Skyrizi Drug Description: Detailed Datasheet
- Generic Name: risankizumab-rzaa injection
- Brand Name: Skyrizi
side effects drug center skyrizi (risankizumab-rzaa injection) drug
Drug Description
What is Skyrizi and how is it used?
Skyrizi is a prescription medicine used to treat the symptoms of Plaque Psoriasis. Skyrizi may be used alone or with other medications.
Skyrizi belongs to a class of drugs called Antipsoriatics, Systemic; Interleukin Inhibitors; Monoclonal Antibodies.
It is not known if Skyrizi is safe and effective in children younger than 18 years of age.
What are the possible side effects of Skyrizi?
Skyrizi may cause serious side effects including:
- fever,
- chills,
- sweating,
- body aches,
- shortness of breath,
- cough,
- bloody mucus,
- mouth sores,
- red or swollen gums,
- stomach pain,
- diarrhea,
- increased urination,
- burning when you urinate,
- pale skin,
- easy bruising,
- unusual bleeding,
- skin sores from psoriasis,
- rash,
- redness, blisters, itching, burning, cracking or peeling of the skin,
- changes in skin color,
- fever,
- cough,
- night sweats,
- loss of appetite,
- weight loss, and
- feeling very tired
Get medical help right away, if you have any of the symptoms listed above.
The most common side effects of Skyrizi include:
- upper respiratory infections,
- headache,
- fatigue,
- injection site reactions (bruising, redness, fluid leakage, bleeding, infection, inflammation, irritation, pain, itching, swelling, warmth), and
- tinea infections (ringworm, athlete’s foot and jock itch)
Tell the doctor if you have any side effect that bothers you or that does not go away.
These are not all the possible side effects of Skyrizi. For more information, ask your doctor or pharmacist.
DESCRIPTION
Risankizumab-rzaa, an interleukin-23 antagonist, is a humanized immunoglobulin G1 (IgG1) monoclonal antibody. Risankizumab-rzaa is produced in a mammalian cell line using recombinant DNA technology.
SKYRIZI (risankizumab-rzaa) injection is a sterile, preservative-free, colorless to slightly yellow, and clear to slightly opalescent solution for subcutaneous use.
Each prefilled syringe delivers 0.83 mL containing 75 mg risankizumab-rzaa, disodium succinate hexahydrate (0.88 mg), polysorbate 20 (0.17 mg), sorbitol (34 mg), succinic acid (0.049 mg), and Water for Injection, USP.
Indications & Dosage
INDICATIONS
SKYRIZI® is indicated for the treatment of moderate-to-severe plaque psoriasis in adults who are candidates for systemic therapy or phototherapy.
DOSAGE AND ADMINISTRATION
Procedures Prior To Treatment Initiation
- Evaluate patients for tuberculosis (TB) infection prior to initiating treatment with SKYRIZI [see WARNINGS AND PRECAUTIONS].
- Complete all age-appropriate vaccinations as recommended by current immunization guidelines [see WARNINGS AND PRECAUTIONS].
Dosage
The recommended dosage is 150 mg administered by subcutaneous injection at Week 0, Week 4, and every 12 weeks thereafter.
If a dose is missed, administer the dose as soon as possible. Thereafter, resume dosing at the regular scheduled time.
Preparation Instructions
- Before injecting, remove the carton with SKYRIZI from the refrigerator and without removing the prefilled pen or prefilled syringe(s) from the carton, allow SKYRIZI to reach room temperature out of direct sunlight (30 to 90 minutes for the prefilled pen and 15 to 30 minutes for the prefilled syringe(s)).
- Visually inspect SKYRIZI for particulate matter and discoloration prior to administration.
SKYRIZI 150 mg/mL is a colorless to yellow and clear to slightly opalescent solution. SKYRIZI 75 mg/0.83 mL is a colorless to slightly yellow and clear to slightly opalescent solution.
The solution may contain a few translucent to white particles. Do not use if the solution contains large particles or is cloudy or discolored.
Administration Instructions
- SKYRIZI is intended for use under the guidance and supervision of a healthcare professional. Patients may self-inject SKYRIZI after training in subcutaneous injection technique. Provide proper training to patients and/or caregivers on the subcutaneous injection technique of SKYRIZI according to the “Instructions for Use” [see Instructions for Use].
- Administer SKYRIZI subcutaneously. Do not inject into areas where the skin is tender, bruised, erythematous, indurated or affected by psoriasis. Administration of SKYRIZI in the upper, outer arm may only be performed by a healthcare professional or caregiver.
- When using SKYRIZI 150 mg/mL prefilled pen or prefilled syringe, inject one 150 mg single-dose prefilled pen or prefilled syringe.
- When using SKYRIZI 75 mg/0.83 mL prefilled syringes, for a 150 mg dose, two 75 mg prefilled syringes are required. Inject one prefilled syringe after the other in different anatomic locations (such as thighs or abdomen).
- Discard prefilled pen or prefilled syringe(s) after use. Do not reuse.
HOW SUPPLIED
Dosage Forms And Strengths
SKYRIZI Pen
Injection: 150 mg/mL as a colorless to yellow and clear to slightly opalescent solution in each single-dose prefilled pen.
SKYRIZI Prefilled Syringe
Injection: 150 mg/mL as a colorless to yellow and clear to slightly opalescent solution in each single-dose prefilled syringe.
Injection: 75 mg/0.83 mL as a colorless to slightly yellow and clear to slightly opalescent solution in each single-dose prefilled syringe.
