Cefuroxime Drug Description: Detailed Datasheet
- Generic Name: cefuroxime injection
- Brand Name: Cefuroxime
side effects drug center cefuroxime (cefuroxime injection) drug
Drug Description
What is Cefuroxime and how is it used?
Cefuroxime and dextrose injection (Brand name: Ceftin) is an antibacterial used to treat lower respiratory tract infections, urinary tract infections, skin infections, septicemia, meningitis, gonorrhea and bone and joint infections. Cefuroxime and dextrose injection is available in generic form.
What are side effects of Cefuroxime?
Common side effects of cefuroxime and dextrose injection include:
- injection site reactions (inflammation, blood clot),
- diarrhea,
- watery or bloody stools,
- stomach cramps,
- nausea,
- vomiting,
- stomach or abdominal pain,
- gas,
- upset stomach,
- fever,
- cough,
- stuffy nose,
- stiff or tight muscles,
- muscle pain,
- joint pain or swelling,
- headache,
- drowsiness,
- restlessness,
- irritability,
- hyperactivity,
- white patches or sores inside your mouth or on your lips,
- unusual or unpleasant taste in your mouth,
- diaper rash in an infant taking liquid cefuroxime,
- itching or skin rash,
- hives,
- anemia,
- vaginal itching or discharge, or
- vaginal yeast infection.
Dosage for Cefuroxime
The recommended dosage of cefuroxime and dextrose is 750 mg to 1.5 grams every 8 hours for 5 to 10 days.
To reduce the development of drug-resistant bacteria and maintain the effectiveness of Cefuroxime (cefuroxime (cefuroxime injection) injection) for Injection USP and Dextrose Injection USP and other antibacterial drugs, Cefuroxime (cefuroxime (cefuroxime injection) injection) for Injection USP and Dextrose Injection USP should be used only to treat or prevent infections that are proven or strongly suspected to be caused by bacteria.
DESCRIPTION
Cefuroxime (cefuroxime (cefuroxime injection) injection) for Injection USP and Dextrose Injection USP is a sterile, nonpyrogenic, single use, packaged combination of Cefuroxime Sodium USP (crystalline) and Dextrose Injection USP (diluent) in the DUPLEX sterile container. The DUPLEX Container is a flexible dual chamber container.
The drug chamber is filled with sterile crystalline Cefuroxime (cefuroxime (cefuroxime injection) injection) for Injection USP, a semisynthetic, broad-spectrum, cephalosporin antibiotic for parenteral administration. It is the sodium salt of (6R,7R)-7-[2-(2-furyl)glyoxylamido]-3-(hydroxymethyl)-8-oxo-5-thia-1-azabicyclo[4.2.0]oct-2-ene-2-carboxylate, 72-(Z)-(O-methyloxime), carbamate (ester).
Cefuroxime (cefuroxime (cefuroxime injection) injection) Sodium USP has the following structural formula:
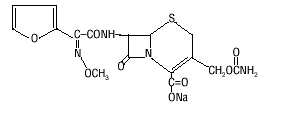 |
The empirical formula is C16H15N4NaO8S, representing a molecular weight of 446.4.
Cefuroxime (cefuroxime (cefuroxime injection) injection) contains approximately 54.2 mg (2.4 mEq) of sodium per gram of cefuroxime (cefuroxime (cefuroxime injection) injection) activity.
The diluent chamber contains Dextrose Injection USP. The concentration of Hydrous Dextrose USP has been adjusted to render the reconstituted drug product iso-osmotic. Dextrose Injection USP is sterile, nonpyrogenic, and contains no bacteriostatic or antimicrobial agents.
Hydrous Dextrose USP has the following structural (molecular) formula:
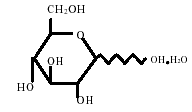 |
The molecular weight of Hydrous Dextrose USP is 198.17.
Dextrose hydrous USP has been added to the diluent to adjust osmolality (approximately 1.45 g and 2.05 g to 750 mg and 1.5 g dosages, respectively).
After removing the peelable foil strip, activating the seals, and thoroughly mixing, the reconstituted drug product is intended for single intravenous use. When reconstituted, the approximate osmolality of the reconstituted solution for Cefuroxime (cefuroxime (cefuroxime injection) injection) for Injection USP and Dextrose Injection USP is 290 mOsmol/kg.
The DUPLEX Container is Latex-free, PVC-free, and Di (2-ethylhexyl) phthalate (DEHP)-free.
The DUPLEX dual chamber container is made from a specially formulated material. The product (diluent and drug) contact layer is a mixture of thermoplastic rubber and a polypropylene ethylene copolymer that contains no plasticizers. The safety of the container system is supported by USP biological evaluation procedures.
Indications
Cefuroxime (cefuroxime (cefuroxime injection) injection) for Injection USP and Dextrose Injection USP is indicated for the treatment of patients with infections caused by susceptible strains of the designated organisms in the following diseases:
- Lower Respiratory Tract Infections, including pneumonia, caused by Streptococcus pneumoniae, Haemophilus influenzae (including ampicillin-resistant strains), Klebsiella spp., Staphylococcus aureus (penicillinase- and non-penicillinase-producing strains), Streptococcus pyogenes, and Escherichia coli.
