Zovirax Cream
- Generic Name: acyclovir cream, 5%
- Brand Name: Zovirax Cream
side effects drug center zovirax cream (acyclovir cream, 5%) drug
Drug Description
What is Zovirax Cream and how is it used?
Zovirax Cream is a prescription medicine used to treat the symptoms of cold sores (Herpes Labialis) and Genital Herpes. Zovirax Cream may be used alone or with other medications.
Zovirax Cream belongs to a class of drugs called Antivirals.
It is not known if Zovirax Cream is safe and effective in children younger than 12 years of age.
What are the possible side effects of Zovirax Cream?
Zovirax Cream may cause serious side effects including:
- easy bruising or bleeding,
- purple or red pinpoint spots under the skin,
- little or no urination,
- painful or difficult urination,
- swelling in your feet or ankles,
- feeling tired, and
- shortness of breath
Get medical help right away, if you have any of the symptoms listed above.
The most common side effects of Zovirax Cream include:
- nausea,
- vomiting,
- diarrhea,
- general ill feeling,
- headache, and
- mouth pain while using an acyclovir buccal tablet
Tell the doctor if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of Zovirax Cream. For more information, ask your doctor or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
DESCRIPTION
ZOVIRAX is the brand name for acyclovir, a synthetic nucleoside analogue active against herpes viruses. ZOVIRAX Cream, 5% is a formulation for topical administration.
The chemical name of acyclovir is 2-amino-1,9-dihydro-9-[(2-hydroxyethoxy)methyl]-6H-purin-6-one; it has the following structural formula:
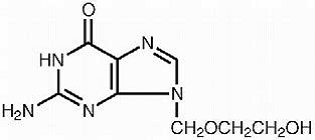 |
Acyclovir is a white, crystalline powder with the molecular formula C8H11N5O3 and a molecular weight of 225. The maximum solubility in water at 37°C is 2.5 mg/mL. The pKa's of acyclovir are 2.27 and 9.25.
Each gram of ZOVIRAX Cream, 5% contains 50 mg of acyclovir and the following inactive ingredients: cetostearyl alcohol, mineral oil, poloxamer 407, propylene glycol, sodium lauryl sulfate, water, and white petrolatum.
Indications & Dosage
INDICATIONS
ZOVIRAX Cream is a herpes simplex virus (HSV) deoxynucleoside analogue DNA polymerase inhibitor indicated for the treatment of recurrent herpes labialis (cold sores) in immunocompetent adults and adolescents 12 years of age and older.
DOSAGE AND ADMINISTRATION
ZOVIRAX Cream should be applied 5 times per day for 4 days. Therapy should be initiated as early as possible following the onset of signs or symptoms of herpes labialis, i.e. during the prodrome or when lesions appear.
For adolescents 12 years of age and older, the dosage is the same as in adults.
HOW SUPPLIED
Dosage Forms And Strengths
Each gram of ZOVIRAX Cream contains 50 mg (equivalent to 5% w/w) of acyclovir.
Storage And Handling
Each gram of ZOVIRAX Cream contains 50 mg (equivalent to 5% w/w) of acyclovir in an aqueous cream base. ZOVIRAX Cream is supplied as follows:
NDC 0187-0994-45: 5 g tubes
Store at or below 25°C (77°F); excursions permitted to 15° to 30°C (59° to 86°F) [see USP Controlled Room Temperature].
Distributed by: Bausch Health US, LLC Bridgewater, NJ 08807 USA. Manufactured by: Bausch Health Companies Inc. Laval, Quebec H7L 4A8, Canada. Revised: Dec 2020
Side Effects & Drug Interactions
SIDE EFFECTS
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug, and may not reflect the rates observed in clinical practice.
In five double-blind, placebo-controlled trials, 1124 patients were treated with ZOVIRAX Cream and 1161 with placebo (vehicle) cream. Local application site reactions were reported by 5% of patients receiving ZOVIRAX Cream and 4% of patients receiving placebo. The most common adverse reactions at the site of topical application were dry lips, desquamation, dryness of skin, cracked lips, burning skin, pruritus, flakiness of skin, and stinging on skin; each adverse reaction occurred in less than 1% of patients receiving ZOVIRAX Cream and placebo. Three patients on ZOVIRAX Cream and one patient on placebo discontinued treatment due to an adverse event.