SKYRIZI (risankizumab-rzaa) injection is supplied in the following strengths:
| Strength | Pack Size | NDC |
| 150 mg/mL single-dose pen | Carton of 1 | 0074-2100-01 |
| 150 mg/mL single-dose prefilled syringe | Carton of 1 | 0074-1050-01 |
| 75 mg/0.83 mL single-dose prefilled syringe | Carton of 2 | 0074-2042-02 |
SKYRIZI (risankizumab-rzaa) injection 150 mg/mL prefilled syringe and prefilled pen contain a sterile, preservative-free, colorless to yellow, and clear to slightly opalescent solution. Each single-dose prefilled syringe or prefilled pen consists of a 1 mL glass syringe with a fixed 27gauge ½ inch needle with needle guard.
SKYRIZI (risankizumab-rzaa) injection 75 mg/0.83 mL prefilled syringe contains a sterile, preservative-free, colorless to slightly yellow and clear to slightly opalescent solution. Each single-dose prefilled syringe consists of a 1 mL glass syringe with a fixed 29-gauge ½ inch needle with needle guard.
Storage And Handling
- Store in a refrigerator at 2°C to 8°C (36°F to 46° F).
- Do not freeze.
- Do not shake.
- Keep prefilled pens and prefilled syringes in the original cartons to protect from light.
- Not made with natural rubber latex.
Manufactured by: AbbVie Inc. North Chicago, IL 60064, USA. Revised: Apr 2021
Side Effects & Drug Interactions
SIDE EFFECTS
The following adverse reactions are discussed in greater detail in other sections of labeling:
- Infections [see WARNINGS AND PRECAUTIONS]
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse drug reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
A total of 2234 subjects were treated with SKYRIZI in clinical development trials in plaque psoriasis. Of these, 1208 subjects with psoriasis were exposed to SKYRIZI for at least one year.
Data from placebo-and active-controlled trials were pooled to evaluate the safety of SKYRIZI for up to 16 weeks. In total, 1306 subjects were evaluated in the SKYRIZI 150 mg group.
Table 1 summarizes the adverse drug reactions that occurred at a rate of at least 1% and at a higher rate in the SKYRIZI group than the placebo group during the 16-week controlled period of pooled clinical trials.
Table 1: Adverse Drug Reactions Occurring in ≥ 1% of Subjects on SKYRIZI through Week 16
| Adverse Drug Reactions | SKYRIZI N = 1306 n (%) |
Placebo N = 300 n (%) |
| Upper respiratory infectionsa | 170 (13.0) | 29 (9.7) |
| Headacheb | 46 (3.5) | 6 (2.0) |
| Fatiguec | 33 (2.5) | 3 (1.0) |
| Injection site reactionsd | 19 (1.5) | 3 (1.0) |
| Tinea infectionse | 15 (1.1) | 1 (0.3) |
| a Includes: respiratory tract infection (viral, bacterial or unspecified), sinusitis (including acute), rhinitis, nasopharyngitis, pharyngitis (including viral), tonsillitis b Includes: headache, tension headache, sinus headache, cervicogenic headache c Includes: fatigue, asthenia d Includes: injection site bruising, erythema, extravasation, hematoma, hemorrhage, infection, inflammation, irritation, pain, pruritus, reaction, swelling, warmth e Includes: tinea pedis, tinea cruris, body tinea, tinea versicolor, tinea manuum, tinea infection, onychomycosis |
||
Adverse drug reactions that occurred in < 1% but > 0.1% of subjects in the SKYRIZI group and at a higher rate than in the placebo group through Week 16 were folliculitis and urticaria.
Specific Adverse Drug Reactions
Infections
In the first 16 weeks, infections occurred in 22.1% of the SKYRIZI group (90.8 events per 100 subject-years) compared to 14.7% of the placebo group (56.5 events per 100 subject-years) and did not lead to discontinuation of SKYRIZI. The rates of serious infections for the SKYRIZI group and the placebo group were ≤0.4%. Serious infections in the SKYRIZI group included cellulitis, osteomyelitis, sepsis, and herpes zoster. In ULTIMMA-1 and ULTIMMA-2, through Week 52, the rate of infections (73.9 events per 100 subject-years) was similar to the rate observed during the first 16 weeks of treatment.
Safety Through Week 52
Through Week 52, no new adverse reactions were identified, and the rates of the adverse reactions were similar to those observed during the first 16 weeks of treatment. During this period, serious infections that led to study discontinuation included pneumonia.
Immunogenicity
As with all therapeutic proteins, there is potential for immunogenicity. The detection of antibody formation is highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of antibody (including neutralizing antibody) positivity in an assay may be influenced by several factors including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of the incidence of antibodies in the studies described below with the incidence of antibodies in other studies or to other products, including other risankizumab products, may be misleading.
By Week 52, approximately 24% (263/1079) of subjects treated with SKYRIZI at the recommended dose developed antibodies to risankizumab-rzaa. Of the subjects who developed antibodies to risankizumab-rzaa, approximately 57% (14% of all subjects treated with SKYRIZI) had antibodies that were classified as neutralizing. Higher antibody titers in approximately 1% of subjects treated with SKYRIZI were associated with lower risankizumab-rzaa concentrations and reduced clinical response.
DRUG INTERACTIONS
No Information provided
Warnings & Precautions
WARNINGS
Included as part of the "PRECAUTIONS" Section
PRECAUTIONS
Infections
SKYRIZI may increase the risk of infections. In clinical studies, infections occurred in 22.1% of the SKYRIZI group compared to 14.7% of the placebo group through 16 weeks of treatment. Upper respiratory tract infections and tinea infections occurred more frequently in the SKYRIZI group than in the placebo group. Subjects with known chronic or acute infections were not enrolled in clinical studies [see ADVERSE REACTIONS].
The rate of serious infections for the SKYRIZI group and the placebo group was ≤ 0.4%. Treatment with SKYRIZI should not be initiated in patients with any clinically important active infection until the infection resolves or is adequately treated.