- Urinary Tract Infections caused by Escherichia coli and Klebsiella spp.
- Skin and Skin-Structure Infections caused by Staphylococcus aureus (penicillinase- and non-penicillinase-producing strains), Streptococcus pyogenes, Escherichia coli, Klebsiella spp., and Enterobacter spp.
- Septicemia caused by Staphylococcus aureus (penicillinase- and non-penicillinase-producing strains), Streptococcus pneumoniae, Escherichia coli, Haemophilus influenzae (including ampicillin-resistant strains), and Klebsiella spp.
- Meningitis caused by Streptococcus pneumoniae, Haemophilus influenzae (including ampicillin-resistant strains), Neisseria meningitidis, and Staphylococcus aureus (penicillinase- and non-penicillinase-producing strains).
- Gonorrhea: Uncomplicated and disseminated gonococcal infections due to Neisseria gonorrhoeae (penicillinase- and non-penicillinase-producing strains) in both males and females.
- Bone and Joint Infections caused by Staphylococcus aureus (penicillinase- and non-penicillinase producing strains).
Clinical microbiological studies in skin and skin-structure infections frequently reveal the growth of susceptible strains of both aerobic and anaerobic organisms. Cefuroxime (cefuroxime (cefuroxime injection) injection) has been used successfully in these mixed infections in which several organisms have been isolated.
In certain cases of confirmed or suspected gram-positive or gram-negative sepsis or in patients with other serious infections in which the causative organism has not been identified, cefuroxime (cefuroxime (cefuroxime injection) injection) may be used concomitantly with an aminoglycoside (see PRECAUTIONS). The recommended doses of both antibiotics may be given depending on the severity of the infection and the patient's condition.
To reduce the development of drug-resistant bacteria and maintain the effectiveness of Cefuroxime (cefuroxime (cefuroxime injection) injection) for Injection USP and Dextrose Injection USP and other antibacterial drugs, Cefuroxime (cefuroxime (cefuroxime injection) injection) for Injection USP and Dextrose Injection USP should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
Prevention: The preoperative prophylactic administration of Cefuroxime (cefuroxime (cefuroxime injection) injection) for Injection USP and Dextrose Injection USP may prevent the growth of susceptible disease-causing bacteria and thereby may reduce the incidence of certain postoperative infections in patients undergoing surgical procedures (e.g., vaginal hysterectomy) that are classified as clean-contaminated or potentially contaminated procedures. Effective prophylactic use of antibiotics in surgery depends on the time of administration. Cefuroxime (cefuroxime (cefuroxime injection) injection) for Injection USP and Dextrose Injection USP should usually be given one-half to 1 hour before the operation to allow sufficient time to achieve effective antibiotic concentrations in the wound tissues during the procedure. The dose should be repeated intraoperatively if the surgical procedure is lengthy.
Prophylactic administration is usually not required after the surgical procedure ends and should be stopped within 24 hours. In the majority of surgical procedures, continuing prophylactic administration of any antibiotic does not reduce the incidence of subsequent infections but will increase the possibility of adverse reactions and the development of bacterial resistance.
The perioperative use of Cefuroxime (cefuroxime (cefuroxime injection) injection) for Injection USP and Dextrose Injection USP has also been effective during open heart surgery for surgical patients in whom infections at the operative site would present a serious risk. For these patients it is recommended that cefuroxime (cefuroxime (cefuroxime injection) injection) therapy be continued for at least 48 hours after the surgical procedure ends. If an infection is present, specimens for culture should be obtained for the identification of the causative organism, and appropriate antimicrobial therapy should be instituted.
Dosage
DOSAGE AND ADMINISTRATION
This product is intended for intravenous administration only.
Dosage: Adults: The usual adult dosage range for cefuroxime (cefuroxime (cefuroxime injection) injection) is 750 mg to 1.5 grams every 8 hours, usually for 5 to 10 days. In uncomplicated urinary tract infections, skin and skin-structure infections, disseminated gonococcal infections, and uncomplicated pneumonia, a 750 mg dose every 8 hours is recommended. In severe or complicated infections, a 1.5 gram dose every 8 hours is recommended.
In bone and joint infections, a 1.5 gram dose every 8 hours is recommended. In clinical trials, surgical intervention was performed when indicated as an adjunct to cefuroxime (cefuroxime (cefuroxime injection) injection) therapy. A course of oral antibiotics was administered when appropriate following the completion of parenteral administration of cefuroxime (cefuroxime (cefuroxime injection) injection) .
In life-threatening infections or infections due to less susceptible organisms, 1.5 grams every 6 hours may be required. In bacterial meningitis, the dosage should not exceed 3 grams every 8 hours. For preventive use for clean-contaminated or potentially contaminated surgical procedures, a 1.5 gram dose administered intravenously just before surgery (approximately one-half to 1 hour before the initial incision) is recommended. Thereafter, give 750 mg intravenously every 8 hours when the procedure is prolonged.
For preventive use during open heart surgery, a 1.5 gram dose administered intravenously at the induction of anesthesia and every 12 hours thereafter for a total of 6 grams is recommended.