An additional study, enrolling 22 healthy adults, was conducted to evaluate the dermal tolerance of ZOVIRAXCream compared with vehicle using single occluded and semi-occluded patch testing methodology. Both ZOVIRAX Cream and placebo showed a high and cumulative irritation potential. Another study, enrolling 251 healthy adults, was conducted to evaluate the contact sensitization potential of ZOVIRAX Cream using repeat insult patch testing methodology. Of 202 evaluable subjects, possible cutaneous sensitization reactions were observed in the same 4 (2%) subjects with both ZOVIRAX Cream and placebo, and these reactions to both ZOVIRAX Cream and placebo were confirmed in 3 subjects upon rechallenge. The sensitizing ingredient(s) has not been identified.
The safety profile in patients 12 to 17 years of age was similar to that observed in adults.
Postmarketing Experience
In addition to adverse events reported from clinical trials, the following events have been identified during postapproval use of acyclovir cream. Because they are reported voluntarily from a population of unknown size, estimates of frequency cannot be made. These events have been chosen for inclusion due to a combination of their seriousness, frequency of reporting, or potentialcausal connection to acyclovir cream.
General: Angioedema, anaphylaxis.
Skin: Contact dermatitis, eczema.
DRUG INTERACTIONS
Clinical experience has identified no interactions resulting from topical or systemic administration of other drugs concomitantly with ZOVIRAX Cream. Due to minimal systemic absorption of ZOVIRAX Cream, systemic drug interactions are unlikely.
Warnings & Precautions
WARNINGS
Included as part of the PRECAUTIONS section.
PRECAUTIONS
General
ZOVIRAX Cream should only beapplied on the affected external aspects of the lips and face in patients with herpes labialis. Because nodata areavailable,application to human mucous membranes is not recommended. ZOVIRAX Cream is intended for cutaneous use only and should not be used in the eye or inside the mouth or nose.
Contact Sensitization
ZOVIRAX Cream has a potential for irritation and contact sensitization [see ADVERSE REACTIONS].
The effect of ZOVIRAX Cream has not been established in immunocompromised patients.
Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (PATIENT INFORMATION).
General
Patients should beinformed that ZOVIRAX Cream is aprescription topical cream for the treatment of cold sores (recurrent herpes labialis) that occur on the face and lips. ZOVIRAX Cream is not a cure for cold sores. Patients should be instructed that ZOVIRAX Cream is intended for cutaneous use only for herpes labialis of the lips and around the mouth. Patients should be advised that ZOVIRAX Cream should not be used in the eye, inside the mouth or nose, or on the genitals. Patients should be instructed to avoid applying other topical products to the affected area while using ZOVIRAX Cream.
Do not use if you are allergic to ZOVIRAX Cream or any of the ingredients in ZOVIRAX Cream. Before you use ZOVIRAX Cream, tell your doctor if you are pregnant, planning to become pregnant, or are breast-feeding.
Instructions For Use
Treatment should be initiated at theearliestsign or symptom of recurrence. Instruct patients to wash hands prior to application and ensure the face and/or lips are clean and dry. Advise patients to apply ZOVIRAX Cream topically 5 times per day for 4 days. Instruct patients to topically apply a quantity of ZOVIRAX Cream sufficient to cover the affected area, including the outer margin. Advise patients to avoid unnecessary rubbing of the affected area to avoid aggravating or transferring the infection. Instruct patients to wash their hands with soap and water after using ZOVIRAX Cream. Keep out of reach of children.
Possible Side Effects
Common skin-related side effects that occurred when ZOVIRAX Cream was applied include application site reactions. ZOVIRAX Cream has the potential for irritation and contact sensitization.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment Of Fertility
Systemic exposure following topical administration of acyclovir is minimal. Dermalcarcinogenicity studies were not conducted. Results from the studies of carcinogenesis, mutagenesis and fertility are not included in the full prescribing information for ZOVIRAX Cream due to the minimal exposures of acyclovir that result from dermal application. Information on these studies is available in the full prescribing information for ZOVIRAX Capsules, Tablets, and Suspension and ZOVIRAX for Injection.
Use In Specific Populations
Pregnancy
Risk Summary
Acyclovir is minimally absorbed systemically following topical route of administration, and maternal use is not expected to result in fetal exposure to the ZOVIRAX Cream [see CLINICAL PHARMACOLOGY]. Experience with topical acyclovir use in pregnant women over several decades, based on published literature including observationalstudies, has not identified a drug-associated risk of major birth defects, miscarriage or adverse maternal or fetal outcomes. Animal reproduction studies with systemic exposure of acyclovir have been conducted. Refer to acyclovir prescribing information for additional details.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Lactation
Risk Summary
Acyclovir is minimally absorbed systemically following topical route of administration, and breastfeeding is not expected to result in exposure of the child to ZOVIRAX Cream [see CLINICAL PHARMACOLOGY]. There are no data on the effects of ZOVIRAX on the breastfed infant or on milk production. The developmental and health benefits of breastfeeding should beconsidered along with the mother’s clinical need for ZOVIRAX Cream and any potentialadverse effects on the breastfed child from ZOVIRAX Cream or from the underlying maternal condition.