In patients with a chronic infection or a history of recurrent infection, consider the risks and benefits prior to prescribing SKYRIZI. Instruct patients to seek medical advice if signs or symptoms of clinically important infection occur. If a patient develops such an infection or is not responding to standard therapy, monitor the patient closely and do not administer SKYRIZI until the infection resolves.
Pre-Treatment Evaluation For Tuberculosis
Evaluate patients for tuberculosis (TB) infection prior to initiating treatment with SKYRIZI. Across the Phase 3 psoriasis clinical studies, of the 72 subjects with latent TB who were concurrently treated with SKYRIZI and appropriate TB prophylaxis during the studies, none developed active TB during the mean follow-up of 61 weeks on SKYRIZI. Two subjects taking isoniazid for treatment of latent TB discontinued treatment due to liver injury. Of the 31 subjects from the IMMHANCE study with latent TB who did not receive prophylaxis during the study, none developed active TB during the mean follow-up of 55 weeks on SKYRIZI. Consider anti-TB therapy prior to initiating SKYRIZI in patients with a past history of latent or active TB in whom an adequate course of treatment cannot be confirmed. Monitor patients for signs and symptoms of active TB during and after SKYRIZI treatment. Do not administer SKYRIZI to patients with active TB.
Immunizations
Prior to initiating therapy with SKYRIZI, consider completion of all age appropriate immunizations according to current immunization guidelines. Avoid use of live vaccines in patients treated with SKYRIZI. No data are available on the response to live or inactive vaccines.
Patient Counseling Information
Advise the patient and/or caregiver to read the FDA-approved patient labeling (Medication Guide and Instructions for Use) before starting SKYRIZI therapy and to reread the Medication Guide each time the prescription is renewed. Advise patients of the potential benefits and risks of SKYRIZI.
Infections
Inform patients that SKYRIZI may lower the ability of their immune system to fight infections. Instruct patients of the importance of communicating any history of infections to the healthcare provider and contacting their healthcare provider if they develop any symptoms of an infection [see WARNINGS AND PRECAUTIONS].
Administration Instruction
Instruct patients or caregivers to perform the first self-injected dose under the supervision and guidance of a qualified healthcare professional for training in preparation and administration of SKYRIZI, including choosing anatomical sites for administration, and proper subcutaneous injection technique [see Instructions for Use].
Instruct patients or caregivers to administer two 75 mg single-dose syringes to achieve the 150 mg dose of SKYRIZI [see Instructions for Use].
Instruct patients or caregivers in the technique of needle and syringe disposal [see Instructions for Use].
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment Of Fertility
Carcinogenicity and mutagenicity studies have not been conducted with SKYRIZI.
No effects on male fertility parameters were observed in sexually mature male cynomolgus monkeys subcutaneously treated with 50 mg/kg risankizumab-rzaa (at 20 times the clinical exposure at the MRHD, based on mg/kg comparison) once weekly for 26 weeks.
Use In Specific Populations
Pregnancy
Risk Summary
Limited available data with SKYRIZI use in pregnant women are insufficient to evaluate a drug associated risk of major birth defects, miscarriage, or adverse maternal or fetal outcome. Human IgG is known to cross the placental barrier; therefore, SKYRIZI may be transmitted from the mother to the developing fetus.
In an enhanced pre- and post-natal developmental toxicity study, pregnant cynomolgus monkeys were administered subcutaneous doses of 5 and 50 mg/kg risankizumab-rzaa once weekly during the period of organogenesis up to parturition. At the 50 mg/kg dose [20 times the maximum recommended human dose (MRHD); 2.5 mg/kg based on administration of a 150 mg dose to a 60 kg individual], increased fetal/infant loss was noted in pregnant monkeys (see Data). No risankizumab-rzaa-related effects on functional or immunological development were observed in infant monkeys from birth through 6 months of age. The clinical significance of these findings for humans is unknown.
All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Data
Animal Data
An enhanced pre- and post-natal developmental toxicity study was conducted in cynomolgus monkeys. Pregnant cynomolgus monkeys were administered weekly subcutaneous doses of risankizumab-rzaa of 5 or 50 mg/kg from gestation day 20 to parturition and the cynomolgus monkeys (mother and infants) were monitored for 6 months after delivery. No maternal toxicity was noted in this study. There were no treatment-related effects on growth and development, malformations, developmental immunotoxicology or neurobehavioral development. However, a dose-dependent increase in fetal/infant loss was noted in the risankizumab-rzaa-treated groups (32% and 43% in the 5 mg/kg and 50 mg/kg groups, respectively) compared to the vehicle control group (19%). The increased fetal/infant loss in the 50 mg/kg group was considered to be related to risankizumab-rzaa treatment. The no observed adverse effect level (NOAEL) for maternal toxicity was identified as 50 mg/kg (20 times the MRHD, based on mg/kg comparison) and the NOAEL for developmental toxicity was identified as 5 mg/kg (2 times the MRHD, based on mg/kg comparison). In the infants, mean serum concentrations increased in a dose-dependent manner and were approximately 17-86% of the respective maternal concentrations. Following delivery, most adult female cynomolgus monkeys and all infants from the risankizumabrzaa-treated groups had measurable serum concentrations of risankizumab-rzaa up to 91 days postpartum. Serum concentrations were below detectable levels at 180 days postpartum.
Lactation
Risk Summary
There are no data on the presence of risankizumab-rzaa in human milk, the effects on the breastfed infant, or the effects on milk production. Maternal IgG is known to be present in human milk. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for SKYRIZI and any potential adverse effects on the breastfed infant from SKYRIZI or from the underlying maternal condition.
