Braftovi Drug Description: Detailed Datasheet
- Generic Name: encorafenib capsules
- Brand Name: Braftovi
- Drug Class: Antineoplastics BRAF Kinase Inhibitor
side effects drug center braftovi (encorafenib capsules) drug
Drug Description
What is Braftovi and how is it used?
Braftovi is a prescription medicine used to treat the symptoms of Melanoma and Metastatic Colorectal Cancer. Braftovi may be used alone or with other medications.
Braftovi belongs to a class of drugs called Antineoplastics, BRAF Kinase Inhibitor.
It is not known if Braftovi is safe and effective in children.
What are the possible side effects of Braftovi?
Braftovi may cause serious side effects including:
- hives,
- difficulty breathing,
- swelling of your face, lips, tongue, or throat,
- eye pain,
- swelling of the eye,
- vision changes,
- seeing halos around lights,
- seeing color “dots” in your vision,
- severe skin rash,
- skin pain or swelling,
- redness and peeling skin on your hands or feet,
- fast or pounding heartbeats,
- fluttering in your chest,
- shortness of breath,
- sudden dizziness,
- weakness,
- dizziness,
- headache,
- nosebleeds,
- rectal bleeding,
- bloody or tarry stools,
- coughing up blood, and
- vomit that looks like coffee grounds
Get medical help right away, if you have any of the symptoms listed above.
The most common side effects of Braftovi include:
- nausea,
- vomiting,
- stomach pain,
- tiredness, and
- joint pain or swelling
Tell the doctor if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of Braftovi. For more information, ask your doctor or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
DESCRIPTION
Encorafenib is a kinase inhibitor. The chemical name is methyl N-{(2S)-1-[(4-{3-[5-chloro-2-fluoro-3( methanesulfonamido)phenyl]-1-(propan-2-yl)-1H-pyrazol-4-yl}pyrimidin-2-yl)amino]propan-2yl} carbamate. The molecular formula is C22H27ClFN7O4S and the molecular weight is 540 daltons. The chemical structure of encorafenib is shown below:
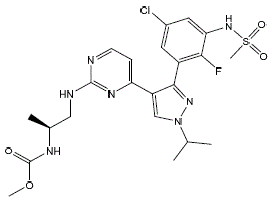 |
Encorafenib is a white to almost white powder. In aqueous media, encorafenib is slightly soluble at pH 1, very slightly soluble at pH 2, and insoluble at pH 3 and higher.
BRAFTOVI (encorafenib) capsules for oral use contain 50 mg or 75 mg of encorafenib with the following inactive ingredients: copovidone, poloxamer 188, microcrystalline cellulose, succinic acid, crospovidone, colloidal silicon dioxide, magnesium stearate (vegetable origin). The capsule shell contains gelatin, titanium dioxide, iron oxide red, iron oxide yellow, ferrosoferric oxide, monogramming ink (pharmaceutical glaze, ferrosoferric oxide, propylene glycol).
Indications & Dosage
INDICATIONS
BRAF V600E Or V600K Mutation-Positive Unresectable Or Metastatic Melanoma
BRAFTOVI® is indicated, in combination with binimetinib, for the treatment of patients with unresectable or metastatic melanoma with a BRAF V600E or V600K mutation, as detected by an FDA-approved test [see DOSAGE AND ADMINISTRATION].
BRAF V600E Mutation-Positive Metastatic Colorectal Cancer (CRC)
BRAFTOVI® is indicated, in combination with cetuximab, for the treatment of adult patients with metastatic colorectal cancer (CRC) with a BRAF V600E mutation, as detected by an FDA-approved test, after prior therapy [see DOSAGE AND ADMINISTRATION].
Limitations Of Use
BRAFTOVI is not indicated for treatment of patients with wild-type BRAF melanoma or wild-type BRAF CRC [see WARNINGS AND PRECAUTIONS].
DOSAGE AND ADMINISTRATION
Patient Selection
BRAF V600E Or V600K Mutation-Positive Unresectable Or Metastatic Melanoma
Confirm the presence of a BRAF V600E or V600K mutation in tumor specimens prior to initiating BRAFTOVI [see WARNINGS AND PRECAUTIONS, Clinical Studies]. Information on FDA-approved tests for the detection of BRAF V600E and V600K mutations in melanoma is available at: http://www.fda.gov/CompanionDiagnostics.
BRAF V600E Mutation-Positive Metastatic Colorectal Cancer (CRC)
Confirm the presence of a BRAF V600E mutation in tumor specimens prior to initiating BRAFTOVI [see WARNINGS AND PRECAUTIONS, Clinical Studies]. Information on FDA-approved tests for the detection of BRAF V600E mutations in CRC is available at: http://www.fda.gov/CompanionDiagnostics.
Recommended Dosage For BRAF V600E Or V600K Mutation-Positive Unresectable Or Metastatic Melanoma
The recommended dosage of BRAFTOVI is 450 mg (six 75 mg capsules) orally once daily in combination with binimetinib until disease progression or unacceptable toxicity. Refer to the binimetinib prescribing information for recommended binimetinib dosing information.
Recommended Dosage For BRAF V600E Mutation-Positive Metastatic Colorectal Cancer (CRC)
The recommended dosage of BRAFTOVI is 300 mg (four 75 mg capsules) orally once daily in combination with cetuximab until disease progression or unacceptable toxicity. Refer to the cetuximab prescribing information for recommended cetuximab dosing information.
Administration
BRAFTOVI may be taken with or without food [see CLINICAL PHARMACOLOGY]. Do not take a missed dose of BRAFTOVI within 12 hours of the next dose of BRAFTOVI.
Do not take an additional dose if vomiting occurs after BRAFTOVI administration but continue with the next scheduled dose.
