairment on the pharmacokinetics of atoltivimab, maftivimab, and odesivimab is unknown.
Microbiology
Mechanism Of Action
INMAZEB is a combination of three recombinant human IgG1κ monoclonal antibodies each targeting the Zaire ebolavirus glycoprotein (GP). Zaire ebolavirus encodes a sole envelope protein, the glycoprotein, which mediates virus attachment and membrane fusion with the host cell membranes. In addition, GP is expressed on the surface of Zaire ebolavirus infected host cells making it a target for antibodies that can mediate killing of these cells by antibody dependent cellular cytotoxicity and/or other effector functions. The 3 antibodies that make up the combination can bind the GP simultaneously. The mean KD values for atoltivimab, odesivimab, and maftivimab were 7.84 nM, 8.26 nM, and 3.34 nM, respectively, as determined by surface plasmon resonance. Maftivimab is a neutralizing antibody that blocks entry of the virus into susceptible cells. Odesivimab is a non-neutralizing antibody that induces antibody-dependent effector function through FcyRIIIa signaling when bound to its target. Odesivimab also binds to the soluble form of Zaire ebolavirus glycoprotein (sGP). Atoltivimab combines both neutralization and FcyRIIIa signaling activities.
Antiviral Activity
In a live virus infection assay on Vero cells, maftivimab neutralized Mayinga, Kikwit, and Makona strains of Zaire ebolavirus, with a concentration between 0.2 and 1.2 nM (0.03 and 0.18 μg/mL) providing 80% inhibition of viral infection in a plaque-reduction neutralization test (PRNT-80). Atoltivimab and odesivimab did not demonstrate any neutralizing activity in this assay. Effector function activity of INMAZEB individual antibodies was assessed with an EBOV Makona-GP expressing cell line and Jurkat/NFAT-Luc/FcγRIIIa reporter effector cells. The EC50 values of atoltivimab and odesivimab were 2.9 nM and 1.6 nM, respectively, whereas maftivimab did not exhibit any FcγRIIIa signaling activity at the maximum concentration tested, 40 nM.
Treatment of Zaire ebolavirus infected rhesus macaques with a single intravenous dose of INMAZEB (50 mg of atolivimab, 50 mg of maftivimab, and 50 mg of odesivimab per kg) generally protected infected animals from Zaire ebolavirus mediated death when drug was administered 5 days post-infection.
Resistance
No clinical data are available on the development of EBOV resistance to INMAZEB. The cell culture development of EBOV resistance to INMAZEB has not been assessed to date. A GP_E280G amino acid substitution identified by routine surveillance in the Democratic Republic of the Congo resulted in a loss of neutralization activity of at least 134-fold mediated by the single human monoclonal antibody atoltivimab in a lentivirus-based pseudovirus system. A GP_E564K substitution identified in an infected NHP PK study resulted in a loss of neutralization activity of at least 215-fold mediated by the single human monoclonal antibody maftivimab in a lentivirus-based pseudovirus system. The clinical significance of these substitutions is unknown.
Immune Response
Interaction studies with recombinant live EBOV vaccines and INMAZEB have not been conducted [see DRUG INTERACTIONS].
Clinical Studies
The efficacy of INMAZEB was evaluated in PALM, a multi-center, open-label, randomized controlled trial sponsored by the National Institute of Allergy and Infectious Diseases (NIAID; NCT03719586). The trial was conducted in the Democratic Republic of Congo, where an outbreak began in August 2018, and enrolled 681 subjects of all ages, including pregnant women, with documented Zaire ebolavirus infection and symptoms of any duration who were receiving optimized standard of care (oSOC). Subjects were randomized to receive INMAZEB (50 mg of atoltivimab, 50 mg of maftivimab, and 50 mg of odesivimab per kg) intravenously as a single infusion, an investigational control 50 mg/kg intravenously every third day, for a total of 3 doses, or other investigational drugs. Eligible subjects had a positive reverse transcriptasepolymerase chain reaction (RT-PCR) for the nucleoprotein (NP) gene of Zaire ebolavirus and had not received other investigational treatments (with the exception of experimental vaccines) within the previous 30 days. Neonates ≤7 days of age were eligible if the mother had documented infection. Neonates born to a mother who had cleared Zaire ebolavirus following a course of her assigned investigational medication were also eligible to be enrolled at investigator discretion regarding the likelihood that the neonate was infected. Randomization was stratified by reverse transcription-PCR cycle threshold calculated using NP targets (CtNP ≤22.0 vs <22.0; corresponding to high and low viral load, respectively) and Ebola Treatment Unit (ETU) site. All subjects received oSOC consisting of a minimum of intravenous fluids, daily clinical laboratory testing, correction of hypoglycemia and electrolyte imbalances, and broad-spectrum antibiotics and antimalarials, as indicated .
The primary efficacy endpoint was 28-day mortality. The primary analysis population includes all subjects who were randomized and concurrently eligible to receive either INMAZEB or the investigational control during the same time period of the trial.
The demographics and baseline characteristics are provided in Table 6 below.