Pediatric Use
The safety and efficacy of SKYRIZI in pediatric patients less than 18 years of age have not yet been established.
Geriatric Use
Of the 2234 subjects with plaque psoriasis exposed to SKYRIZI, 243 subjects were 65 years or older and 24 subjects were 75 years or older. No overall differences in risankizumab-rzaa exposure, safety or effectiveness were observed between older and younger subjects who received SKYRIZI. However, the number of subjects aged 65 years and older was not sufficient to determine whether they respond differently from younger subjects.
Overdosage & Contraindications
OVERDOSE
In the event of overdosage, monitor the patient for any signs or symptoms of adverse reactions and administer appropriate symptomatic treatment immediately.
CONTRAINDICATIONS
None.
Clinical Pharmacology
Mechanism Of Action
Risankizumab-rzaa is a humanized immunoglobulin G1 (IgG1) monoclonal antibody that selectively binds to the p19 subunit of human interleukin 23 (IL-23) cytokine and inhibits its interaction with the IL-23 receptor. IL-23 is a naturally occurring cytokine that is involved in inflammatory and immune responses.
Risankizumab-rzaa inhibits the release of pro-inflammatory cytokines and chemokines.
Pharmacodynamics
No formal pharmacodynamics studies have been conducted with risankizumab-rzaa.
Pharmacokinetics
Risankizumab-rzaa plasma concentrations increased dose-proportionally from 90 to 180 mg and from 18 to 300 mg (0.6 to 1.2 and 0.12 to 2.0 times the approved recommended dosage) following subcutaneous administration in subjects with plaque psoriasis and healthy volunteers, respectively. Steady-state concentrations were achieved by Week 16 following subcutaneous administration of risankizumab-rzaa at Weeks 0, 4, and every 12 weeks thereafter. At the 150 mg dose, the estimated steady-state peak concentration (Cmax) and trough concentration (Ctrough) were approximately 12 mcg/mL and 2 mcg/mL, respectively.
Absorption
The absolute bioavailability of risankizumab-rzaa was estimated to be 89% following subcutaneous injection. Cmax was reached by 3-14 days.
Distribution
The estimated steady-state volume of distribution (inter-subject CV%) was 11.2 L (34%) in subjects with plaque psoriasis.
Elimination
The estimated systemic clearance (inter-subject CV%) was 0.31 L/day (24%) and terminal elimination half-life was approximately 28 days in subjects with plaque psoriasis.
Metabolism
The metabolic pathway of risankizumab-rzaa has not been characterized. As a humanized IgG1 monoclonal antibody, risankizumab-rzaa is expected to be degraded into small peptides and amino acids via catabolic pathways in a manner similar to endogenous IgG.
Specific Populations
No clinically significant differences in the pharmacokinetics of risankizumab-rzaa were observed based on age (≥18 years). No specific studies have been conducted to determine the effect of renal or hepatic impairment on the pharmacokinetics of risankizumab-rzaa.
Body Weight
Risankizumab-rzaa clearance and volume of distribution increase and plasma concentrations decrease as body weight increases; however, no dose adjustment is recommended based on body weight.
Drug Interaction Studies
Cytochrome P450 Substrates
No clinically significant changes in exposure of caffeine (CYP1A2 substrate), warfarin (CYP2C9 substrate), omeprazole (CYP2C19 substrate), metoprolol (CYP2D6 substrate), or midazolam (CYP3A substrate) were observed when used concomitantly with risankizumab-rzaa 150 mg administered subcutaneously at Weeks 0, 4, 8 and 12 (more frequent than the approved recommended dosing frequency) in subjects with plaque psoriasis.
Clinical Studies
Four multicenter, randomized, double-blind studies [ULTIMMA-1 (NCT02684370), ULTIMMA-2 (NCT02684357), IMMHANCE (NCT02672852), and IMMVENT (NCT02694523)] enrolled 2109 subjects 18 years of age and older with moderate to severe plaque psoriasis who had a body surface area (BSA) involvement of ≥10%, a static Physician’s Global Assessment (sPGA) score of ≥3 (“moderate”) in the overall assessment (plaque thickness/induration, erythema, and scaling) of psoriasis on a severity scale of 0 to 4, and a Psoriasis Area and Severity Index (PASI) score ≥12.
Overall, subjects had a median baseline PASI score of 17.8 and a median BSA of 20.0%. Baseline sPGA score was 4 (“severe”) in 19% of subjects. A total of 10% of study subjects had a history of diagnosed psoriatic arthritis.
Across all studies, 38% of subjects had received prior phototherapy, 48% had received prior non-biologic systemic therapy, and 42% had received prior biologic therapy for the treatment of psoriasis.
ULTIMMA-1 And ULTIMMA-2
In ULTIMMA-1 and ULTIMMA-2, 997 subjects were enrolled (including 598 subjects randomized to the SKYRIZI 150 mg group, 200 subjects randomized to the placebo group, and 199 to the biologic active control group). Subjects received treatment at Weeks 0, 4, and every 12 weeks thereafter.
Both studies assessed the responses at Week 16 compared to placebo for the two co-primary endpoints:
- the proportion of subjects who achieved an sPGA score of 0 (“clear”) or 1 (“almost clear”)
- the proportion of subjects who achieved at least a 90% reduction from baseline PASI (PASI 90)
Secondary endpoints included the proportion of subjects who achieved PASI 100, sPGA 0, and PSS 0 at Week 16.
The results are presented in Table 2.
