Cyclocort Drug Description: Detailed Datasheet
- Generic Name: amcinonide
- Brand Name: Cyclocort Ointment
side effects drug center cyclocort ointment (amcinonide) drug
Drug Description
PrCYCLOCORT®
(amcinonide) USP
DESCRIPTION
Pharmaceutical Information
Drug Substance
Proper name: amcinonide
Chemical name: Pregna-1,4-diene-3,20-dione,21-(acetyloxy)-16,17 [cyclopentylidenebis(oxy)]-9-fluoro-11-hydroxy-,(11β,16α)-
Molecular formula: C28H35FO7
Molecular mass: 502.57
Structural formula:
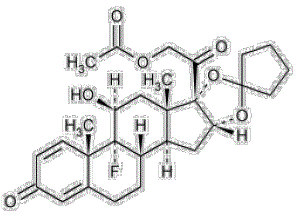 |
Physicochemical properties: White to cream coloured crystals or crystalline powder.
Indications
CYCLOCORT® (amcinonide) is a high potency topical corticosteroid indicated for the relief of inflammatory and pruritic manifestations of corticosteroid-responsive dermatoses for a maximum duration of 5 days on the face, axillae, scrotum and scalp and a maximum of 3 weeks on the body.
Geriatrics (> 65 Years Of Age)
Safety and effectiveness of CYCLOCORT® in geriatric patients over 65 years of age have not been established (see WARNINGS AND PRECAUTIONS, Special Populations, Geriatrics (> 65 Years Of Age)).
Pediatrics (<18 Years Of Age)
Safety and effectiveness of CYCLOCORT® in pediatric patients less than 18 years of age have not been established (see WARNINGS AND PRECAUTIONS, Special Populations, Pediatrics (<18 Years Of Age)).
Dosage
DOSAGE AND ADMINISTRATION
Dosing Considerations
- Patients/caregivers should be instructed to use the minimum quantity of CYCLOCORT® for the shortest duration of time necessary to achieve the desired therapeutic benefit because of the potential for corticosteroids to suppress the hypothalamic-pituitary-adrenal (HPA) axis and cause skin atrophy (See WARNINGS AND PRECAUTIONS).
- If the condition worsens or does not improve within 2 weeks, treatment and diagnosis should be re-evaluated.
- Use in pediatric patients is not recommended. Pediatric patients are more likely to develop local and systemic toxicity from equivalent doses of topical corticosteroids because of their larger skin surface to body weight ratios.
- Geriatric patients may be more susceptible to percutaneous absorption and the potential effects of systemic absorption. The greater frequency of decreased hepatic or renal function in the elderly may delay elimination if systemic absorption occurs.
Recommended Dose And Dosage Adjustment
Apply a thin layer to affected area once or twice daily and gently rub in. Allow adequate time for absorption after each application before applying an emollient.
CYCLOCORT® should not be used for longer than 5 days on the face, axillae, scrotum and scalp (see WARNINGS AND PRECAUTIONS).
CYCLOCORT® should not be used for longer than 2 to 3 weeks on the body (see WARNINGS AND PRECAUTIONS). If the condition worsens or does not improve within 2 weeks, treatment and diagnosis should be re-evaluated.
Avoid abrupt discontinuation of CYCLOCORT® therapy once control is achieved as rebound of pre-existing dermatoses can occur. Continue an emollient as maintenance therapy.
Pediatrics (< 18 Years Of Age)
The safety and effectiveness of CYCLOCORT® in pediatric patients less than 18 years of age have not been established (see WARNINGS AND PRECAUTIONS - Special Populations, Pediatrics (< 18 Years Of Age)).
Geriatrics (> 65 Years Of Age)
CYCLOCORT® should be used with caution in geriatric patients due to increased risk of renal or hepatic impairment in this population. The minimum quantity should be used for the shortest duration to achieve the desired therapeutic benefit (see WARNINGS AND PRECAUTIONS - Special Populations, Geriatrics (> 65 Years Of Age)).
Renal/Hepatic Impairment
In patients with renal or hepatic impairment, the minimum quantity should be used for the shortest duration to achieve the desired therapeutic benefit (see WARNINGS AND PRECAUTIONS - Special Populations, Patients With Renal / Hepatic Impairment).