Dosage Modifications For Adverse Reactions
BRAF V600E Or V600K Mutation-Positive Unresectable Or Metastatic Melanoma
If binimetinib is withheld, reduce BRAFTOVI to a maximum dose of 300 mg (four 75 mg capsules) once daily until binimetinib is resumed [see WARNINGS AND PRECAUTIONS].
Dose reductions for adverse reactions associated with BRAFTOVI are presented in Table 1.
Table 1: Recommended Dose Reductions for BRAFTOVI for Adverse Reactions – Melanoma
| Action | Recommended Dose |
| First Dose Reduction | 300 mg (four 75 mg capsules) orally once daily |
| Second Dose Reduction | 225 mg (three 75 mg capsules) orally once daily |
| Subsequent Modification | Permanently discontinue if unable to tolerate BRAFTOVI 225 mg (three 75 mg capsules) once daily |
BRAF V600E Mutation-Positive Metastatic Colorectal Cancer (CRC)
If cetuximab is discontinued, discontinue BRAFTOVI.
Dose reductions for adverse reactions associated with BRAFTOVI are presented in Table 2.
Table 2: Recommended Dose Reductions for BRAFTOVI for Adverse Reactions – CRC
| Action | Recommended Dose |
| First Dose Reduction | 225 mg (three 75 mg capsules) orally once daily |
| Second Dose Reduction | 150 mg (two 75 mg capsules) orally once daily |
| Subsequent Modification | Permanently discontinue if unable to tolerate BRAFTOVI 150 mg (two 75 mg capsules) once daily |
BRAF V600E Or V600K Mutation-Positive Unresectable Or Metastatic Melanoma And BRAF V600E Mutation-Positive Metastatic Colorectal Cancer (CRC)
Dosage modifications for adverse reactions associated with BRAFTOVI are presented in Table 3.
Table 3: Recommended Dosage Modifications for BRAFTOVI for Adverse Reactions
| Severity of Adverse Reactiona | Dose Modification for BRAFTOVI |
| New Primary Malignancies [see WARNINGS AND PRECAUTIONS] | |
| Non-Cutaneous RAS Mutation-positive Malignancies | Permanently discontinue BRAFTOVI. |
| Uveitis [see WARNINGS AND PRECAUTIONS] | |
| If Grade 1 or 2 does not respond to specific ocular therapy, or for Grade 3 uveitis, withhold BRAFTOVI for up to 6 weeks.
|
| Permanently discontinue BRAFTOVI. |
| QTc Prolongation [see WARNINGS AND PRECAUTIONS] | |
| Withhold BRAFTOVI until QTcF less than or equal to 500 ms. Resume at reduced dose.
|
| Permanently discontinue BRAFTOVI. |
| Hepatotoxicity | |
| Maintain BRAFTOVI dose.
|
| See Other Adverse Reactions. |
| Dermatologic (other than Hand-foot Skin Reaction [HFSR]) | |
| If no improvement within 2 weeks, withhold BRAFTOVI until Grade 0-1. Resume at same dose. |
| Withhold BRAFTOVI until Grade 0-1. Resume at same dose if first occurrence or reduce dose if recurrent. |
| Permanently discontinue BRAFTOVI. |
| Other Adverse Reactions (including Hemorrhage [see WARNINGS AND PRECAUTIONS] and HFSR)b | |
| Withhold BRAFTOVI for up to 4 weeks.
|
| Permanently discontinue BRAFTOVI or Withhold BRAFTOVI for up to 4 weeks.
|
| Consider permanently discontinuing BRAFTOVI. |
| Permanently discontinue BRAFTOVI. |
| a National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE) version 4.03. b Dose modification of BRAFTOVI when administered with binimetinib or with cetuximab is not recommended for new primary cutaneous malignancies; ocular events other than uveitis, iritis, and iridocyclitis; interstitial lung disease/pneumonitis; cardiac dysfunction; creatine phosphokinase (CPK) elevation; rhabdomyolysis; and venous thromboembolism. | |
Refer to the binimetinib or cetuximab prescribing information for dose modifications for adverse reactions associated with each product, as appropriate.
Dose Modifications For Coadministration With Strong Or Moderate CYP3A4 Inhibitors
Avoid coadministration of BRAFTOVI with strong or moderate CYP3A4 inhibitors. If coadministration is unavoidable, reduce the BRAFTOVI dose according to the recommendations in Table 4. After the inhibitor has been discontinued for 3 to 5 elimination half-lives, resume the BRAFTOVI dose that was taken prior to initiating the CYP3A4 inhibitor [see DRUG INTERACTIONS, CLINICAL PHARMACOLOGY].
Table 4: Recommended Dose Reductions for BRAFTOVI for Coadministration with Strong or Moderate CYP3A4 Inhibitors
| Current Daily Dosea | Dose for Coadministration with Moderate CYP3A4 Inhibitor | Dose for Coadministration with Strong CYP3A4 Inhibitor |
| 450 mg | 225 mg (three 75 mg capsules) | 150 mg (two 75 mg capsules) |
| 300 mg | 150 mg (two 75 mg capsules) | 75 mg |
| 225 mg | 75 mg | 75 mg |
| 150 mg | 75 mg | 75 mgb |
| a Current daily dose refers to recommended dose of BRAFTOVI based on indication or reductions for adverse reactions based on dosing recommendations in Table 1 (Melanoma) and Table 2 (CRC). b Encorafenib exposure at the 75 mg QD BRAFTOVI dosage when coadministered with a strong CYP3A4 inhibitor is expected to be higher than at the 150 mg QD dosage in the absence of a CYP3A4 inhibitor and similar to exposure at the 225 mg QD dosage in the absence of a CYP3A4 inhibitor. Monitor patients closely for adverse reactions and use clinical judgement when using BRAFTOVI with strong CYP3A4 inhibitors at the 150 mg dose level. | ||
HOW SUPPLIED
Dosage Forms And Strengths
Capsules: 75 mg, hard gelatin, stylized “A” on beige cap and “LGX 75mg” on white body.