Table 2: Efficacy Results at Week 16 in Adults with Plaque Psoriasis in ULTIMMA-1 and ULTIMMA-2
| ULTIMMA-1 | ULTIMMA-2 | |||
| SKYRIZI (N=304) n (%) |
Placebo (N=102) n (%) |
SKYRIZI (N=294) n (%) |
Placebo (N=98) n (%) |
|
| sPGA 0 or 1 (“clear or almost clear”)a | 267 (88) | 8 (8) | 246 (84) | 5 (5) |
| PASI 90a | 229 (75) | 5 (5) | 220 (75) | 2 (2) |
| sPGA 0 (“clear”) | 112 (37) | 2 (2) | 150 (51) | 3 (3) |
| PASI 100 | 109 (36) | 0 (0) | 149 (51) | 2 (2) |
| a Co-primary endpoints | ||||
Examination of age, gender, race, body weight, baseline PASI score and previous treatment with systemic or biologic agents did not identify differences in response to SKYRIZI among these subgroups at Week 16.
In ULTIMMA-1 and ULTIMMA-2 at Week 52, subjects receiving SKYRIZI achieved sPGA 0 (58% and 60%, respectively), PASI 90 (82% and 81%, respectively), and PASI 100 (56% and 60%, respectively).
Patient Reported Outcomes
Improvements in signs and symptoms related to pain, redness, itching and burning at Week 16 compared to placebo were observed in both studies as assessed by the Psoriasis Symptom Scale (PSS). In ULTIMMA-1 and ULTIMMA-2, about 30% of the subjects who received SKYRIZI achieved PSS 0 (“none”) at Week 16 compared to 1% of the subjects who received placebo.
IMMHANCE
IMMHANCE enrolled 507 subjects (407 randomized to SKYRIZI 150 mg and 100 to placebo). Subjects received treatment at Weeks 0, 4, and every 12 weeks thereafter.
At Week 16, SKYRIZI was superior to placebo on the co-primary endpoints of sPGA 0 or 1 (84% SKYRIZI and 7% placebo) and PASI 90 (73% SKYRIZI and 2% placebo). The respective response rates for SKYRIZI and placebo at Week 16 were: sPGA 0 (46% SKYRIZI and 1% placebo); PASI 100 (47% SKYRIZI and 1% placebo); and PASI 75 (89% SKYRIZI and 8% placebo).
Maintenance And Durability Of Response
In ULTIMMA-1 and ULTIMMA-2, among the subjects who received SKYRIZI and had PASI 100 at Week 16, 80% (206/258) of the subjects who continued on SKYRIZI had PASI 100 at Week 52. For PASI 90 responders at Week 16, 88% (398/450) of the subjects had PASI 90 at Week 52.
In IMMHANCE, subjects who were originally on SKYRIZI and had sPGA 0 or 1 at Week 28 were re-randomized to continue SKYRIZI every 12 weeks or withdrawal of therapy. At Week 52, 87% (97/111) of the subjects re-randomized to continue treatment with SKYRIZI had sPGA 0 or 1 compared to 61% (138/225) who were re-randomized to withdrawal of SKYRIZI.
Medication Guide
PATIENT INFORMATION
SKYRIZI®
(sky-RIZZ-ee)
(risankizumab-rzaa) injection, for subcutaneous use
What is the most important information I should know about SKYRIZI?
SKYRIZI may cause serious side effects, including:
Infections. SKYRIZI may lower the ability of your immune system to fight infections and may increase your risk of infections. Your healthcare provider should check you for infections and tuberculosis (TB) before starting treatment with SKYRIZI and may treat you for TB before you begin treatment with SKYRIZI if you have a history of TB or have active TB. Your healthcare provider should watch you closely for signs and symptoms of TB during and after treatment with SKYRIZI. Tell your healthcare provider right away if you have an infection or have symptoms of an infection, including:
- fever, sweats, or chills
- cough
- shortness of breath
- blood in your mucus (phlegm)
- muscle aches
- warm, red, or painful skin or sores on your body different from your psoriasis
- weight loss
- diarrhea or stomach pain
- burning when you urinate or urinating more often than normal
See “What are the possible side effects of SKYRIZI?” for more information about side effects.
What is SKYRIZI?
SKYRIZI is a prescription medicine used to treat adults with moderate to severe plaque psoriasis who may benefit from taking injections or pills (systemic therapy) or treatment using ultraviolet or UV light (phototherapy).
It is not known if SKYRIZI is safe and effective in children under 18 years of age.
Before using SKYRIZI, tell your healthcare provider about all of your medical conditions,including if you:
- have any of the conditions or symptoms listed in the section “What is the most important information I should know about SKYRIZI?”
- have an infection that does not go away or that keeps coming back.
- have TB or have been in close contact with someone with TB.
- have recently received or are scheduled to receive an immunization (vaccine). Medications that interact with the immune system may increase your risk of getting an infection after receiving live vaccines. You should avoid receiving live vaccines right before, during, or right after treatment with SKYRIZI. Tell your healthcare provider that you are taking SKYRIZI before receiving a vaccine.
- are pregnant or plan to become pregnant. It is not known if SKYRIZI can harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if SKYRIZI passes into your breast milk.
- If you become pregnant while taking SKYRIZI, you are encouraged to enroll in the Pregnancy Registry. The purpose of the pregnancy registry is to collect information about the health of you and your baby. Talk to your healthcare provider or call 1-877-302-2161 to enroll in this registry.
Tell your healthcare provider about all the medicines you take, including prescription and over-the counter medicines, vitamins, and herbal supplements.
How should I use SKYRIZI?
See the detailed “Instructions for Use” that comes with SKYRIZI for information on how to prepare and inject a dose of SKYRIZI, and how to properly throw away (dispose of) used SKYRIZI prefilled pen or prefilled syringe.
- Use SKYRIZI exactly as your healthcare provider tells you to use it.
- If you miss your SKYRIZI dose, inject a dose as soon as you remember. Then, take your next dose at your regular scheduled time. Call your healthcare provider if you are not sure what to do.