Missed Dose
In the event of a missed dose, CYCLOCORT® should be applied as soon as possible after the missed dose is remembered. If this is close to the scheduled application time or the next dose, the patient should wait and apply the next scheduled dose. The usual schedule should be resumed thereafter.
Administration
- CYCLOCORT ® is for topical use only.
- Use with occlusive dressings is not recommended (see WARNINGS AND PRECAUTIONS).
- CYCLOCORT® is not for use in or near the eye or on other mucous membranes.
HOW SUPPLIED
Storage And Stability
Store between 15°C and 25°C. Do not freeze. Keep out of the sight and reach of children.
Special Handling Instructions
CYCLOCORT® Lotion must be shaken before use.
Dosage Forms, Composition And Packaging
CYCLOCORT® Cream is a white, smooth homogeneous opaque cream, free from gritty particles and visible contamination. CYCLOCORT® Ointment is a white, smooth homogeneous ointment, free from visible contamination. CYCLOCORT® Lotion is a white, smooth homogeneous opaque emulsion, free from visible contamination.
CYCLOCORT® Cream contains amcinonide 0.1% w/w, benzyl alcohol, emulsifying wax, glycerin, isopropyl palmitate, lactic acid, purified water, and sorbitol solution.
CYCLOCORT® Ointment contains amcinonide 0.1% w/w, benzyl alcohol, emulsifying wax, TENOX II (butylated hydroxyanisole, citric acid, propylene glycol, and propyl gallate), and white petrolatum.
CYCLOCORT® Lotion contains amcinonide 0.1% w/w, benzyl alcohol, emulsifying wax, glycerin, isopropyl palmitate, lactic acid, purified water, and sorbitol solution.
CYCLOCORT® 0.1% w/w Cream is available in 60 g tubes.
CYCLOCORT® 0.1% w/w Ointment is available in 60 g tubes.
CYCLOCORT® 0.1% w/w Lotion is available in 60 mL bottles.
Manufactured by: GlaxoSmithKline Inc. 7333 Mississauga Road, Mississauga, Ontario, L5N 6L4, 1-800-387-7374. Revised: Nov 2014
Side Effects
Post-Market Adverse Drug Reactions
The following adverse reactions are reported when topical corticosteroids are used as recommended. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish causal relationship to drug exposure.
Endocrine Disorders
Hypothalamic-pituitary adrenal (HPA) axis suppression, cushingoid features (e.g. moon face, central obesity), delayed weight gain/growth retardation in children, osteoporosis, hyperglycemia/glucosuria, hypertension, increased weight/obesity, decreased endogenous cortisol levels, steroid withdrawal syndrome
Eye Disorders
glaucoma, cataract subcapsular
Gastrointestinal Disorders
upper abdominal pain
General Disorders And Administration Site Conditions
Application site irritation/pain.
Immune System Disorders
local hypersensitivity (see Skin And Subcutaneous Tissue Disorders)
Infections And Infestations
secondary infection
Investigations
Decreased glucose tolerance has also been reported
Skin And Subcutaneous Tissue Disorders
skin striae, skin atrophy, telangiectasia, purpura-like bleeding, acne, dermatitis, hypertrichosis, skin depigmentation, erythema, burning sensation, pruritus, eczema, dryness, itching, local irritation, atrophy of the subcutaneous tissues, pustules, miliaria, folliculitis, pyoderma, allergic contact dermatitis, acneiform eruptions, perioral dermatitis, rash, urticaria, pustular psoriasis, skin wrinkling, alopecia, trichorrhexis, skin infection.
Vascular Disorders
Drug Interactions
Overview
No clinical trials were specifically designed to assess potential drug-drug, drug-food, drug-herb, or drug-laboratory interactions with CYCLOCORT®.
Co-administered drugs that can inhibit CYP3A4 (e.g. ritonavir, itraconazole) have been shown to inhibit the metabolism of corticosteroids leading to increased systemic exposure. The extent to which this interaction is clinically relevant depends on the dose and route of administration of the corticosteroids and the potency of the CYP3A4 inhibitor.
Latex
Treatment with CYCLOCORT® in the genital or anal area with the simultaneous use of latex products (e.g. condoms, diaphragms) can result in reduction in the reliability and therefore impairment of the safety of these products due to the excipients.
Drug-Drug Interactions
Interactions with other drugs have not been established.
Drug-Food Interactions
Interactions with food have not been established.
Drug-Herb Interactions
Interactions with herbal products have not been established.