Storage And Handling
BRAFTOVI (encorafenib) is supplied as 75 mg hard gelatin capsules.
75 mg: stylized “A” on beige cap and “LGX 75mg” on white body, available in cartons (NDC 70255-025-01) containing two bottles of 90 capsules each (NDC 70255-025-02) and cartons (NDC 70255-025-03) containing two bottles of 60 capsules each (NDC 70255-025-04).
Store at 20°C to 25°C (68°F to 77°F); excursions permitted between 15°C and 30°C (59°F and 86°F) [see USP Controlled Room Temperature]. Do not use if safety seal under cap is broken or missing. Dispense in original bottle. Do not remove desiccant. Protect from moisture. Keep container tightly closed.
Distributed by: Array BioPharma Inc., a wholly owned subsidiary of Pfizer Inc., 3200 Walnut Street, Boulder, CO 80301. Revised: Apr 2020
Warnings & Precautions
WARNINGS
Included as part of the PRECAUTIONS section.
PRECAUTIONS
New Primary Malignancies
New primary malignancies, cutaneous and non-cutaneous, have been observed in patients treated with BRAF inhibitors and can occur with BRAFTOVI.
Cutaneous Malignancies
In COLUMBUS, cutaneous squamous cell carcinoma (cuSCC), including keratoacanthoma (KA), occurred in 2.6%, and basal cell carcinoma occurred in 1.6% of patients who received BRAFTOVI in combination with binimetinib. Median time to first occurrence of cuSCC/KA was 5.8 months (range 1 to 9 months) [see ADVERSE REACTIONS].
For patients who received BRAFTOVI as a single agent, cuSCC/KA was reported in 8%, basal cell carcinoma in 1%, and a new primary melanoma in 5% of patients.
In BEACON CRC, cuSCC/KA occurred in 1.4% of patients with CRC, and a new primary melanoma occurred in 1.4% of patients who received BRAFTOVI in combination with cetuximab.
Perform dermatologic evaluations prior to initiating treatment, every 2 months during treatment, and for up to 6 months following discontinuation of treatment. Manage suspicious skin lesions with excision and dermatopathologic evaluation. Dose modification is not recommended for new primary cutaneous malignancies.
Non-Cutaneous Malignancies
Based on its mechanism of action, BRAFTOVI may promote malignancies associated with activation of RAS through mutation or other mechanisms [see WARNINGS AND PRECAUTIONS]. Monitor patients receiving BRAFTOVI for signs and symptoms of non-cutaneous malignancies. Discontinue BRAFTOVI for RAS mutation-positive non-cutaneous malignancies [see DOSAGE AND ADMINISTRATION].
Tumor Promotion In BRAF Wild-Type Tumors
In vitro experiments have demonstrated paradoxical activation of MAP-kinase signaling and increased cell proliferation in BRAF wild-type cells, which are exposed to BRAF inhibitors. Confirm evidence of BRAF V600E or V600K mutation prior to initiating BRAFTOVI [see INDICATIONS AND USAGE, DOSAGE AND ADMINISTRATION].
Hemorrhage
In COLUMBUS, hemorrhage occurred in 19% of patients receiving BRAFTOVI in combination with binimetinib; Grade 3 or greater hemorrhage occurred in 3.2% of patients. The most frequent hemorrhagic events were gastrointestinal, including rectal hemorrhage (4.2%), hematochezia (3.1%), and hemorrhoidal hemorrhage (1%). Fatal intracranial hemorrhage in the setting of new or progressive brain metastases occurred in 1.6% of patients.
In BEACON CRC, hemorrhage occurred in 19% of patients receiving BRAFTOVI in combination with cetuximab; Grade 3 or higher hemorrhage occurred in 1.9% of patients, including fatal gastrointestinal hemorrhage in 0.5% of patients. The most frequent hemorrhagic events were epistaxis (6.9%), hematochezia (2.3%) and rectal hemorrhage (2.3%).
Withhold, reduce dose, or permanently discontinue based on severity of adverse reaction [see DOSAGE AND ADMINISTRATION, ADVERSE REACTIONS].
Uveitis
Uveitis, including iritis and iridocyclitis, has been reported in patients treated with BRAFTOVI in combination with binimetinib. In COLUMBUS, the incidence of uveitis among patients treated with BRAFTOVI in combination with binimetinib was 4%.
Assess for visual symptoms at each visit. Perform an ophthalmologic evaluation at regular intervals and for new or worsening visual disturbances, and to follow new or persistent ophthalmologic findings. Withhold, reduce dose, or permanently discontinue based on severity of adverse reaction [see DOSAGE AND ADMINISTRATION, ADVERSE REACTIONS].
QT Prolongation
BRAFTOVI is associated with dose-dependent QTc interval prolongation in some patients [see CLINICAL PHARMACOLOGY]. In COLUMBUS, an increase in QTcF to > 500 ms was measured in 0.5% (1/192) of patients who received BRAFTOVI in combination with binimetinib.
Monitor patients who already have or who are at significant risk of developing QTc prolongation, including patients with known long QT syndromes, clinically significant bradyarrhythmias, severe or uncontrolled heart failure and those taking other medicinal products associated with QT prolongation. Correct hypokalemia and hypomagnesemia prior to and during BRAFTOVI administration. Withhold, reduce dose, or permanently discontinue for QTc > 500 ms [see DOSAGE AND ADMINISTRATION, ADVERSE REACTIONS].