- If you inject more SKYRIZI than prescribed, call your healthcare provider right away.
What are the possible side effects of SKYRIZI?
SKYRIZI may cause serious side effects. See “What is the most important information I should know about SKYRIZI?”
The most common side effects of SKYRIZI include:
- upper respiratory infections
- headache
- feeling tired
- injection site reactions
- fungal skin infections
These are not all of the possible side effects of SKYRIZI. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store SKYRIZI?
- Store SKYRIZI in the refrigerator between 36°F to 46°F (2°C to 8°C).
- Do not freeze SKYRIZI.
- Do not shake SKYRIZI.
- Keep SKYRIZI in the original carton to protect it from light.
- SKYRIZI is not made with natural rubber latex.
Keep SKYRIZI and all medicines out of the reach of children.
General information about the safe and effective use of SKYRIZI
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use SKYRIZI for a condition for which it was not prescribed. Do not give SKYRIZI to other people, even if they have the same symptoms that you have. It may harm them. You can ask your healthcare provider or pharmacist for information about SKYRIZI that is written for health professionals.
What are the ingredients in SKYRIZI?
Active ingredient: risankizumab-rzaa
SKYRIZI 150 mg/mL inactive ingredients: acetic acid, polysorbate 20, sodium acetate trihydrate, trehalose dihydrate, and Water for Injection, USP.
SKYRIZI 75 mg/0.83 mL inactive ingredients: disodium succinate hexahydrate, polysorbate 20, sorbitol, succinic acid, and Water for Injection, USP.
INSTRUCTIONS FOR USE
SKYRIZI®
(sky-RIZZ-ee) Pen
(risankizumab-rzaa) injection, for subcutaneous use
Read Before First Use
Refer to the Medication Guide for product information.
Read this Instructions for Use before using SKYRIZI Pen (risankizumab-rzaa) injection.
Before using SKYRIZI, you should receive training from your healthcare provider on how to inject SKYRIZI.
SKYRIZI Single-Dose Pen
 |
Important Information
- Store SKYRIZI in the refrigerator at 36° to 46°F (2° to 8°C).
- Keep SKYRIZI in the original carton to protect from light until you are ready to use.
- Before injecting, take the SKYRIZI carton out of the refrigerator. Leave the carton at room temperature and out of direct sunlight for 30 to 90 minutes.
- The liquid in the inspection window should look clear to yellow and may contain tiny white or clear particles.
- Do not use SKYRIZI if liquid is cloudy or contains flakes or large particles.
- Do not use SKYRIZI if expiration date (EXP) has passed.
- Do not use SKYRIZI if liquid has been frozen, even if it has been thawed.
- Do not shake SKYRIZI.
- Do not use if SKYRIZI Pen has been dropped or damaged.
- Do not use SKYRIZI if carton perforations are broken. Return product to pharmacy.
- Do not remove the dark gray cap until right before injection.
- SKYRIZI is not made with natural rubber latex.
Prepare SKYRIZI injection
Take the SKYRIZI carton out of the refrigerator. Leave the carton at room temperature and out of direct sunlight for 30 to 90 minutes before injecting.
- Do not remove the Pen from the carton while allowing SKYRIZI to reach room temperature.
- Do not warm SKYRIZI in any other way. For example, do not warm it in a microwave or in hot water.
- Do not use the Pen if liquid has been frozen, even if it has been thawed.
 |
Check expiration date (EXP). Do not use the Pen if expiration date has passed.
Place the following on a clean, flat surface:
- 1 single-dose SKYRIZI Pen (included)
- 1 alcohol swab (not included)
- 1 cotton ball or gauze pad (not included)
- FDA-cleared sharps disposal container (not included). See Used SKYRIZI Prefilled Pen Disposal for information on how to throw away (dispose of) used Pens.
Wash and dry your hands.
 |
Choose an injection site:
- on the front of your thighs or
- your abdomen (belly) at least 2 inches from your navel (belly button)
Wipe the injection site in a circular motion with the alcohol swab and let it dry.
- Do not touch or blow on the injection site after it is cleaned. Allow the skin to dry before injecting.
- Do not inject through clothes.
- Do not inject into skin that is sore, bruised, red, hard, scarred, has stretch marks, or areas with psoriasis.
 |
Hold the Pen with the dark gray cap pointing up.
- Pull the dark gray cap straight off.
- Throw the dark gray cap away.
Check the liquid through the inspection window.
- It is normal to see one or more bubbles in the liquid.
- The liquid should look clear to yellow and may contain tiny white or clear particles.
- Do not use if the liquid is cloudy or contains flakes or large particles.
 |
Give SKYRIZI injection
Hold the Pen with your fingers on the gray hand grips.
Turn the Pen so that the white needle sleeve points toward the injection site and you can see the green activator button.
Pinch the skin at your injection site to make a raised area and hold it firmly.
Place the white needle sleeve straight (90° angle) against the raised injection site.
 |
Hold the Pen so that you can see the green activator button and inspection window.
Push and keep pressing the Pen down against the raised injection site.
- The Pen will activate only if the white needle sleeve is pressed down against the injection site before pressing the green activator button.
Press the green activator button and hold the Pen for 15 seconds.
- The first loud “click” means the start of the injection.
 |
Keep pressing the Pen down against the injection site.
The injection is complete when:
- the Pen has made a second “click” or
- the yellow indicator has filled the inspection window
This takes upto 15 seconds.
 |
After SKYRIZI injection
When the injection is complete, slowly pull the Pen straight out from the skin.
The white needle sleeve will cover the needle tip and make another “click.”
After completing the injection, place a cotton ball or gauze pad on the skin at the injection site.
- Do not rub the injection site.