Drug-Laboratory Interactions
Interactions with laboratory tests have not been established.
Warnings & Precautions
WARNINGS
Included as part of the "PRECAUTIONS" Section
PRECAUTIONS
General
Patients should be advised to inform subsequent physicians of the prior use of corticosteroids.
CYCLOCORT® Cream, Ointment and Lotion should not be used under occlusion due to increased risk of systemic exposure and infection. When used under occlusive dressing, over extensive areas, or on the face, scalp, axillae, or scrotum, sufficient absorption may occur to result in adrenal suppression and other systemic effects (see Endocrine And Metabolism, Immune, And Ophthalmologic).
Cardiovascular
Suitable precautions should be taken when using topical corticosteroids in patients with stasis dermatitis and other skin diseases with impaired circulation.
Use of corticosteroids around chronic leg ulcers may be associated with a higher occurrence of local hypersensitivity reactions and an increased risk of local infection.
Endocrine And Metabolism
Manifestations of hypercortisolism (Cushing’s syndrome) and reversible hypothalamic-pituitary-adrenal (HPA) axis suppression, leading to glucocorticosteroid insufficiency, can occur in some individuals as a result of increased systemic absorption of topical corticosteroids. Hyperglycemia and glucosuria can also be produced in some patients by systemic absorption of topical corticosteroids (see ADVERSE REACTIONS).
Conditions which augment systemic absorption include the formulation and potency of the topical corticosteroid, the application of topical corticosteroids over large body surface areas, application to intertriginous areas (such as the axillae), frequency of application, prolonged use, or the use of occlusive dressings. Other risk factors for increased systemic effects include increasing hydration of the stratum corneum, use on thin skin areas (such as the face), and use on broken skin or in conditions where the skin barrier may be impaired.
If patients must be treated over large body surface areas, they should be evaluated periodically for evidence of HPA axis suppression (see Monitoring And Laboratory Tests). If HPA axis suppression or Cushing’s syndrome is observed, an attempt should be made to withdraw the drug by reducing the frequency of application. Abrupt withdrawal of treatment may result in glucocorticosteroid insufficiency (see ADVERSE REACTIONS).
Recovery of HPA axis function is generally prompt upon discontinuation of topical corticosteroids. Infrequently, signs and symptoms of glucocorticosteroid insufficiency may occur, requiring supplemental systemic corticosteroids. For information on systemic corticosteroid supplementation, see the prescribing information for those products.
Under treatment with CYCLOCORT®, systemic undesirable effects (see ADVERSE REACTIONS and OVERDOSE) may occur. This is dependent on the absorption of large quantities of the active ingredient through the skin. It must therefore be ensured that the specified duration of treatment (see DOSAGE AND ADMINISTRATION) is not exceeded and the size of the area treated is not more than 10-20% of the body surface area.
Pediatric patients may absorb larger amounts of topical corticosteroids and thus be more susceptible to systemic toxicity from equivalent doses because of their larger skin surface to body mass ratios as compared with adult patients (see Special Populations, Pediatrics).
Immune
Topical corticosteroids may increase the risk of infections including aggravation of cutaneous infection, masked infection and secondary infections. In particular, bacterial infection is encouraged by the warm, moist conditions within skin-fold areas, or caused by occlusive dressings. If concomitant skin infections develop, CYCLOCORT® should be discontinued and antimicrobial therapy should be administered.
Ophthalmologic
Topical corticosteroids should be used with caution on lesions close to the eye because systemic absorption may cause increased intraocular pressure, glaucoma or cataracts.
Sensitivity
Local hypersensitivity reactions (see ADVERSE REACTIONS) may resemble symptoms of the condition under treatment. If hypersensitivity reactions occur, CYCLOCORT® should be discontinued and appropriate therapy initiated.
Allergic contact dermatitis with corticosteroids is usually diagnosed by observing a failure to heal rather than noticing a clinical exacerbation. Such an observation should be corroborated with appropriate diagnostic patch testing.
Sexual Function/Reproduction
There are no data in humans to evaluate the effect of topical corticosteroids on fertility. Corticosteroids have been shown to impair fertility in animal studies.
Skin
Topical corticosteroids should be used with caution in psoriasis as rebound relapses, development of tolerances, risk of generalised pustular psoriasis and development of local or systemic toxicity due to impaired barrier function of the skin have been reported in some cases. If used in psoriasis careful patient supervision is important.