Pediatric Use
An open-label, uncontrolled trial with ZOVIRAX Cream was conducted in 113 patients aged 12 to 17 years with recurrent herpes labialis. In this trial, therapy was applied using the same dosing regimen as in adults and subjects were followed for adverse events. The safety profile was similar to that observed in adults. Safety and effectiveness in pediatric patients less than 12 years of age have not been established.
Geriatric Use
Clinical studies of acyclovir cream did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. Systemic absorption of acyclovir after topical administration is minimal [see CLINICAL PHARMACOLOGY].
Overdosage & Contraindications
OVERDOSE
Overdosage by topical application of ZOVIRAX Cream is unlikely because of minimalsystemic exposure [see CLINICAL PHARMACOLOGY]. There is no information available for overdose.
CONTRAINDICATIONS
ZOVIRAX Cream is contraindicated in patients with known hypersensitivity to acyclovir, valacyclovir, or any component of the formulation.
Clinical Pharmacology
Mechanism Of Action
Acyclovir is an antiviral drug active against α-herpesviruses [see Microbiology].
Pharmacokinetics
A clinical pharmacology study was performed with ZOVIRAX Cream in adult volunteers to evaluate the percutaneous absorption of acyclovir. In this study, which included 6 male volunteers, the cream was applied to an area of 710 cm² on the backs of the volunteers 5 times daily at intervals of 2 hours for a total of 4 days. The weight of cream applied and urinary excretion of acyclovir were measured daily. Plasma concentration of acyclovir was assayed 1 hour after the final application. The average daily urinary excretion of acyclovir was approximately 0.04% of the daily applied dose. Plasma acyclovir concentrations were below the limit of detection (0.01 μM) in 5 subjects and barely detectable (0.014 μM) in 1 subject. Systemic absorption of acyclovir from ZOVIRAX Cream is minimal in adults.
The systemic absorption of acyclovir following topical application of cream has not been evaluated in patients <18 years of age.
Microbiology
Mechanism Of Action
Acyclovir is a synthetic purine deoxynucleoside analogue with cell culture and in vivo inhibitory activity against HSV types 1 (HSV-1) and 2 (HSV-2) DNA polymerases. It inhibits HSV-1 and HSV-2 replication in cell culture and in vivo.
The inhibitory activity of acyclovir is selective due to its affinity for the enzyme thymidine kinase (TK) encoded by HSV. This viral enzyme converts acyclovir into acyclovir monophosphate, a deoxynucleotide analogue. The monophosphate is further converted into diphosphate by cellular guanylate kinase and into triphosphate by a number of cellular enzymes. In biochemical assays, acyclovir triphosphate inhibits replication of α-herpes viral DNA. This inhibition is accomplished in 3 ways: 1) competitive inhibition of viralDNApolymerase, 2) incorporation into and termination of the growing viralDNA chain, and 3) inactivation of the viral DNA polymerase.
Antiviral Activity
The quantitative relationship between the susceptibility of herpes viruses to antivirals in cell culture and the clinical response to therapy has not been established in humans, and virus sensitivity testing has not been standardized. Sensitivity testing results, expressed as the concentration of drug required to inhibit by 50% the growth of virus in cell culture (EC50 value), vary greatly depending upon anumber of factors. Using plaque-reduction assays on Vero cells, the EC50 values of acyclovir against herpes simplex virus isolates range from 0.09 to 59.9 μM (0.02 to 13.5 μg/mL) for HSV-1 and from 0.04 to 44.0 μM (0.01 to 9.9 μg/mL) for HSV-2.
Resistance
In Cell Culture
Acyclovir-resistant HSV-1 and HSV-2 strains were isolated in cellculture. Acyclovir-resistant HSV resulted from mutations in the viral thymidine kinase (TK; pUL23) and DNA polymerase (POL; pUL30) genes. Frameshifts were commonly isolated and result in premature truncation of the HSV TKproduct with consequent decreased susceptibility to acyclovir. Mutations in theviralTKgene may lead to complete loss of TK activity (TK negative), reduced levels of TK activity (TK partial),or alteration in the ability of viralTKto phosphorylate the drug without an equivalentloss in the ability to phosphorylate thymidine (TKaltered). In cellculture the following resistance-associated substitutions in TK of HSV-1 and HSV-2 were observed (Table 1).