- Slight bleeding at the injection site is normal.
 |
Throw away (dispose of) the used Pen in a FDA-cleared sharps disposal container right away after use.
- Do not dispose of used Pens in your household trash unless your community guidelines permit this.
- Do not recycle your sharps disposal container.
The dark gray cap, alcohol swab, cotton ball or gauze pad, and packaging may be placed in your household trash.
For more information, see Used SKYRIZI Prefilled Pen Disposal.
 |
Important Information
- Store SKYRIZI in the refrigerator at 36° to 46°F (2° to 8°C).
- Keep SKYRIZI in the original carton to protect from light until you are ready to use.
- Before injecting, take the SKYRIZI carton out of the refrigerator. Leave the carton at room temperature and out of direct sunlight for 30 to 90 minutes.
- The liquid in the inspection window should look clear to yellow and may contain tiny white or clear particles.
- Do not use SKYRIZI if liquid is cloudy or contains flakes or large particles.
- Do not use SKYRIZI if expiration date (EXP) has passed.
- Do not use SKYRIZI if liquid has been frozen, even if it has been thawed.
- Do not shake SKYRIZI.
- Do not use if SKYRIZI Pen has been dropped or damaged.
- Do not use SKYRIZI if carton perforations are broken. Return product to pharmacy.
- Do not remove the dark gray cap until right before injection.
- SKYRIZI is not made with natural rubber latex.
Keep the SKYRIZI Pen and sharps disposal container out of the reach of children.
Call your healthcare provider or (866) SKYRIZI or (866) 759-7494 if you need help or do not know how to proceed.
Questions About Using the SKYRIZI Pen
Q. What if I need help on how to inject SKYRIZI?
A. Call your healthcare provider or (866) SKYRIZI or (866) 759-7494 if you need help.
Q. I have removed the dark gray cap and pressed the green activator button. Why isn’t myinjection starting?
A. The green activator button will not start the injection unless the white needle sleeve is pressed firmly against the injection site.
Q. How do I know when the injection is complete?
A. The injection is complete if the Pen makes a second “click” or the yellow indicator fills the inspection window. This takes up to 15 seconds.
Q. What should I do if there are more than a few drops of liquid on the injection site?
A. Call (866) SKYRIZI or (866) 759-7494 for help.
Q. What should I do with the used Pen after my injection?
A. Dispose of the used Pen in a sharps disposal container right after use. Do not dispose of the used Pen in your household trash. You can sign up to receive sharps containers for SKYRIZI Pen disposal at no additional cost by going to www.SKYRIZI.com or calling (866) SKYRIZI or (866) 759-7494.
Call (866) SKYRIZI or (866) 759-7494 or go to www.SKYRIZI.com for help with your injection.
To help remember when to inject, mark your calendar with the date you give your SKYRIZI injection.
 |
Keep the SKYRIZI Pen and sharps disposal container out of the reach of children.
Call your healthcare provider or (866) SKYRIZI or (866) 759-7494 if you need help or have questions about the use of SKYRIZI.
Used SKYRIZI Prefilled Pen Disposal
If you do not have a FDA-cleared sharps disposal container, you may use a household container that is:
- made of a heavy-duty plastic,
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- upright and stable during use,
- leak-resistant, and
- properly labeled to warn of hazardous waste inside the container.
When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used Pens.
For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA’s website at: www.fda.gov/safesharpsdisposal.
Do not recycle your used sharps disposal container.
Instructions for Use
SKYRIZI®
(sky-RIZZ-ee)
(risankizumab-rzaa) injection, for subcutaneous use 150 mg/mL prefilled syringe
Read Before First Use
Refer to the Medication Guide for product information.
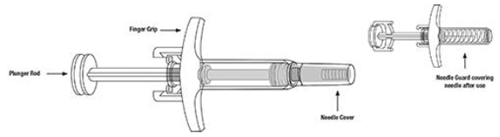
SKYRIZI Single-Dose Prefilled Syringe
 |
Important Information
- Keep SKYRIZI in the original carton to protect from light until time to use.
- The liquid should look clear to yellow and may contain tiny white or clear particles.
- Do not use SKYRIZI if the liquid is cloudy or contains flakes or large particles.
- Do not use SKYRIZI if the expiration date (EXP:) shown on the carton and prefilled syringe has passed.
- Do not use SKYRIZI if the liquid has been frozen (even if thawed).
- Do not shake SKYRIZI.
- Do not use SKYRIZI if the syringe has been dropped or damaged.
- Do not use SKYRIZI if carton perforations are broken. Return product to the pharmacy.
- Do not remove the needle cover until right before giving the injection.
Keep SKYRIZI and all medicines out of the reach of children.
Please Read Complete Instructions for Use Before Using SKYRIZI Syringe
Before Injecting
- Receive training on how to inject SKYRIZI before giving injection. Call your healthcare provider or (866) SKYRIZI or (866) 759-7494 if you need help.
- Mark your calendar ahead of time to remember when to take SKYRIZI.
- Leave the carton at room temperature and out of direct sunlight for 15 to 30 minutes to warm.
- Do not remove the syringe from the carton while allowing SKYRIZI to reach room temperature.
- Do not warm SKYRIZI in any other way (for example, do not warm it in a microwave or in hot water).
Important Information
- The liquid should look clear to yellow and may contain tiny white or clear particles.
- Do not use SKYRIZI if the liquid is cloudy or contains flakes or large particles.
- Do not use SKYRIZI if the expiration date (EXP:) shown on the carton and prefilled syringe has passed.
- Do not use SKYRIZI if the syringe has been dropped or damaged.
- Do not use SKYRIZI if carton perforations are broken. Return product to the pharmacy.
Storage Information
- Store SKYRIZI in your refrigerator between 36° F to 46°F (2° to 8°C).