Impaired Renal Function: A reduced dosage must be employed when renal function is impaired. Dosage should be determined by the degree of renal impairment and the susceptibility of the causative organism (see Table 2).
Table 2: Dosage of Cefuroxime (cefuroxime (cefuroxime injection) injection) in Adults with Reduced Renal
Function
| Creatinine Clearance (mL/min) | Dose | Frequency |
| >20 | 750 mg-1.5 grams | q8h |
| 10-20 | 750 mg | q12h |
| <10 | 750 mg | q24h* |
*Since cefuroxime (cefuroxime (cefuroxime injection) injection) is dialyzable, patients on hemodialysis should be given a further dose at the end of the dialysis.
When only serum creatinine is available, the following formula2 (based on sex, weight, and age of the patient) may be used to convert this value into creatinine clearance. The serum creatinine should represent a steady state of renal function.
| Males: Creatinine clearance (mL/min) = | Weight (kg) x (140 - age) |
| 72 x serum creatinine (mg/dL) |
Females: 0.85 x male value
Note: As with antibiotic therapy in general, administration of Cefuroxime (cefuroxime (cefuroxime injection) injection) for Injection USP and Dextrose Injection USP should be continued for a minimum of 48 to 72 hours after the patient becomes asymptomatic or after evidence of bacterial eradication has been obtained; a minimum of 10 days of treatment is recommended in infections caused by Streptococcus pyogenes in order to guard against the risk of rheumatic fever or glomerulonephritis; frequent bacteriologic and clinical appraisal is necessary during therapy of chronic urinary tract infection and may be required for several months after therapy has been completed; persistent infections may require treatment for several weeks; and doses smaller than those indicated above should not be used. In staphylococcal and other infections involving a collection of pus, surgical drainage should be carried out where indicated.
Pediatric Patients Above 3 Months of Age: Administration of 50 to 100 mg/kg/day in equally divided doses every 6 to 8 hours has been successful for most infections susceptible to cefuroxime (cefuroxime (cefuroxime injection) injection) . The higher dosage of 100 mg/kg/day (not to exceed the maximum adult dosage) should be used for the more severe or serious infections.
In bone and joint infections, 150 mg/kg/day (not to exceed the maximum adult dosage) is recommended in equally divided doses every 8 hours. In clinical trials, a course of oral antibiotics was administered to pediatric patients following the completion of parenteral administration of cefuroxime (cefuroxime (cefuroxime injection) injection) .
In cases of bacterial meningitis, a larger dosage of cefuroxime (cefuroxime (cefuroxime injection) injection) is recommended, 200 to 240 mg/kg/day intravenously in divided doses every 6 to 8 hours.
In pediatric patients with renal insufficiency, the frequency of dosing should be modified consistent with the recommendations for adults.
Cefuroxime (cefuroxime (cefuroxime injection) injection) for Injection USP and Dextrose for Injection USP in the DUPLEX® Container is designed to deliver a 750 mg or 1.5 g dose of cefuroxime (cefuroxime (cefuroxime injection) injection) . To prevent unintentional overdose, this product should not be used in pediatric patients who require less than the full adult dose.
For intermittent IV infusion with a Y-type administration set, dosing can be accomplished through the tubing system by which the patient may be receiving other IV solutions. However, during infusion of the solution containing Cefuroxime (cefuroxime (cefuroxime injection) injection) , it is advisable to temporarily discontinue administration of any other solutions at the same site.
Solutions of cefuroxime (cefuroxime (cefuroxime injection) injection) , like those of most beta-lactam antibiotics, should not be added to solutions of aminoglycoside antibiotics because of potential interaction.
However, if concurrent therapy with cefuroxime (cefuroxime (cefuroxime injection) injection) and an aminoglycoside is indicated, each of these antibiotics can be administered separately to the same patient.
Use sterile equipment.
Caution: Do not use plastic containers in series connections. Such use could result in air embolism due to residual air being drawn from the primary container before administration of the fluid from the secondary container is complete.
DUPLEX® Drug Delivery System Directions for Use
Removal from Multi-Pack Tray
- Tear tape strips from one or both sides of the trays. Remove top tray.
- To avoid inadvertent activation, DUPLEX Container should remain in the folded position until activation is intended.
Patient Labeling and Drug Powder/Diluent Inspection
- Apply patient-specific label on foil side of container. USE CARE to avoid activation. Do not cover any portion of foil strip with patient label.
- Unlatch side tab and unfold DUPLEX Container. (See Diagram 1.)
- Visually inspect diluent chamber for particulate matter.
- Use only if container and seals are intact.
- To inspect the drug powder for foreign matter or discoloration, peel foil strip from drug chamber. (See Diagram 2.)
- Protect from light after removal of foil strip.
 |
 |
Note: If foil strip is removed, product must be used within 30 days, but not beyond the labeled expiration date.
- The product should be re-folded and the side tab latched until ready to activate.
Reconstitution (Activation)
- Do not use directly after storage by refrigeration, allow the product to equilibrate to room temperature before patient use.
- Unfold the Duplex Container and point the set port in a downward direction. Starting at the hanger tab end, fold the DUPLEX Container just below the diluent meniscus trapping all air above the fold. To activate, squeeze the folded diluent chamber until the seal between the diluent and powder opens, releasing diluent into the drug powder chamber. (See Diagram 3.)