Embryo-Fetal Toxicity
Based on its mechanism of action, BRAFTOVI can cause fetal harm when administered to a pregnant woman. Encorafenib produced embryo-fetal developmental changes in rats and rabbits and was an abortifacient in rabbits at doses greater than or equal to those resulting in exposures approximately 26 (in the rat) and 178 (in the rabbit) times the human exposure at the recommended dose of 450 mg, with no clear findings at lower doses.
Advise women of the potential risk to a fetus. Advise females of reproductive potential to use an effective, non-hormonal method of contraception since BRAFTOVI can render hormonal contraceptives ineffective, during treatment and for 2 weeks after the final dose of BRAFTOVI [see Use In Specific Populations].
Risks Associated With BRAFTOVI As A Single Agent
BRAFTOVI when used as a single agent is associated with an increased risk of certain adverse reactions compared to when BRAFTOVI is used in combination with binimetinib. In COLUMBUS, Grades 3 or 4 dermatologic reactions occurred in 21% of patients treated with BRAFTOVI single agent compared to 2% of patients treated with BRAFTOVI in combination with binimetinib [see WARNINGS AND PRECAUTIONS, ADVERSE REACTIONS].
If binimetinib is temporarily interrupted or permanently discontinued, reduce the dose of BRAFTOVI as recommended [see DOSAGE AND ADMINISTRATION].
Risks Associated With Combination Treatment
BRAFTOVI is indicated for use as part of a regimen in combination with binimetinib or cetuximab. Refer to the prescribing information for binimetinib and cetuximab for additional risk information.
Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Inform patients of the following:
New Primary Cutaneous Malignancies
Advise patients to contact their healthcare provider immediately for change in or development of new skin lesions [see WARNINGS AND PRECAUTIONS].
Hemorrhage
Advise patients to notify their healthcare provider immediately with any symptoms suggestive of hemorrhage, such as unusual bleeding [see WARNINGS AND PRECAUTIONS].
Uveitis
Advise patients to contact their healthcare provider if they experience any changes in their vision [see WARNINGS AND PRECAUTIONS].
QT Prolongation
Advise patients that BRAFTOVI can cause QTc interval prolongation and to inform their physician if they have any QTc interval prolongation symptoms, such as syncope [see WARNINGS AND PRECAUTIONS].
Embryo-Fetal Toxicity
Advise females with reproductive potential of the potential risk to a fetus. Advise females to contact their healthcare provider if they become pregnant, or if pregnancy is suspected, during treatment with BRAFTOVI [see WARNINGS AND PRECAUTIONS, Use In Specific Populations].
Advise females of reproductive potential to use effective non-hormonal contraception during treatment with BRAFTOVI and for 2 weeks after the final dose [Use In Specific Populations].
Lactation
Advise women not to breastfeed during treatment with BRAFTOVI and for 2 weeks after the final dose [see Use In Specific Populations].
Infertility
Advise males of reproductive potential that BRAFTOVI may impair fertility [see Use In Specific Populations].
Strong Or Moderate CYP3A Inducers Or Inhibitors
Coadministration of BRAFTOVI with a strong or moderate CYP3A inhibitor may increase encorafenib concentrations; while coadministration of BRAFTOVI with a strong or moderate CYP3A inducer may decrease encorafenib concentrations. Advise patients that they need to avoid certain medications while taking BRAFTOVI and to inform their healthcare provider of all concomitant medications, including prescription medicines, over-the-counter drugs, vitamins, and herbal products. Advise patients to avoid grapefruit or grapefruit juice while taking BRAFTOVI [see DRUG INTERACTIONS].
Storage
BRAFTOVI is moisture sensitive. Advise patients to store BRAFTOVI in the original bottle with desiccant and to keep the cap of the bottle tightly closed. Do not remove the desiccants from the bottle.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment Of Fertility
Carcinogenicity studies with encorafenib have not been conducted. Encorafenib was not genotoxic in studies evaluating reverse mutations in bacteria, chromosomal aberrations in mammalian cells, or micronuclei in bone marrow of rats.
No dedicated fertility studies were performed with encorafenib in animals. In a general toxicology study in rats, decreased testes and epididymis weights, tubular degeneration in testes, and oligospermia in epididymides were observed at doses approximately 13 times the human exposure at the 450 mg clinical dose based on AUC. No effects on reproductive organs were observed in either sex in any of the non-human primate toxicity studies.
Use In Specific Populations
Pregnancy
Risk Summary
Based on its mechanism of action, BRAFTOVI can cause fetal harm when administered to a pregnant woman [see CLINICAL PHARMACOLOGY]. There are no available clinical data on the use of BRAFTOVI during pregnancy. In animal reproduction studies, encorafenib produced embryo-fetal developmental changes in rats and rabbits and was an abortifacient in rabbits at doses greater than or equal to those resulting in exposures approximately 26 (in the rat) and 178 (in the rabbit) times the human exposure at the clinical dose of 450 mg, with no clear findings at lower doses (see Data). Advise pregnant women of the potential risk to a fetus.
In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Data
Animal Data
In reproductive toxicity studies, administration of encorafenib to rats during the period of organogenesis resulted in maternal toxicity, decreased fetal weights, and increased incidence of total skeletal variations at a dose of 20 mg/kg/day (approximately 26 times the human exposure based on area under the concentrationtime curve [AUC] at the recommended clinical dose of 450 mg once daily). In pregnant rabbits, administration of encorafenib during the period of organogenesis resulted in maternal toxicity, decreased fetal body weights, increased incidence of total skeletal variations and increased post-implantation loss, including total loss of pregnancy at a dose of 75 mg/kg/day (approximately 178 times the human exposure based on AUC at the recommended clinical dose of 450 mg once daily). While formal placental transfer studies have not been performed, encorafenib exposure in the fetal plasma of both rats and rabbits was up to 1.7% and 0.8%, respectively, of maternal exposure.