If significant irritation develops, CYCLOCORT® should be discontinued and appropriate therapy should be instituted.
Prolonged use of topical corticosteroid preparations may produce striae or atrophy of the skin or subcutaneous tissue. Topical corticosteroids should be used with caution on lesions of the face, groin, and axillae as these areas are more prone to atrophic changes than other areas of the body. Frequent observation is important if these areas are to be treated. If skin atrophy is observed, treatment should be discontinued.
Special Populations
Pregnant Women
Topical administration of corticosteroids to pregnant animals can cause abnormalities of fetal development. The relevance of this finding to humans has not been established.
There are no adequate and well-controlled studies of CYCLOCORT® in pregnant women. Administration of CYCLOCORT® during pregnancy should only be considered if the expected benefit to the mother outweighs the potential risk to the fetus. The minimum quantity should be used for the minimum duration.
Due to the expected systemic re-sorption of the active ingredient, intrauterine growth disorders and atrophy of the adrenal cortex of the fetus as observed following long-term oral therapy with glucocorticoids cannot be excluded with long-term use of CYCLOCORT® and its use on large areas.
Nursing Women
The safe use of topical corticosteroids during lactation has not been established.
Systemically administered corticosteroids appear in human milk and could suppress growth, interfere with endogenous corticosteroid production, or cause other untoward effects. It is not known whether topical administration of corticosteroids could result in sufficient systemic absorption to produce detectable quantities in human breast milk.
Because many drugs are excreted in human milk, caution should be exercised when CYCLOCORT® is administered to a nursing woman. Administration of CYCLOCORT® during lactation should only be considered if the expected benefit to the mother outweighs the risk to the infant. Nursing mothers must not apply CYCLOCORT® to the breast area in order to prevent direct contact of the nursing infant with the active ingredient.
It is not known whether CYCLOCORT® is excreted into breast milk. If its use is required on large areas during lactation, nursing mothers should cease nursing.
Pediatrics (<18 Years Of Age)
The safety of CYCLOCORT® has not been studied in pediatric patients.
Because of a higher ratio of skin surface area to body mass, pediatric patients are at a greater risk than adults of HPA axis suppression and Cushing’s syndrome when they are treated with topical corticosteroids. They are therefore also at greater risk of adrenal insufficiency during and/or after withdrawal of treatment.
Adverse effects including striae have been reported with use of topical corticosteroids in infants and children. HPA axis suppression, Cushing’s syndrome, linear growth retardation, delayed weight gain, and intracranial hypertension have been reported in children receiving topical corticosteroids. Manifestations of adrenal suppression in children include low plasma cortisol levels and an absence of response to ACTH stimulation. Manifestations of intracranial hypertension include bulging fontanelles, headaches, and bilateral papilledema. Chronic corticosteroid therapy may interfere with the growth and development of children.
Geriatrics (> 65 Years Of Age)
The safety of CYCLOCORT® has not been studied in geriatric patients.
In general, topical corticosteroids should be used cautiously in elderly patients, reflecting their increased skin fragility and greater frequency of hepatic, renal, or cardiac dysfunction, and of concomitant disease or other drug therapy. The greater frequency of decreased hepatic or renal function in the elderly may delay elimination if systemic absorption occurs.
There are no adequate and well-controlled studies of CYCLOCORT® in geriatric patients. For geriatric patients over 65 years of age, the minimum quantity should be used for the minimum duration (see DOSAGE AND ADMINISTRATION).
Patients With Renal / Hepatic Impairment
The safety of CYCLOCORT® has not been studied in patients with renal or hepatic impairment.
In case of systemic absorption, metabolism and elimination may be delayed leading to increased risk of systemic toxicity.
There are no adequate and well controlled studies of CYCLOCORT® in patients with renal or hepatic impairment. For patients with renal or hepatic impairment, the minimum quantity should be used for the minimum duration (see DOSAGE AND ADMINISTRATION).
Monitoring And Laboratory Tests
The cosyntropin (ACTH1-24) stimulation test may be helpful in evaluating patients for HPA axis suppression.
Overdosage
For management of a suspected drug overdose, contact your regional Poison Control Centre.
Topically applied corticosteroids can be absorbed in sufficient amounts to produce systemic effects (see WARNINGS AND PRECAUTIONS). In the event of overdose or misuse, the features of hypercortisolism may occur (see ADVERSE REACTIONS).