- Agitate the liquid-powder mixture until the drug powder is completely dissolved.
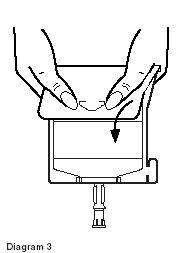 |
Note: Following reconstitution (activation), product must be used within 24 hours if stored at room temperature or within 7 days if stored under refrigeration.
Administration
- Visually inspect the reconstituted solution for particulate matter.
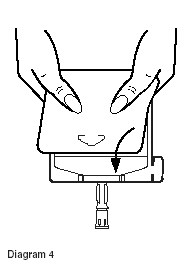
- Point the set port in a downwards direction. Starting at the hanger tab end, fold the DUPLEX® Container just below the solution meniscus trapping all air above the fold. Squeeze the folded DUPLEX Container until the seal between reconstituted drug solution and set port opens, releasing liquid to set port. (See Diagram 4.)
- Prior to attaching the IV set, check for minute leaks by squeezing container firmly. If leaks are found, discard container and solution as sterility may be impaired.
- Using aseptic technique, remove the set port cover from the set port and attach sterile administration set.
- Refer to Directions for Use accompanying the administration set.
 |
Precautions
- As with other cephalosporins, reconstituted Cefuroxime (cefuroxime (cefuroxime injection) injection) for Injection USP and Dextrose Injection USP tends to darken depending on storage conditions, within the stated recommendations. However, product potency is not adversely affected.
- Use only if prepared solution is clear and free from particulate matter.
- Do not use in series connection.
- Do not introduce additives into the DUPLEX Container.
- Do not freeze.
HOW SUPPLIED
Cefuroxime (cefuroxime (cefuroxime injection) injection) for Injection USP and Dextrose Injection USP in the DUPLEX Drug Delivery System is a flexible dual chamber container supplied in two concentrations. After reconstitution, the concentrations are equivalent to 750 mg and 1.5 g cefuroxime (cefuroxime (cefuroxime injection) injection) . The diluent chamber contains approximately 50 mL of Dextrose Injection USP. Dextrose Injection USP has been adjusted to 4.1% and 2.9% for the 750 mg and 1.5 g doses, respectively, such that the reconstituted solution is iso-osmotic.
Cefuroxime (cefuroxime (cefuroxime injection) injection) for Injection USP and Dextrose Injection USP is supplied sterile and nonpyrogenic in the DUPLEX Drug Delivery System containers packaged 12 units per tray, 2 trays per case.
| NDC | Cat. No. | Dose | Volume |
| Cefuroxime for Injection USP and Dextrose Injection USP | |||
| 0264-3112-11 | 3112-11 | 750 mg | 50 mL |
| Cefuroxime for Injection USP and Dextrose Injection USP | |||
| 0264-3114-11 | 3114-11 | 1.5 g | 50 mL |
Store the unactivated unit at 20-25°C (68-77°F). Excursions permitted to 15-30°C (59-86°F).
REFERENCES2.Cockcroft, DW., and Gault MH.: Prediction of creatinine clearance from serum creatinine. Nephron. 16:31-41, 1976.
DUPLEX® is a registered trademark of B. Braun Medical Inc., Clinitest® is a registered trademark of Ames Division, Miles Laboratories, Inc., Made in USA. Revised: January 2007, B. Braun Medical Inc., Irvine, CA USA 92614-5895. FDA revision date: 9/10/2007
Side Effects & Drug Interactions
SIDE EFFECTS
Cefuroxime (cefuroxime (cefuroxime injection) injection) is generally well tolerated. The most common adverse effects have been local reactions following IV administration. Other adverse reactions have been encountered only rarely.
Local Reactions: Thrombophlebitis has occurred with IV administration in 1 in 60 patients.
Gastrointestinal: Gastrointestinal symptoms occurred in 1 in 150 patients and included diarrhea (1 in 220 patients) and nausea (1 in 440 patients). The onset of pseudomembranous colitis may occur during or after antibacterial treatment (see WARNINGS).
Hypersensitivity Reactions: Hypersensitivity reactions have been reported in fewer than 1% of the patients treated with cefuroxime (cefuroxime (cefuroxime injection) injection) and include rash (1 in 125). Pruritus, urticaria, and positive Coombs' test each occurred in fewer than 1 in 250 patients, and, as with other cephalosporins, rare cases of anaphylaxis, drug fever, erythema multiforme, interstitial nephritis, toxic epidermal necrolysis, and Stevens-Johnson syndrome have occurred.
Blood: A decrease in hemoglobin and hematocrit has been observed in 1 in 10 patients and transient eosinophilia in 1 in 14 patients. Less common reactions seen were transient neutropenia (fewer than 1 in 100 patients) and leukopenia (1 in 750 patients). A similar pattern and incidence were seen with other cephalosporins used in controlled studies. As with other cephalosporins, there have been rare reports of thrombocytopenia.