Lactation
Risk Summary
There are no data on the presence of encorafenib or its metabolites in human milk or the effects of encorafenib on the breastfed infant, or on milk production. Because of the potential for serious adverse reactions from BRAFTOVI in breastfed infants, advise women not to breastfeed during treatment with BRAFTOVI and for 2 weeks after the final dose.
Females And Males Of Reproductive Potential
Pregnancy Testing
Verify the pregnancy status of females of reproductive potential prior to initiating BRAFTOVI [see Use In Specific Populations].
Contraception
BRAFTOVI can cause fetal harm when administered to a pregnant woman [see Use In Specific Populations].
Females
Advise females of reproductive potential to use effective contraception during treatment with BRAFTOVI and for 2 weeks after the final dose. Counsel patients to use a non-hormonal method of contraception since BRAFTOVI has the potential to render hormonal contraceptives ineffective [see DRUG INTERACTIONS].
Infertility
Males
Based on findings in male rats at doses approximately 13 times the human exposure at the 450 mg clinical dose, use of BRAFTOVI may impact fertility in males [see Nonclinical Toxicology].
Pediatric Use
The safety and effectiveness of BRAFTOVI have not been established in pediatric patients.
Geriatric Use
Of the 690 patients with BRAF mutation-positive melanoma who received BRAFTOVI at doses between 300 mg and 600 mg once daily in combination with binimetinib (45 mg twice daily) across multiple clinical trials, 20% were aged 65 to 74 years and 8% were aged 75 years and older [see Clinical Studies].
Of the 216 patients with BRAF V600E mutation positive metastatic CRC who received BRAFTOVI 300 mg QD in combination with cetuximab, 62 (29%) were 65 years of age to up to 75 years of age, while 20 (9%) were 75 years of age and over [see Clinical Studies].
No overall differences in the safety or effectiveness of BRAFTOVI plus binimetinib or BRAFTOVI plus cetuximab were observed in elderly patients as compared to younger patients.
Hepatic Impairment
No BRAFTOVI dosage adjustment is recommended in patients with mild hepatic impairment (Child-Pugh Class A) [see CLINICAL PHARMACOLOGY]. A recommended dosage has not been established in patients with moderate (Child-Pugh Class B) or severe (Child-Pugh Class C) hepatic impairment.
Renal Impairment
No BRAFTOVI dosage adjustment is recommended in patients with mild to moderate renal impairment (CLcr 30 to < 90 mL/min) [see CLINICAL PHARMACOLOGY]. A recommended dosage has not been established in patients with severe renal impairment (CLcr <30 mL/min).
Overdosage & Contraindications
OVERDOSE
Since encorafenib is 86% bound to plasma proteins, hemodialysis is likely to be ineffective in the treatment of overdose with BRAFTOVI.
CONTRAINDICATIONS
None.
Clinical Pharmacology
Mechanism Of Action
Encorafenib is a kinase inhibitor that targets BRAF V600E, as well as wild-type BRAF and CRAF in in vitro cell-free assays with IC50 values of 0.35, 0.47, and 0.3 nM, respectively. Mutations in the BRAF gene, such as BRAF V600E, can result in constitutively activated BRAF kinases that may stimulate tumor cell growth. Encorafenib was also able to bind to other kinases in vitro including JNK1, JNK2, JNK3, LIMK1, LIMK2, MEK4, and STK36 and substantially reduce ligand binding to these kinases at clinically achievable concentrations (≤ 0.9 μM).
Encorafenib inhibited in vitro growth of tumor cell lines expressing BRAF V600 E, D, and K mutations. In mice implanted with tumor cells expressing BRAF V600E, encorafenib induced tumor regressions associated with RAF/MEK/ERK pathway suppression.
Encorafenib and binimetinib target two different kinases in the RAS/RAF/MEK/ERK pathway. Compared with either drug alone, co-administration of encorafenib and binimetinib resulted in greater anti-proliferative activity in vitro in BRAF mutation-positive cell lines and greater anti-tumor activity with respect to tumor growth inhibition in BRAF V600E mutant human melanoma xenograft studies in mice. Additionally, the combination of encorafenib and binimetinib delayed the emergence of resistance in BRAF V600E mutant human melanoma xenografts in mice compared to either drug alone.
Pharmacodynamics
Cardiac Electrophysiology
A dedicated study to evaluate the QT prolongation potential of BRAFTOVI has not been conducted. BRAFTOVI is associated with dose-dependent QTc interval prolongation. Following administration of the recommended dose of BRAFTOVI in combination with binimetinib, based on a central tendency analysis of QTc in a study of adult patients with melanoma, the largest mean (90% CI) QTcF change from baseline (ΔQTcF) was 18 (14 to 22) ms [see WARNINGS AND PRECAUTIONS].
Pharmacokinetics
The pharmacokinetics of encorafenib were studied in healthy subjects and patients with solid tumors, including advanced and unresectable or metastatic cutaneous melanoma harboring a BRAF V600E or V600K mutation. After a single dose, systemic exposure of encorafenib was dose proportional over the dose range of 50 mg to 700 mg. After once-daily dosing, systemic exposure of encorafenib was less than dose proportional over the dose range of 50 mg to 800 mg. Steady-state was reached within 15 days, with exposure being 50% lower compared to Day 1; intersubject variability (CV%) of AUC ranged from 12% to 69%.
Absorption
After oral administration, the median Tmax of encorafenib is 2 hours. At least 86% of the dose is absorbed.
Effect Of Food
Administration of a single dose of BRAFTOVI 100 mg (0.2 times the recommended dose) with a high-fat, high-calorie meal (comprised of approximately 150 calories from protein, 350 calories from carbohydrates, and 500 calories from fat) decreased the mean maximum encorafenib concentration (Cmax) by 36% with no effect on AUC.
Distribution
Encorafenib is 86% bound to human plasma proteins in vitro. The blood-to-plasma concentration ratio is 0.58. The geometric mean (CV%) of apparent volume of distribution is 164 L (70%).