Table 1: Summary of Acyclovir (ACV) Resistance-associated Amino Acid Substitutions in Cell Culture
| HSV-1 | TK | P5A, H7Q, L50V, G56V, G59A, G61A, K62N, T63A, E83K, P84S, D116N, P131S, R163H, A167V, P173L, Q185R, R216S, R220H, T245M, R281stop, T287M, M322K |
| HSV-2 | TK | L69P, C172R, T288M |
| HSV-1 | POL | D368A, Y557S, E597D, V621S, L702H, N815S, V817M, G841C |
| HSV-2 | POL | - |
In HSV-Infected Patients
Clinical HSV-1 and HSV-2 isolates obtained from patients who failed treatment for their α-herpesvirus infections were evaluated for genotypic changes in the TK and POL genes and for phenotypic resistance to acyclovir(Table 2). HSV isolates with frameshift mutations and resistance-associated substitutions in TK and POLwere identified. The listing of substitutions in HSV TK and POL leading to decreased susceptibility to acyclovir is not all inclusive and additional changes willlikely beidentified in HSVvariants isolated from patients who fail acyclovir-containing regimens. The possibility of viralresistance to acyclovir should be considered in patients who fail to respond or experience recurrent viral shedding during therapy.
Table 2: Summary of ACV Resistance-associated Amino Acid Substitutions Observed in Treated Patients
| HSV-1 | TK | G6C, R32H, R41H, R51W, Y53C/D/H, Y53stop, D55N, G56D/S, P57H, H58/N/R/Y, G59R, G61A, K62N, T63I, Q67stop, S74stop, Y80N, E83K, P84L, Y87H, W88R, R89Q/W, E95stop, T103P, Q104H, Q104stop, H105P, D116N, M121L/R, S123R, Q125H, M128L, G129D, I143V, A156V, D162A/H/N, R163G/H, L170P, Y172C, P173L, A174P, A175V, R176Q/W, R176stop, L178R, S181N, V187M, A189V, V192A, G200C/D/S, T201P, V204G, A207P, L208F/H, R216C/H, R220C/H, R221H, R222C/H, L227F, T245M/P, L249P, Q250Stop, C251G, R256W, E257K, Q261R, T287M, L288Stop, L291P/R, L297S, L315S, L327R, C336Y, Q342Stop, T354P, L364P, A365T |
| HSV-2 | TK | R34C, G39E, R51W, Y53N, G59P, G61W, S66P, A72S, D78N, P85S, A94V, N100H, I101S, Q105P, T131P, D137stop, F140L, L158P, S169P, R177W, S182N, M183I, V192M, G201D, R217H, R221C/H, Q222stop, R223H, Y239stop, R271V, P272S, D273R, T287M, C337Y |
| HSV-1 | POL | K532T, Q570R, L583V, A605V, A657T, D672N, V715G, A719T/V, S724N, F733C, E771Q, S775N, L778M, E798K, V813M, N815S, G841S, I890M, G901V, V958L H1228D |
| Note: Additional substitutions to acyclovir resistance may exist. | ||
Cross-resistance
Cross-resistance has been observed among HSV isolates carrying frameshift mutations and resistance-associated substitutions, which confer reduced susceptibility to penciclovir (PCV), famciclovir (FCV), and foscarnet (FOS) [Table 3].
Table 3: Summary of Amino Acid Substitutions Conferring Cross-Resistance to PCV, FCV or FOS
| Cross-resistant to PCV/FCV | HSV-1 TK | G6C, R32H, R51W, Y53C/H, H58N, G61A, S74Stop, E83K, P84L, T103P, Q104Stop, D116N, M121R, I143V, R163H, L170P, Y172C, A174P, R176Q/W, Q185R, A189V, G200D, L208H, R216C, R220H, R222C/H, T245M, Q250Stop, R256W, R281Stop, T287M, L315S, M322K, C336Y |
| Cross-resistant to PCV/FCV | HSV-1 POL | A657T, D672N, V715G, A719V, S724N, E798K, N815S, G841S |
| Cross-resistant to PCV/FCV | HSV-2 TK | G39E, R51W, Y53N, R177W, R221H, T288M |
| Cross-resistant to PCV/FCV | HSV-2 POL | K533E, A606V, C625R, R628C, S729N, Q732R, M789K/T, V818A, N820S, F923L, T934A |
| Cross-resistant to FOS | HSV-1 POL | D368A, A605V, D672N, L702H, V715G, A719T/V, S724N, L778M, E798K, V813M, N815S, V817M, G841C/S, I890M, |
| Cross-resistant to FOS | HSV-2 POL | K533E, A606V, C625R, R628C, A724V, S725G, S729N, I731F, Q732R, M789K/T, V818A, Y823C, D912V, F923L, T934A, R964H |
Clinical Studies
Adult Subjects
ZOVIRAX Cream was evaluated in two double-blind, randomized, placebo (vehicle)-controlled trials for the treatment of recurrent herpes labialis. The average patient had five episodes of herpes labialis in the previous 12 months. In the first trial, the median ageof subjects was 37 years (range 18 to 81 years), 74% were female, and 94% were Caucasian. In the second trial, median age of subjects was 38 years (range 18 to 87 years), 73% were female, and 94% were Caucasian. Subjects were instructed to initiate treatment within 1 hour of noticing signs or symptoms and continue treatment for 4 days, with application of study medication 5 times per day. In both studies, the mean duration of the recurrent herpes labialis episode was approximately one-half day shorter in the subjects treated with ZOVIRAX Cream (n = 682) compared with subjects treated with placebo (n =703) for approximately 4.5 days versus 5 days, respectively. No significant difference was observed between subjects receiving ZOVIRAX Cream or placebo in the prevention of progression of cold sore lesions.