Hepatic: Transient rise in SGOT and SGPT (1 in 25 patients), alkaline phosphatase (1 in 50 patients), LDH (1 in 75 patients), and bilirubin (1 in 500 patients) levels has been noted.
Kidney: Elevations in serum creatinine and/or blood urea nitrogen and a decreased creatinine clearance have been observed, but their relationship to cefuroxime (cefuroxime (cefuroxime injection) injection) is unknown.
Postmarketing Experience with Cefuroxime (cefuroxime (cefuroxime injection) injection) : In addition to the adverse events reported during clinical trials, the following events have been observed during clinical practice in patients treated with cefuroxime (cefuroxime (cefuroxime injection) injection) and were reported spontaneously. Data are generally insufficient to allow an estimate of incidence or to establish causation.
Neurologic: Seizure.
Non-site specific: Angioedema.
Cephalosporin-class Adverse Reactions: In addition to the adverse reactions listed above that have been observed in patients treated with cefuroxime (cefuroxime (cefuroxime injection) injection) , the following adverse reactions and altered laboratory tests have been reported for cephalosporin-class antibiotics:
Adverse Reactions: Vomiting, abdominal pain, colitis, vaginitis including vaginal candidiasis, toxic nephropathy, hepatic dysfunction including cholestasis, aplastic anemia, hemolytic anemia, and hemorrhage.
Several cephalosporins, including cefuroxime (cefuroxime (cefuroxime injection) injection) , have been implicated in triggering seizures, particularly in patients with renal impairment when the dosage was not reduced (see DOSAGE AND ADMINISTRATION). If seizures associated with drug therapy should occur, the drug should be discontinued. Anticonvulsant therapy can be given if clinically indicated.
Altered Laboratory Tests: Prolonged prothrombin time, pancytopenia, agranulocytosis.
DRUG INTERACTIONS
Drug/Laboratory Test Interactions
A false-positive reaction for glucose in the urine may occur with copper reduction tests (Benedict's or Fehling's solution or with Clinitest® tablets) but not with enzyme-based tests for glycosuria. As a false-negative result may occur in the ferricyanide test, it is recommended that either the glucose oxidase or hexokinase method be used to determine blood plasma glucose levels in patients receiving cefuroxime (cefuroxime injection) .
Cefuroxime (cefuroxime (cefuroxime injection) injection) does not interfere with the assay of serum and urine creatinine by the alkaline picrate method.
Warnings
BEFORE THERAPY WITH CEFUROXIME (cefuroxime (cefuroxime injection) injection) FOR INJECTION USP AND DEXTROSE INJECTION USP IS INSTITUTED, CAREFUL INQUIRY SHOULD BE MADE TO DETERMINE WHETHER THE PATIENT HAS HAD PREVIOUS HYPERSENSITIVITY REACTIONS TO CEPHALOSPORINS, PENICILLINS OR OTHER DRUGS. THIS PRODUCT SHOULD BE GIVEN CAUTIOUSLY TO PENICILLIN-SENSITIVE PATIENTS. ANTIBIOTICS SHOULD BE ADMINISTERED WITH CAUTION TO ANY PATIENT WHO HAS DEMONSTRATED SOME FORM OF ALLERGY, PARTICULARLY TO DRUGS. IF AN ALLERGIC REACTION TO CEFUROXIME (cefuroxime (cefuroxime injection) injection) OCCURS, DISCONTINUE THE DRUG. SERIOUS ACUTE HYPERSENSITIVITY REACTIONS MAY REQUIRE EPINEPHRINE AND OTHER EMERGENCY MEASURES.
Clostridium difficile associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including Cefuroxime (cefuroxime (cefuroxime injection) injection) for Injection USP and Dextrose Injection USP, and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon leading to overgrowth of C. difficile.
C. difficile produces toxins A and B which contribute to the development of CDAD. Hypertoxin producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibiotic use. Careful medical history is necessary since CDAD has been reported to occur over two months after the administration of antibacterial agents.
If CDAD is suspected or confirmed, ongoing antibiotic use not directed against C. difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibiotic treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated.
Precautions
General
Although Cefuroxime (cefuroxime (cefuroxime injection) injection) for Injection USP and Dextrose Injection USP rarely produces alterations in kidney function, evaluation of renal status during therapy is recommended, especially in seriously ill patients receiving the maximum doses. Cephalosporins should be given with caution to patients receiving concurrent treatment with potent diuretics as these regimens are suspected of adversely affecting renal function.
The total daily dose of cefuroxime (cefuroxime (cefuroxime injection) injection) should be reduced in patients with transient or persistent renal insufficiency (see DOSAGE AND ADMINISTRATION), because high and prolonged serum antibiotic concentrations can occur in such individuals from usual doses.
As with other antibiotics, prolonged use of cefuroxime (cefuroxime (cefuroxime injection) injection) may result in overgrowth of nonsusceptible organisms. Careful observation of the patient is essential. If superinfection occurs during therapy, appropriate measures should be taken.
Broad-spectrum antibiotics should be prescribed with caution in individuals with a history of gastrointestinal disease, particularly colitis.
Nephrotoxicity has been reported following concomitant administration of aminoglycoside antibiotics and cephalosporins.