Elimination
The mean (CV%) terminal half-life (t½) of encorafenib is 3.5 hours (17%), and the apparent clearance is 14 L/h (54%) at day 1, increasing to 32 L/h (59%) at steady-state.
Metabolism
The primary metabolic pathway is N-dealkylation, with CYP3A4 as the main contributor (83%) to total oxidative clearance of encorafenib in human liver microsomes, followed by CYP2C19 (16%) and CYP2D6 (1%).
Excretion
Following a single oral dose of 100 mg radiolabeled encorafenib, 47% (5% unchanged) of the administered dose was recovered in the feces and 47% (2% unchanged) was recovered in the urine.
Specific Populations
Age (19 to 89 years), sex, body weight, mild hepatic impairment (Child-Pugh Class A), and mild or moderate renal impairment (CLcr 30 to < 90 mL/min) do not have a clinically meaningful effect on the pharmacokinetics of encorafenib. The effect of race or ethnicity, moderate or severe hepatic impairment (Child-Pugh Class B or C), and severe renal impairment (CLcr < 30 mL/min) on encorafenib pharmacokinetics have not been studied.
Drug Interaction Studies
Clinical Studies
Effect Of CYP3A4 Inhibitors On Encorafenib
Coadministration of a strong (posaconazole) or moderate (diltiazem) CYP3A4 inhibitor with BRAFTOVI increased the AUC of encorafenib by 3- and 2-fold, respectively, and increased the Cmax by 68% and 45%, respectively, after a single BRAFTOVI dose of 50 mg (0.1 times the recommended dose).
Effect Of CYP3A4 Inducers On Encorafenib
The effect of coadministration of a CYP3A4 inducer on encorafenib exposure has not been studied. In clinical trials, steady-state encorafenib exposures were lower than encorafenib exposures after the first dose, suggesting CYP3A4 auto-induction.
Effect Of Acid Reducing Agents On Encorafenib
Coadministration of a proton pump inhibitor, rabeprazole, had no effect on AUC and Cmax of encorafenib.
Combination Treatment
Coadministration of BRAFTOVI (UGT1A1 inhibitor) with binimetinib (UGT1A1 substrate) had no effect on binimetinib exposure.
In Vitro Studies
Effect Of Encorafenib On CYP/UGT Substrates
Encorafenib is a reversible inhibitor of UGT1A1, CYP1A2, CYP2B6, CYP2C8/9, CYP2D6, and CYP3A, and a time-dependent inhibitor of CYP3A4 at clinically relevant plasma concentrations. Encorafenib induced CYP2B6, CYP2C9, and CYP3A4 at clinically relevant plasma concentrations.
Effect Of Transporters On Encorafenib
Encorafenib is a substrate of P-glycoprotein (P-gp). Encorafenib is not a substrate of breast cancer resistance protein (BCRP), multidrug resistance-associated protein 2 (MRP2), organic anion transporting polypeptide (OATP1B1, OATP1B3) or organic cation transporter (OCT1) at clinically relevant plasma concentrations.
Effect Of Encorafenib On Transporters
Encorafenib inhibited P-gp, BCRP, OCT2, organic anion transporter (OAT1, OAT3), OATP1B1, and OATP1B3, but not OCT1 or MRP2 at clinically relevant plasma concentrations.
Animal Toxicology And/Or Pharmacology
Adverse histopathology findings of hyperplasia and hyperkeratosis occurred in the stomach of rats at encorafenib doses of 20 mg/kg/day (approximately 14 times the human exposure at the 450 mg clinical dose based on AUC) or greater, in both 4 and 13-week studies.
Clinical Studies
BRAFTOVI in combination with binimetinib was evaluated in a randomized, active-controlled, open-label, multicenter trial (COLUMBUS; NCT01909453). Eligible patients were required to have BRAF V600E or V600K mutation-positive unresectable or metastatic melanoma, as detected using the bioMerieux THxID™BRAF assay. Patients were permitted to have received immunotherapy in the adjuvant setting and one prior line of immunotherapy for unresectable locally advanced or metastatic disease. Prior use of BRAF inhibitors or MEK inhibitors was prohibited. Randomization was stratified by American Joint Committee on Cancer (AJCC) Stage (IIIB, IIIC, IVM1a or IVM1b, versus IVM1c), Eastern Cooperative Oncology Group (ECOG) performance status (0 versus 1), and prior immunotherapy for unresectable or metastatic disease (yes versus no).
Patients were randomized (1:1:1) to receive BRAFTOVI 450 mg once daily in combination with binimetinib 45 mg twice daily (BRAFTOVI in combination with binimetinib), BRAFTOVI 300 mg once daily, or vemurafenib 960 mg twice daily. Treatment continued until disease progression or unacceptable toxicity. Only the results of the approved dosing (BRAFTOVI 450 mg in combination with binimetinib 45 mg) are described below.
The major efficacy outcome measure was progression-free survival (PFS), as assessed by a blinded independent central review, to compare BRAFTOVI in combination with binimetinib with vemurafenib. Additional efficacy outcome measures included overall survival (OS), as well as objective response rate (ORR) and duration of response (DoR) which were assessed by central review.
A total of 577 patients were randomized, 192 to the BRAFTOVI in combination with binimetinib arm, 194 to the BRAFTOVI arm, and 191 to the vemurafenib arm. Of the 383 patients randomized to either the BRAFTOVI in combination with binimetinib or the vemurafenib arms, the median age was 56 years (20 to 89 years), 59% were male, 91% were White, and 72% had baseline ECOG performance status of 0. Ninety-five percent (95%) had metastatic disease, 65% were Stage IVM1c, and 4% received prior CTLA-4, PD-1, or PD-L1 directed antibodies. Twenty-eight percent (28%) had elevated baseline serum lactate dehydrogenase (LDH), 45% had ≥ 3 organs with tumor involvement at baseline, and 3% had brain metastases. Based on centralized testing, 100% of patients’ tumors tested positive for BRAF mutations; BRAF V600E (88%), BRAF V600K (11%), or both (<1%).