Excessive prolonged use or misuse may suppress hypothalamic-pituitary-adrenal (HPA) axis function, resulting in secondary adrenal insufficiency. If symptoms of HPA axis suppression occur, amcinonide should be withdrawn gradually by reducing the frequency of application, or by substituting a less potent corticosteroid because of the risk of glucocorticosteroid insufficiency. Further management should be as clinically indicated. If toxic effects occur, CYCLOCORT® should be discontinued and symptomatic therapy should be administered.
Contraindications
- Patients who are hypersensitive to this drug or to any ingredient in the formulation or component of the container. For a complete listing, see the Dosage Forms, Composition And Packaging section of the Product Monograph.
- Patients who are hypersensitive to other corticosteroids.
- Patients with viral (e.g. herpes or varicella) lesions of the skin, bacterial or fungal skin infections, parasitic infections, skin manifestations relating to tuberculosis or syphilis, eruptions following vaccinations, pruritus without inflammation, perioral dermatitis, rosacea, acne vulgaris, perianal and genital pruritus.
- Topical application to the eye.
Clinical Pharmacology
No Information Provided
Medication Guide
PATIENT INFORMATION
PrCYCLOCORT®
(amcinonide) USP
Cream, USP 0.1% w/w
Ointment, USP 0.1% w/w
Lotion 0.1% w/w
This leaflet is part III of a three-part “Product Monograph” and is designed specifically for Consumers. This leaflet is a summary and will not tell you everything about CYCLOCORT®. Contact your doctor or pharmacist if you have any questions about the drug.
ABOUT THIS MEDICATION
What the medication is used for:
CYCLOCORT® is used to help relieve the redness and itchiness of certain skin problems.
What it does:
CYCLOCORT® contains amcinonide which belongs to a group of medicines called steroids. Steroids help to reduce redness, swelling and irritation of the skin.
When it should not be used:
Do not use CYCLOCORT® if you are allergic to amcinonide, other corticosteroids, or to any other ingredients in CYCLOCORT® (see What the nonmedicinal ingredients are).
Do not apply CYCLOCORT® on the areas of the skin that have:
- bacterial, fungal, parasitic, viral skin infections (e.g. herpes simplex, chicken pox), tuberculosis or syphilis skin lesions, or a skin reaction following a recent vaccination.
- acne, rosacea (a facial skin condition where the nose, cheeks, chin, forehead or entire face are unusually red, with or without tiny visible blood vessels, bumps (papules) or pus-filled bumps (pustules)), rashes around the mouth, itchy skin which is not inflamed, itchy skin around the anus or genitals.
Do not apply in or near the eye.
If you think any of these apply to you, don’t use CYCLOCORT® until you have checked with your doctor or pharmacist.
What the medicinal ingredient is:
Amcinonide
What the nonmedicinal ingredients are:
The nonmedicinal ingredients of CYCLOCORT® Cream are benzyl alcohol, emulsifying wax, glycerin, isopropyl palmitate, lactic acid, purified water, and sorbitol solution.
The nonmedicinal ingredients of CYCLOCORT®Ointment are benzyl alcohol, emulsifying wax, TENOX II (butylated hydroxyanisole, citric acid, propylene glycol, and propyl gallate), and white petrolatum.
The nonmedicinal ingredients of CYCLOCORT® Lotion are benzyl alcohol, emulsifying wax, glycerin, isopropyl palmitate, lactic acid, purified water, and sorbitol solution.
What dosage forms it comes in:
CYCLOCORT® Cream and Ointment are available in 60 g tubes. CYCLOCORT® Lotion is available in 60 mL bottles.
WARNINGS AND PRECAUTIONS
Apply just enough CYCLOCORT® to cover the affected areas. CYCLOCORT® can get into the blood and cause side effects.
Always follow your doctor’s instructions.
Do NOT use CYCLOCORT® with occlusive dressings such as a bandage, or cover the treated areas tightly.
CYCLOCORT® is more likely to cause side effects when used:
- over large areas
- on sensitive areas such as the face, scalp, skin fold areas like the armpit and groin
- on broken skin
- for a long time
Inform any doctor you consult that you are using or you have previously used corticosteroids.
Before using CYCLOCORT®, talk to your doctor or pharmacist if:
- you are pregnant or planning to become pregnant.