As with other therapeutic regimens used in the treatment of meningitis, mild-to-moderate hearing loss has been reported in a few pediatric patients treated with cefuroxime (cefuroxime (cefuroxime injection) injection) . Persistence of positive CSF (cerebrospinal fluid) cultures at 18 to 36 hours has also been noted with cefuroxime (cefuroxime (cefuroxime injection) injection) injection, as well as with other antibiotic therapies; however, the clinical relevance of this is unknown.
Cephalosporins may be associated with a fall in prothrombin activity. Those at risk include patients with renal or hepatic impairment, or poor nutritional state, as well as patients receiving a protracted course of antimicrobial therapy, and patients previously stabilized on anticoagulant therapy. Prothrombin time should be monitored in patients at risk and exogenous Vitamin K administered as indicated.
As with other dextrose-containing solutions, Cefuroxime (cefuroxime (cefuroxime injection) injection) for Injection USP and Dextrose Injection USP should be prescribed with caution in patients with overt or known subclinical diabetes mellitus or carbohydrate intolerance for any reason.
Prescribing Cefuroxime (cefuroxime (cefuroxime injection) injection) for Injection USP and Dextrose Injection USP in the absence of a proven or strongly suspected bacterial infection or a prophylactic indication is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Although lifetime studies in animals have not been performed to evaluate carcinogenic potential, no mutagenic activity was found for cefuroxime (cefuroxime (cefuroxime injection) injection) in the mouse lymphoma assay and a battery of bacterial mutation tests. Positive results were obtained in an in vitro chromosome aberration assay, however, negative results were found in an in vivo micronucleus test at doses up to 10 g/kg. Reproduction studies in mice at doses up to 3,200 mg/kg/day (3.1 times the recommended maximum human dose based on mg/m²) have revealed no impairment of fertility.
Reproductive studies revealed no impairment of fertility in animals.
Pregnancy
Teratogenic Effects - Pregnancy Category B.
Reproduction studies have been performed in mice at doses up to 6,400 mg/kg/day (6.3 times the recommended maximum human dose based on mg/m²), and rabbits at doses up to 400 mg/kg/day (2.1 times the recommended maximum human dose based on mg/m²) and have revealed no evidence of impaired fertility or harm to the fetus due to cefuroxime (cefuroxime (cefuroxime injection) injection) . There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Nursing Mothers
Since cefuroxime (cefuroxime (cefuroxime injection) injection) is excreted in human milk, caution should be exercised when cefuroxime (cefuroxime (cefuroxime injection) injection) is administered to a nursing woman.
Pediatric Use
Safety and effectiveness in pediatric patients below 3 months of age have not been established. Accumulation of other members of the cephalosporin class in newborn infants (with resulting prolongation of drug half-life) has been reported.
Cefuroxime (cefuroxime (cefuroxime injection) injection) for Injection USP and Dextrose Injection USP in the DUPLEX® Container is designed to deliver a 750 mg or 1.5 g dose of cefuroxime (cefuroxime (cefuroxime injection) injection) . To prevent unintentional overdose, this product should not be used in pediatric patients who require less than the full adult dose of cefuroxime (cefuroxime (cefuroxime injection) injection) .
Geriatric Use
Of the 1,914 subjects who received cefuroxime (cefuroxime (cefuroxime injection) injection) in 24 clinical studies of cefuroxime (cefuroxime (cefuroxime injection) injection) , 901 (47%) were 65 and over while 421 (22%) were 75 and over. No overall differences in safety or effectiveness were observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater susceptibility of some older individuals to drug effects cannot be ruled out. This drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function (see DOSAGE AND ADMINISTRATION).
Overdosage & Contraindications
OVERDOSE
Overdosage of cephalosporins can cause cerebral irritation leading to convulsions. Serum levels of cefuroxime (cefuroxime (cefuroxime injection) injection) can be reduced by hemodialysis and peritoneal dialysis.
CONTRAINDICATIONS
Cefuroxime (cefuroxime (cefuroxime injection) injection) for Injection USP and Dextrose Injection USP is contraindicated in patients with known allergy to the cephalosporin group of antibiotics. Solutions containing dextrose may be contraindicated in patients with hypersensitivity to corn products.
Clinical Pharmacology
Following IV doses of 750 mg and 1.5 g, serum concentrations were approximately 50 and 100 mcg/mL, respectively, at 15 minutes. Therapeutic serum concentrations of approximately 2 mcg/mL or more were maintained for 5.3 hours and 8 hours or more, respectively. There was no evidence of accumulation of cefuroxime (cefuroxime (cefuroxime injection) injection) in the serum following IV administration of 1.5 g doses every 8 hours to normal volunteers. The serum half-life after IV injection is approximately 80 minutes.
Approximately 89% of a dose of cefuroxime (cefuroxime (cefuroxime injection) injection) is excreted by the kidneys over an 8 hour period, resulting in high urinary concentrations.
Intravenous doses of 750 mg and 1.5 g produced urinary levels averaging 1,150 and 2,500 mcg/mL, respectively, during the first 8 hour period.