BRAFTOVI in combination with binimetinib demonstrated a statistically significant improvement in PFS compared to vemurafenib. Efficacy results are summarized in Table 6 and Figure 1.
Table 6: Efficacy Results for COLUMBUS
| BRAFTOVI with binimetinib N=192 |
Vemurafenib N=191 |
|
| Progression-Free Survival | ||
| Number of events (%) | 98 (51) | 106 (55) |
| Progressive disease | 88 (46) | 104 (54) |
| Death | 10 (5) | 2 (1) |
| Median PFS, months (95% CI) | 14.9 (11, 18.5) | 7.3 (5.6, 8.2) |
| HR (95% CI)a | 0.54(0.41, 0.71) | |
| P-valueb | <0.0001 | |
| Overall Survivalc | ||
| Number of events (%) | 105 (55) | 127 (67) |
| Median OS, months (95% CI) | 33.6 (24.4, 39.2) | 16.9 (14.0, 24.5) |
| HR (95% CI)a | 0.61 (0.47, 0.79) | |
| Overall Response Rate | ||
| ORR (95% CI) | 63% (56%, 70%) | 40% (33%, 48%) |
| CR | 8% | 6% |
| PR | 55% | 35% |
| Duration of Response | ||
| Median DoR, months (95% CI) | 16.6 (12.2, 20.4) | 12.3 (6.9, 16.9) |
| CI = Confidence interval; CR = Complete response; DoR =
Duration of response; HR = Hazard ratio; NE = Not estimable; ORR = Overall response
rate; OS = Overall survival; PFS = Progression-free survival; PR = Partial
response. a Estimated with Cox proportional hazard model adjusted by the following stratification factors: American Joint Committee on Cancer (AJCC) Stage (IIIB, IIIC, IVM1a or IVM1b, versus IVM1c) and Eastern Cooperative Oncology Group (ECOG) performance status (0 versus 1). b Log-rank test adjusted by the same stratification factors. c Based on a cutoff date of 17.6 months after the date of the PFS analysis. |
||
Figure 1: Kaplan-Meier Curves for Progression-Free
Survival in COLUMBUS
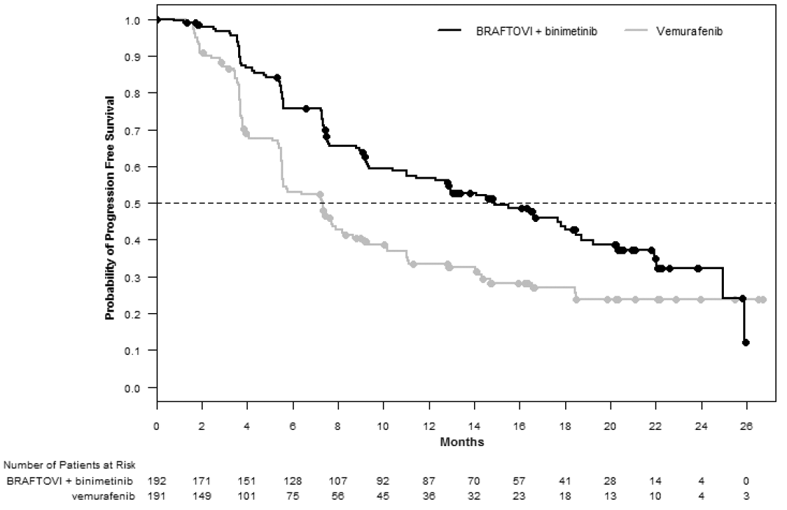 |
Medication Guide
PATIENT INFORMATION
BRAFTOVI®
(braf-TOE-vee)
(encorafenib) capsules
Important information: BRAFTOVI is used with other medicines, either binimetinib or cetuximab. Read the Patient Information leaflet that comes with binimetinib if used with binimetinib, and talk to your healthcare provider about cetuximab if used with cetuximab.
What is the most important information I should know about BRAFTOVI?
BRAFTOVI may cause serious side effects, including:
- Risk of new skin cancers. BRAFTOVI when used alone, or with binimetinib or cetuximab, may cause skin cancers called cutaneous squamous cell carcinoma or basal cell carcinoma.
Talk to your healthcare provider about your risk for these cancers.
Check your skin and tell your healthcare provider right away about any skin changes, including a:
Your healthcare provider should check your skin before treatment with BRAFTOVI, every 2 months during treatment, and for up to 6 months after you stop treatment with BRAFTOVI to look for any new skin cancers.
Your healthcare provider should also check for cancers that may not occur on the skin. Tell your healthcare provider about any new symptoms that develop during treatment with BRAFTOVI.
See “What are the possible side effects of BRAFTOVI?” for more information about side effects.
What is BRAFTOVI?
BRAFTOVI is a prescription medicine used:
- in combination with a medicine called binimetinib to treat people with a type of skin cancer called melanoma:
- that has spread to other parts of the body or cannot be removed by surgery, and
- that has a certain type of abnormal “BRAF” gene
- in combination with a medicine called cetuximab, for the treatment of adults with cancer of your colon or rectum (colorectal cancer):
- that has been previously treated, and
- that has spread to other parts of the body, and
- that has a certain type of abnormal “BRAF” gene
BRAFTOVI should not be used to treat people with wild-type BRAF melanoma or wild-type BRAF colorectal cancer.
Your healthcare provider will perform a test to make sure that BRAFTOVI is right for you.
It is not known if BRAFTOVI is safe and effective in children.