Table 6: Demographics and Baseline Characteristics in PALM Trial
| Parameter | INMAZEB (N=154) | Control (N=153) |
| Mean age (years) | 28 | 31 |
| Age <1 month (%) | 1 (1%) | 2 (1%) |
| Age 1 month to <1 year (%) | 4 (3%) | 1 (1%) |
| Age 1 year to < 6 years (%) | 18 (12%) | 13 (8%) |
| Age 6 years to <12 years (%) | 8 (5%) | 4 (3%) |
| Age 12 years to <18 years (%) | 8 (5%) | 8 (5%) |
| Age 18 years to <50 years (%) | 93 (60%) | 105 (69%) |
| Age 50 years to <65 years (%) | 17 (11%) | 18 (12%) |
| Age ≥65 years (%) | 5 (3%) | 2 (1%) |
| Female (%) | 90 (58%) | 80 (52%) |
| Positive result on pregnancy testa, n (%) | 2/67 (3%) | 4/61 (7%) |
| RT-PCR CtNP cycle threshold ≤22, n | 66 | 64 |
| Median RT-PCR CtNP (IQR) | 22.7 (20.1, 28.1) | 22.9 (18.8, 26.4) |
| Median creatinine (IQR) | 1.0 (0.7, 4.0) | 1.1 (0.7, 3.2) |
| Median AST (IQR) | 225.5 (98.0, 941.0) | 351.0 (109, 1404.0) |
| Median ALT (IQR) | 165.0 (56.0, 418.0) | 223.5 (47.0, 564.0) |
| Median days from onset of symptoms to randomization (IQR) | 5.0 (3.0, 7.0) | 5.0 (3.0, 7.0) |
| Reported Vaccination with rVSV-ZEBOV vaccine, n (%) | 34 (22%) | 41 (27%) |
| <10 days before ETU admission | 20/34 (59%) | 21/41 (51%) |
| ≥10 days before ETU admission | 14/34 (41%) | 18/41 (44%) |
| Timing unknown | 0/34 (0%) | 2/41 (5%) |
| a Pregnancy positive test was calculated based on subjects who had pregnancy test result. CtNP = cycle threshold calculated using NP targets; IQR = interquartile range; AST=Aspartate aminotransferase; ALT=Alanine aminotransferase; ETU=Ebola treatment unit | ||
The PALM trial was stopped early on the basis of a pre-specified interim analysis showing a statistically significant reduction in mortality for INMAZEB compared to control.
Mortality efficacy results are shown in Table 7.
Table 7: Mortality Rates in PALM Trial
| Efficacy Endpoints | INMAZEBa (N=154) | Controla (N=153) |
| Overall | ||
| 28-day mortality, n (%) | 52 (34%) | 78 (51%) |
| Mortality rate difference relative to control (95% CI) | -17.2 (-28.4, -2.6) | |
| p-Valueb | 0.0024 | |
| Baseline Viral Load | ||
| High viral load (CtNP ≤ 22)c | n=66 | n=64 |
| 28-day mortality, n (%) | 42 (64%) | 56 (88%) |
| Mortality rate difference relative to control (95% CI) | -23.9 (-43.8, -6.4) | |
| Low viral load (CtNP > 22)c | n=88 | n=88 |
| 28-day mortality, n (%) | 10 (11%) | 22 (25%) |
| Mortality rate difference relative to control (95% CI) | -13.6 (-31.8, -1.4) | |
| Age group | ||
| Adults (age ≥18 years) | 39/115 (34%) | 67/125 (54%) |
| 12 to < 18 years of age | 2/8 (25%) | 4/8 (50%) |
| 6 to < 12 years of age | 1/8 (13%) | 1/4 (25%) |
| < 6 years of age | 10/23 (43%) | 6/16 (38%) |
| Sex | ||
| Male | 21/64 (33%) | 31/73 (42%) |
| Female | 31/90 (34%) | 47/80 (59%) |
| a Both INMAZEB and Control were administered with optimized standard of care b The result is significant according to the interim stopping boundary, p<0.028 c Cepheid GeneXpert Ebola® Assay used for detection of Zaire ebolavirus RNA | ||
Figure 1: Kaplan-Meier Curve for Overall Mortality
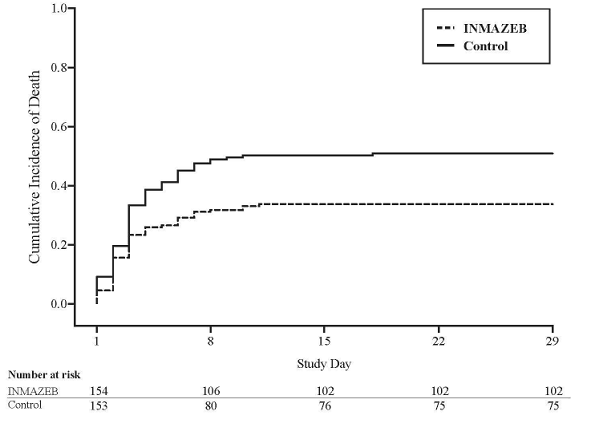 |
Medication Guide
PATIENT INFORMATION
Hypersensitivity Reactions Including Infusion-Associated Events
Inform patients that hypersensitivity reactions including infusion-associated events have been reported during and post-infusion with INMAZEB and to immediately report if they experience any symptoms of systemic hypersensitivity reactions [see WARNINGS AND PRECAUTIONS].
Lactation
Instruct patients with Zaire ebolavirus infection not to breastfeed because of the risk of passing Zaire ebolavirus to the baby [see Use In Specific Populations].