The concomitant oral administration of probenecid with cefuroxime (cefuroxime (cefuroxime injection) injection) slows tubular secretion, decreases renal clearance by approximately 40%, increases the peak serum level by approximately 30%, and increases the serum half-life by approximately 30%. Cefuroxime (cefuroxime (cefuroxime injection) injection) is detectable in therapeutic concentrations in pleural fluid, joint fluid, bile, sputum, bone, cerebrospinal fluid (in patients with meningitis), and aqueous humor.
Cefuroxime (cefuroxime (cefuroxime injection) injection) is detectable in therapeutic concentrations in cerebrospinal fluid (CSF) of adults and pediatric patients with meningitis. The following table shows the concentrations of cefuroxime (cefuroxime (cefuroxime injection) injection) achieved in cerebrospinal fluid during multiple dosing of patients with meningitis.
Table 1. Concentrations of Cefuroxime (cefuroxime (cefuroxime injection) injection) Achieved in Cerebrospinal
Fluid During Multiple Dosing of Patients with Meningitis
| Patients | Dose | Number of Patients |
Mean (Range) CFS Cefuroxime (cefuroxime (cefuroxime injection) injection) Concentrations (mcg/mL) Achieved Within 8 Hours Post Dose |
| Pediatric patients (4 weeks to 6.5 years) |
200 mg/kg/day, divided q 6 hours | 5 | 6.6 (0.9-17.3) |
| Pediatric patients (7 months to 9 years) |
200 to 230 mg/kg/day, divided q 8 hours | 6 | 8.3 (<2-22.5) |
| Adults | 1.5 grams q 8 hours | 2 | 5.2 (2.7-8.9) |
| Adults | 1.5 grams q 6 hours | 10 | 6.0 (1.5-13.5) |
Cefuroxime (cefuroxime (cefuroxime injection) injection) is approximately 50% bound to serum protein.
Microbiology: Cefuroxime (cefuroxime (cefuroxime injection) injection) has in vitro activity against a wide range of gram-positive and gram-negative organisms, and it is highly stable in the presence of beta-lactamases of certain gram-negative bacteria. The bactericidal action of cefuroxime (cefuroxime (cefuroxime injection) injection) results from inhibition of cell-wall synthesis.
Cefuroxime (cefuroxime (cefuroxime injection) injection) is usually active against the following organisms in vitro.
Aerobes, Gram-positive
Staphylococcus aureus
Staphylococcus epidermidis
Streptococcus pneumoniae, and
Streptococcus pyogenes (and other streptococci)
NOTE: Most strains of enterococci, e.g., Enterococcus faecalis (formerly Streptococcus faecalis), are resistant to cefuroxime (cefuroxime (cefuroxime injection) injection) . Methicillin-resistant staphylococci and Listeria monocytogenes are resistant to cefuroxime (cefuroxime (cefuroxime injection) injection) .
Aerobes, Gram-negative
Citrobacter spp.
Enterobacter spp.
Escherichia coli
Haemophilus influenzae (including ampicillin-resistant strains)
Haemophilus parainfluenzae
Klebsiella spp. (including Klebsiella pneumoniae)
Moraxella (Branhamella) catarrhalis (including ampicillin- and
cephalothin-resistant strains)
Morganella morganii (formerly Proteus morganii)
Neisseria gonorrhoeae (including penicillinase- and non-penicillinase-producing
strains)
Neisseria meningitidis
Proteus mirabilis
Providencia rettgeri (formerly Proteus rettgeri)
Salmonella spp., and Shigella spp.
NOTE: Some strains of Morganella morganii, Enterobacter cloacae, and Citrobacter spp. have been shown by in vitro tests to be resistant to cefuroxime (cefuroxime (cefuroxime injection) injection) and other cephalosporins. Pseudomonas and Campylobacter spp., Acinetobacter calcoaceticus, and most strains of Serratia spp. and Proteus vulgaris are resistant to most first- and second-generation cephalosporins.
Anaerobes: Gram-positive and gram-negative cocci (including Peptococcus and Peptostreptococcus spp.), gram-positive bacilli (including Clostridium spp.), and gram-negative bacilli (including Bacteroides and Fusobacterium spp.).
NOTE: Clostridium difficile and most strains of Bacteroides fragilis are resistant to cefuroxime (cefuroxime (cefuroxime injection) injection) .
Susceptibility Tests
Diffusion Techniques
Quantitative methods that require measurement of zone diameters give an estimate of antibiotic susceptibility. One such standard procedure1 that has been recommended for use with disks to test susceptibility of organisms to cefuroxime (cefuroxime injection) uses the 30 mcg cefuroxime (cefuroxime (cefuroxime injection) injection) disk. Interpretation involves the correlation of the diameters obtained in the disk test with the minimum inhibitory concentration (MIC) for cefuroxime (cefuroxime (cefuroxime injection) injection) .
A report of &lequoSusceptible” indicates that the pathogen is likely to be inhibited by generally achievable blood levels. A report of “Moderately Susceptible” suggests that the organism would be susceptible if high dosage is used or if the infection is confined to tissues and fluids in which high antibiotic levels are attained. A report of “ Intermediate” suggests an equivocable or indeterminate result. A report of “ Resistant” indicates that achievable concentrations of the antibiotic are unlikely to be inhibitory and other therapy should be selected.