Pediatric Subjects
An open-label, uncontrolled trial with ZOVIRAX Cream was conducted in 113 patients aged 12 to 17 years with recurrent herpes labialis. In this trial, therapy was applied using the same dosing regimen as in adults and subjects were followed for adverse events. The safety profile was similar to that observed in adults.
Medication Guide
PATIENT INFORMATION
ZOVIRAX
(zho-vahy-rex)
(acyclovir) Cream
Important information: ZOVIRAX Cream is for use on cold sores on the lips and around the mouth only.
ZOVIRAX Cream should not be used in your eyes, mouth, nose, or on your genitals.
What is ZOVIRAX Cream?
- ZOVIRAX Cream is a prescription medicine used to treat cold sores (herpes labialis) that are recurring in adults and children 12 years of age and older, and who have normal immune systems.
- ZOVIRAX Cream is not a cure for cold sores.
It is not known if ZOVIRAX Cream is safe and effective in children less than 12 years of age.
Do not use ZOVIRAX Cream if you are allergic to acyclovir, valacyclovir, or any of the ingredients in ZOVIRAX Cream. See the end of this leaflet for a complete list of ingredients in ZOVIRAX Cream.
What should I tell my healthcare provider before using ZOVIRAX Cream?
Before using ZOVIRAX Cream, tell your healthcare provider about all of your medical conditions, including if you:
- become sick very easily (have a weak immune system).
- are pregnant or plan to become pregnant. It is not known if ZOVIRAX Cream will harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if ZOVIRAX Cream passes into your breast milk. Talk to your healthcare provider about the best way to feed your baby if you use ZOVIRAX Cream.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
How should I use ZOVIRAX Cream?
- Use ZOVIRAX Cream exactly as your healthcare provider tells you to use it.
- Use ZOVIRAX Cream as soon as you have the first symptoms of a cold sore such as itching, redness, burning or tingling, or when the cold sore appears.
- Wash your hands with soap and water before and after applying ZOVIRAX Cream.
- The affected area should be clean and dry before applying ZOVIRAX Cream.
- Apply ZOVIRAX Cream to the affected area 5 times each day for 4 days, including the outer edge.
- You should not apply other skin products to the affected area during treatment with ZOVIRAX Cream.
- Avoid unnecessary rubbing of the cold sore because this may cause the cold sore to spread to other areas around your mouth or make your cold sore worse.
What are the possible side effects of ZOVIRAX Cream?
The most common side effects of ZOVIRAX Cream are skin reactions at the treatment site and may include: dry or cracked lips, peeling, flaking or dryness of the skin, a burning or stinging feeling, and itching.
These are not all the possible side effects of ZOVIRAX Cream.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store ZOVIRAX Cream?
- Store ZOVIRAX Cream at room temperature between 68° to 77°F (20° to 25°C).
Keep ZOVIRAX Cream and all medicines out of reach of children.
General information about the safe and effective use of ZOVIRAX Cream.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use ZOVIRAX Cream for a condition for which it was not prescribed. Do not give ZOVIRAX Cream to other people, even if they have the same symptoms you have. It may harm them. You can ask your pharmacist or healthcare provider for information about ZOVIRAX Cream that is written for health professionals.
What are the ingredients in ZOVIRAX Cream?
Active ingredient: acyclovir
Inactive ingredients: cetostearyl alcohol, mineral oil, poloxamer 407, propylene glycol, sodium lauryl sulfate, water, and white petrolatum
This Patient Information has been approved by the U.S. Food and Drug Administration.