Before taking BRAFTOVI, tell your healthcare provider about all of your medical conditions, including if you:
- have had bleeding problems
- have eye problems
- have heart problems, including a condition called long QT syndrome
- have been told that you have low blood levels of potassium, calcium, or magnesium
- have liver or kidney problems
- are pregnant or plan to become pregnant. BRAFTOVI can harm your unborn baby.
- Females who are able to become pregnant should use effective non-hormonal birth control (contraception) during treatment with BRAFTOVI and for 2 weeks after the final dose of BRAFTOVI. Birth control methods that contain hormones (such as birth control pills, injections or transdermal systems) may not work as well during treatment with BRAFTOVI.
- Talk to your healthcare provider about birth control methods that may be right for you during this time.
- Your healthcare provider will do a pregnancy test before you start taking BRAFTOVI. Tell your healthcare provider right away if you become pregnant or think you might be pregnant during treatment with BRAFTOVI.
- are breastfeeding or plan to breastfeed. It is not known if BRAFTOVI passes into your breast milk. Do not breastfeed during treatment with BRAFTOVI and for 2 weeks after the final dose of BRAFTOVI. Talk to your healthcare provider about the best way to feed your baby during this time.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
BRAFTOVI and certain other medicines can affect each other, causing side effects or affecting how BRAFTOVI or the other medicines work.
Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine.
How should I take BRAFTOVI?
- Take BRAFTOVI exactly as your healthcare provider tells you. Do not change your dose or stop taking BRAFTOVI unless your healthcare provider tells you to.
- Your healthcare provider may change your dose of BRAFTOVI, temporarily stop, or completely stop your treatment with BRAFTOVI if you develop certain side effects.
- For melanoma, take BRAFTOVI in combination with binimetinib by mouth one time each day.
- For colorectal cancer, take BRAFTOVI by mouth one time each day. You will also receive cetuximab through a vein in your arm (intravenously) given by your healthcare provider.
- BRAFTOVI may be taken with or without food.
- Avoid grapefruit during treatment with BRAFTOVI. Grapefruit products may increase the amount of BRAFTOVI in your body.
- If you miss a dose of BRAFTOVI, take it as soon as you remember. If it is within 12 hours of your next scheduled dose, take your next dose at your regular time. Do not make up for the missed dose.
- Do not take an extra dose if you vomit after taking your scheduled dose. Take your next dose at your regular time.
- If you stop treatment with binimetinib or cetuximab, talk to your healthcare provider about your BRAFTOVI treatment. Your BRAFTOVI dose may need to be changed or stopped.
What are the possible side effects of BRAFTOVI?
BRAFTOVI may cause serious side effects, including:
See “What is the most important information I should know about BRAFTOVI?”
- Bleeding problems. BRAFTOVI, when taken with binimetinib or cetuximab, can cause serious bleeding problems, including in your stomach or brain, that can lead to death. Call your healthcare provider and get medical help right away if you have any signs of bleeding, including:
- headaches, dizziness, or feeling weak
- cough up blood or blood clots
- vomit blood or your vomit looks like “coffee grounds”
- red or black stools that look like tar
- Eye problems. Tell your healthcare provider right away if you develop any of these symptoms of eye problems:
- blurred vision, loss of vision, or other vision changes
- see colored dots
- see halos (blurred outline around objects)
- eye pain, swelling, or redness
- Changes in the electrical activity of your heart called QT prolongation. QT prolongation can cause irregular heartbeats that can be life threatening. Your healthcare provider should do tests before you start taking BRAFTOVI with binimetinib or cetuximab and during your treatment to check your body salts (electrolytes). Tell your healthcare provider right away if you feel faint, lightheaded, dizzy or if you feel your heart beating irregularly or fast while taking BRAFTOVI with binimetinib or cetuximab. These symptoms may be related to QT prolongation.
The most common side effects of BRAFTOVI when taken in combination with binimetinib, include:
- fatigue
- nausea
- vomiting
- abdominal pain
- pain or swelling of your joints (arthralgia)
The most common side effects of BRAFTOVI when taken in combination with cetuximab, include:
- fatigue
- nausea
- diarrhea
- acne-like rash (dermatitis acneiform)
- abdominal pain
- decreased appetite
- pain or swelling of your joints (arthralgia)
- rash
BRAFTOVI may cause fertility problems in males. Talk to your healthcare provider if this is a concern for you.
These are not all the possible side effects of BRAFTOVI.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
You may also report side effects to Array BioPharma Inc. at 1-844-792-7729.
How should I store BRAFTOVI?
- Store BRAFTOVI at room temperature between 68°F to 77°F (20°C to 25°C).
- Store BRAFTOVI in the original bottle.
- Keep the BRAFTOVI bottle tightly closed and protect it from moisture.
- BRAFTOVI comes with a desiccant packet in the bottle to help protect your medicine from moisture. Do not remove the desiccant packet from the bottle.
Keep BRAFTOVI and all medicines out of the reach of children.
General information about the safe and effective use of BRAFTOVI.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use BRAFTOVI for a condition for which it was not prescribed. Do not give BRAFTOVI to other people, even if they have the same symptoms that you have. It may harm them. You can ask your healthcare provider or pharmacist for information about BRAFTOVI that is written for health professionals.
What are the ingredients in BRAFTOVI?
Active ingredient: encorafenib
Inactive ingredients: copovidone, poloxamer 188, microcrystalline cellulose, succinic acid, crospovidone, colloidal silicon dioxide, and magnesium stearate of vegetable origin
Capsule shell: gelatin, titanium dioxide, iron oxide red, iron oxide yellow, ferrosoferric oxide, monogramming ink (pharmaceutical glaze, ferrosoferric oxide, propylene glycol)
This Medication Guide has been approved by the U.S. Food and Drug Administration.