Reports from the laboratory giving results of the standard single-disk susceptibility test for organisms other than Haemophilus spp. and Neisseria gonorrhoeae with a 30 mcg cefuroxime (cefuroxime (cefuroxime injection) injection) disk should be interpreted according to the following criteria:
| Zone Diameter (mm) | Interpretation |
| ≥ 18 | (S) Susceptible |
| 15-17 | (MS) Moderately Susceptible |
| ≤ 14 | (R) Resistant |
Results for Haemophilus spp. should be interpreted according to the following criteria:
| Zone Diameter (mm) | Interpretation |
| ≥ 24 | (S) Susceptible |
| 21-23 | (I) Intermediate |
| ≤ 20 | (R) Resistant |
Results for Neisseria gonorrhoeae should be interpreted according to the following criteria:
| Zone Diameter (mm) | Interpretation |
| ≥ 31 | (S) Susceptible |
| 26-30 | (MS) Moderately Susceptible |
| &le25 | (R) Resistant |
Organisms should be tested with the cefuroxime (cefuroxime injection) disk since cefuroxime (cefuroxime (cefuroxime injection) injection) has been shown by in vitro tests to be active against certain strains found resistant when other beta-lactam disks are used. The cefuroxime (cefuroxime (cefuroxime injection) injection) disk should not be used for testing susceptibility to other cephalosporins.
Standardized procedures require the use of laboratory control organisms. The 30 mcg cefuroxime (cefuroxime (cefuroxime injection) injection) disk should give the following zone diameters.
1.Testing for organisms other than Haemophilus spp. and Neisseria gonorrhoeae:
| Organism | Zone Diameter (mm) |
| Staphylococcus aureus ATCC 25923 | 27-35 |
| Escherichia coli ATCC 25922 | 20-26 |
2. Testing for Haemophilus spp.:
| Organism | Zone Diameter (mm) |
| Haemophilus influenzae ATCC 49766 | 28-36 |
3. Testing for Neisseria gonorrhoeae:
| Organism | Zone Diameter (mm) |
| Neisseria gonorrhoeae ATCC 49226 | 33-41 |
| Staphylococcus aureus ATCC 25923 | 29-33 |
Dilution Techniques
Use a standardized dilution method1 (broth, agar, microdilution) or equivalent with cefuroxime (cefuroxime (cefuroxime injection) injection) powder. The MIC values obtained for bacterial isolates other than Haemophilus spp. and Neisseria gonorrhoeae should be interpreted according to the following criteria:
| MIC (mcg/mL) | Interpretation |
| ≤ 8 | (S) Susceptible |
| 16 | (MS) Moderately Susceptible |
| ≥ 32 | (R) Resistant |
MIC values obtained for Haemophilus spp. should be interpreted according to the following criteria:
| MIC (mcg/mL) | Interpretation |
| ≤ 4 | (S) Susceptible |
| 8 | (I) Intermediate |
| ≥ 16 | (R) Resistant |
MIC values obtained for Neisseria gonorrhoeae should be interpreted according to the following criteria:
| MIC (mcg/mL) | Interpretation |
| ≤ 1 | (S) Susceptible |
| 2 | (MS) Moderately Susceptible |
| ≥ 4 | (R) Resistant |
As with standard diffusion techniques, dilution methods require the use of laboratory control organisms. Standard cefuroxime (cefuroxime (cefuroxime injection) injection) powder should provide the following MIC values.
1. For organisms other than Haemophilus spp. and Neisseria gonorrhoeae:
| Organism | MIC (mcg/mL) |
| Staphylococcus aureus ATCC 29213 | 0.5-2.0 |
| Escherichia coli ATCC 25922 | 2.0-8.0 |
2. For Haemophilus spp.:
| Organism | MIC (mcg/mL) |
| Haemophilus influenzae ATCC 49766 | 0.25-1.0 |
3. For Neisseria gonorrhoeae:
| Organism | MIC (mcg/mL) |
| Neisseria gonorrhoeae ATCC 49226 | 0.25-1.0 |
| Staphylococcus aureus ATCC 29213 | 0.25-1.0 |
REFERENCES
1. National Committee for Clinical Laboratory Standards. Performance Standards for Antimicrobial Susceptibility Testing. Third Informational Supplement. NCCLS Document M100-S3, Vol. 11, No. 17, Villanova, PA: NCCLS; 1991.
Medication Guide
PATIENT INFORMATION
Diarrhea is a common problem caused by antibiotics which usually ends when the antibiotic is discontinued. Sometimes after starting treatment with antibiotics, patients can develop watery and bloody stools (with or without stomach cramps and fever) even as late as two or more months after having taken the last dose of the antibiotic. If this occurs, patients should contact their physician as soon as possible.
Patients should be counseled that antibacterial drugs including Cefuroxime (cefuroxime (cefuroxime injection) injection) for Injection USP and Dextrose Injection USP should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When Cefuroxime (cefuroxime (cefuroxime injection) injection) for Injection USP and Dextrose Injection USP is prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by Cefuroxime (cefuroxime (cefuroxime injection) injection) for Injection USP and Dextrose Injection USP or other antibacterial drugs in the future.