- you are breastfeeding. It is not known if CYCLOCORT® will appear in breast milk. You should only use CYCLOCORT® while breastfeeding if you and your doctor decide that the benefits to the mother outweigh the risks to the baby. If you do use CYCLOCORT® when breastfeeding, do not use on your breast area to ensure that the baby does not accidentally get it in their mouth. If CYCLOCORT® is used on large areas of body, you should stop breastfeeding.
- you have inflammatory skin diseases in the leg as a result of impaired circulation (such as stasis dermatitis).
- you have problems with your kidney or liver. You may need to use a smaller amount of CYCLOCORT® or use it less often.
- you have any skin disease around a leg ulcer; use of a topical corticosteroid may increase the risk of an allergic reaction or an infection around the ulcer.
While using CYCLOCORT®, talk to your doctor or pharmacist if:
- you develop any skin infection
- you have an allergic reaction
- you develop significant skin irritation
- you experience skin thinning or softening
- your condition worsens or does not improve
- you develop raised bumps with pus under the skin
CYCLOCORT® should be used with caution on the scalp, face or in skin fold areas, such as the groin or the armpit since these areas are more prone to skin thinning.
Avoid applying CYCLOCORT® in or near the eye, or other mucous membranes. In case of contact, wash with water. Absorption in the body may cause increased pressure in the eye (glaucoma), or a cloudy lens in the eye (cataracts).
Children absorb larger amounts of topical corticosteroids and therefore, may be more likely to develop side effects. CYCLOCORT® is not recommended for use in children under 18 years of age.
INTERACTIONS WITH THIS MEDICATION
It is NOT known whether CYCLOCORT® interacts with other medication. Some medicines may affect how CYCLOCORT® works, or make it more likely that you’ll have side effects. Examples of these medicines include:
- Ritonavir (for HIV)
- Itraconazole (for fungal infections)
Tell your doctor or pharmacist about all your other medications, including medicines that you bought without prescription and natural health products.
Treatment with CYCLOCORT® in the genital or anal area with the simultaneous use of latex products (e.g. condoms, diaphragms) can result in reduction in the reliability and therefore impairment of the safety of these products.
PROPER USE OF THIS MEDICATION
Use the minimum quantity of CYCLOCORT® for the shortest amount of time necessary to achieve the desired results. This is especially important if you are 65 years or older or have liver or kidney disease.
Check with your doctor or pharmacist if you are not sure.
CYCLOCORT® is for use on the skin only. It is NOT for use in the eyes or on other mucous membranes.
Usual dose:
Apply a thin film to the affected areas once or twice a day. The number of times you use your medicine may be reduced as your skin gets better or your doctor may prescribe a weaker steroid for you to use instead.
Use for a maximum of:
- 5 days on the face, scalp, skin-fold areas like the armpit and groin.
- 2-3 weeks on the body. If your condition worsens or no improvement is seen within 2 weeks, contact your doctor.
It is important to not stop using CYCLOCORT® suddenly or your skin condition could flare up again.
Use CYCLOCORT® only as directed by your health care provider. Do NOT use more of it, do NOT use it more often and do NOT use it for a longer period of time than your health care provider recommended. Using too much CYCLOCORT® may increase your chances of unwanted and sometimes dangerous side effects.
This medication has been prescribed specifically for you. Do NOT give it to anyone else. It may harm them, even if their symptoms seem to be similar to yours.
How to Apply CYCLOCORT®:
- Apply a thin layer and gently rub in, using only enough to cover the entire affected area.
- Wash your hands after use unless treating the hands.
- Excess product should not be returned to the container, since it may cause contamination.
- If you are also using an emollient (moisturising) preparation allow time for CYCLOCORT® to be absorbed after each application before applying the emollient.
- Your doctor may recommend using a moisturizer as maintenance therapy.
- Do not use occlusive dressings such as a bandage, or cover the treated areas tightly.
CYCLOCORT® Lotion should be shaken before use.
Overdose:
In case of drug overdose, contact a health care practitioner, hospital emergency department or regional Poison Control Centre immediately, even if there are no symptoms.
Missed Dose:
If you forget to use CYCLOCORT®, apply it as soon as you remember. If it is close to the time scheduled to apply your next dose, wait and apply your next scheduled dose and then continue as before. Do not apply extra CYCLOCORT® to make up for missed doses.
SIDE EFFECTS AND WHAT TO DO ABOUT THEM
Like all medicines CYCLOCORT® can have side effects although not everybody gets them. Side effects will affect your skin and may have an effect on other parts of your body if a sufficient quantity of medicine is absorbed through the skin and enters your blood stream.
If your skin condition gets worse or your skin becomes swollen during treatment. You may be allergic to the medicine or need other treatment. Stop using CYCLOCORT® and tell your doctor as soon as possible.
The following side effects have been reported in patients using topical corticosteroids:
Common side effects
- itchy skin
- local skin burning or pain
Very rare side effects
Use of CYCLOCORT® for a long period of time, over a large body surface, or use under an airtight dressing, may cause the following symptoms:
- increased weight
- moon face/rounding of the face, obesity
- skin thinning (this may cause stretch marks), skin wrinkling, skin dryness, the appearance of blood vessels under the surface of your skin (telangiectasia), changes to the colour of your skin, skin infection
- increased body hair, hair loss/lack of hair growth/damaged looking hair
- allergic reaction, irritation, itching or pain at the site of application
- inflammation of hair follicles
- worsening of condition
- redness, rash or hives
- secondary infection
- allergic contact dermatitis/dermatitis (a type of eczema)
- upper abdominal pain
- prickly heat rash
- steroid withdrawal syndrome (symptoms may include weight loss, fatigue, nausea, diarrhea and abdominal pain)
- acne
If you have psoriasis you may get raised bumps with pus under the skin. This can happen very rarely during or after treatment and is known as pustular psoriasis.
Other symptoms that may only show in blood tests or when your doctor gives you a medical examination are: decreased hormone cortisol levels in your blood, increased sugar levels in your blood or urine, high blood pressure, cloudy lens in the eye (cataract), increased pressure in the eye (glaucoma), as well as weakening of the bones through gradual mineral loss (osteoporosis) and additional tests may be needed after your medical examination to confirm whether you have osteoporosis.
If any of the side effects listed becomes severe or troublesome, tell your doctor or pharmacist.
SERIOUS SIDE EFFECTS, HOW OFTEN THEY HAPPEN AND WHAT TO DO ABOUT THEM
| Symptom / effect | Talk with your doctor or pharmacist | Stop taking drug and call your doctor or pharmacist | ||
| Only if severe | In all cases | |||
| Allergic reactions: rash, hives, swelling of the skin, chills, fever, muscle aches or pains or flu-like symptoms occurring with or before a skin rash. |
|
|||
| Cushing's syndrome: weight gain, moon face / rounding of the face and obesity. |
|
|||
| Hyperglycemia (increased blood sugar): b frequent urination, thirst and hunger. |
|
|||
| Glucosuria (sugar in urine): excessive or sweet smelling urine. |
|
|||
| Hypertension (high blood pressure): headaches, vision disorders, nausea and vomiting. |
|
|||
| Osteoporosis: weakening of the bones potentially leading to an increased risk of bone fracture. |
|
|||
| Glaucoma or cataracts: blurred vision, increased pressure in your eyes, eye pain. |
|
|||
This is not a complete list of side effects. For any unexpected effects while taking CYCLOCORT®, contact your doctor or pharmacist.
HOW TO STORE IT
Store between 15° and 25°C. Do not freeze.
Keep out of the sight and reach of children.
REPORTING SUSPECTED SIDE EFFECTS
You can report any suspected adverse reactions associated with the use of health products to the Canada Vigilance Program by one of the following 3 ways:
- Report online at www.healthcanada.gc.ca/medeffect
- Call toll-free at 1-866-234-2345
- Complete a Canada Vigilance Reporting Form and:
- Fax toll-free to 1-866-678-6789, or
- Mail to: Canada Vigilance Program
Health Canada
Postal Locator 0701E
Ottawa, Ontario
K1A 0K9
Postage paid labels, Canada Vigilance Reporting Form and the adverse reaction reporting guidelines are available on the MedEffect™ Canada Web site at www.healthcanada.gc.ca/medeffect.
NOTE: Should you require information related to the management of side effects, contact your health professional. The Canada Vigilance Program does not provide medical advice.
MORE INFORMATION
This document plus the full product monograph, prepared for health professionals can be found at: http://www.stiefel.ca or by contacting the sponsor,