- Do not shake SKYRIZI.
- Keep SKYRIZI in the original carton to protect from light until time to use.
- SKYRIZI is not made with natural rubber latex.
- Do not use if liquid has been frozen (even if thawed).
Keep SKYRIZI and all medicines out of the reach of children.
Call your healthcare provider or (866) SKYRIZI or (866) 759-7494 if you need help or do not know how to proceed.
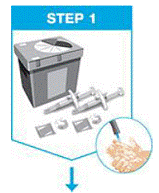
Gather the supplies for the injection.
- Do not hold or pull plunger rod when removing the prefilled syringe from the sleeve.
Place the following on a clean, flat surface:
- 1 prefilled syringe (included)
- 1 alcohol swab (not included)
- 1 cotton ball or gauze pad (not included)
- FDA-cleared sharps disposal container (not included)
Wash and dry your hands.
 |
Remove prefilled syringe from cardboard sleeve by holding the finger grip.
 |
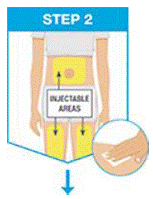
Pick from the 3 injectable areas:
- Front of left thigh or right thigh
- Your abdomen (belly) at least 2 inches from your navel (belly button)
Wipe the injection site in a circular motion with the alcohol swab before the injection.
- Do not touch or blow on the injection site after it is cleaned. Allow the skin to dry before injecting.
- Do not inject through clothes.
- Do not inject into skin that is sore, bruised, red, hard, scarred, or has stretch marks, or into areas affected by psoriasis.
 |
Hold the prefilled syringe with covered needle facing down, as shown.
Check the liquid in the prefilled syringe.
- It is normal to see one or more bubbles in the window.
- The liquid should look clear to yellow and may contain tiny white or clear particles.
- Do not use the prefilled syringe if liquid is cloudy or contains flakes or large particles.
 |
Remove the needle cover.
- Hold the syringe in one hand between the finger grip and needle cover.
- With the other hand, gently pull the needle cover straight off.
- Do not hold or pull plunger rod when removing the needle cover.
- You may see a drop of liquid at the end of the needle. This is normal.
- Throw away the needle cover.
- Do not touch the needle with your fingers or let the needle touch anything.
 |
Hold the body of the prefilled syringe in one hand between the thumb and index fingers.
Pinch the area of cleaned skin with your other hand and hold it firmly.
Insert the needle into the skin at about a 45-degree angle using a quick, short movement. Hold angle steady.
 |
Slowly push the plunger rod all the way in until all of the liquid is injected, and the syringe is empty.
Pull the needle out of the skin while keeping the syringe at the same angle.
Release the plunger rod and allow the prefilled syringe to move up until the entire needle is covered by the needle guard.
The prefilled syringe needle guard will not activateunless all the liquid has been injected.
- Press a cotton ball or gauze pad over the injection site and hold for 10 seconds.
- Do not rub the injection site. You may have slight bleeding. This is normal.
 |
Put your used prefilled syringe in a FDA-cleared sharps disposal container right away after use.
- Do not throw away (dispose of) used prefilled syringe in the household trash.
For more information, see “Used SKYRIZI Prefilled Syringe Disposal” section.
 |
Questions About Using SKYRIZI
Q. What if I need help on how to inject SKYRIZI?
A. Call your healthcare provider or (866) SKYRIZI or (866) 759-7494 if you need help.
Q. What should I do with the used prefilled syringe after my injection?
A. Throw away (dispose of) the used prefilled syringe in a sharps disposal container and not your household trash. You can sign up to receive sharps containers for SKYRIZI syringe disposal at no additional cost by going to www.SKYRIZI.com or calling (866) SKYRIZI or (866) 759-7494.
Q. How do I know when the injection is complete?
A. The injection is complete when the prefilled syringe is empty, the plunger rod is pushed all the way in, and the syringe needle guard is activated.
Used SKYRIZI Prefilled Syringe Disposal
If you do not have a FDA-cleared sharps disposal container, you may use a household container that is:
- made of a heavy-duty plastic,
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- upright and stable during use,
- leak-resistant, and
- properly labeled to warn of hazardous waste inside the container.
When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes.
For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA’s website at: www.fda.gov/safesharpsdisposal.
Do not recycle your used sharps disposal container.
Instructions for Use
SKYRIZI®
(sky-RIZZ-ee) (risankizumab-rzaa) injection, for subcutaneous use 75 mg/0.83 mL prefilled syringe
Read Before First Use
Refer to the Medication Guide for product information.
SKYRIZI Single-Dose Prefilled Syringe
 |
Important Information:
- Keep SKYRIZI in the original carton to protect from light until time to use.
- The liquid should look clear to slightly yellow and may contain tiny white or clear particles.
- DO NOT use SKYRIZI if the liquid is cloudy or contains flakes or large particles.
- DO NOT use SKYRIZI if the expiration date (EXP:) shown on the carton and prefilled syringe has passed.
- DO NOT use SKYRIZI if the liquid has been frozen (even if thawed).
- DO NOT shake SKYRIZI.
- DO NOT use SKYRIZI if the syringe has been dropped or damaged.
- DO NOT use SKYRIZI if carton perforations are broken. Return product to the pharmacy.
- DO NOT remove the needle cover until right before giving the injections.
Keep SKYRIZI and all medicines out of the reach of children.
Please Read Complete Instructions for Use Before Using SKYRIZI Syringe
Before Injecting:
- Receive training on how to inject SKYRIZI before giving injections. Call your healthcare provider or (866) SKYRIZI or (866) 759-7494 if you need help.
- Mark your calendar ahead of time to remember when to take SKYRIZI.
- Leave the carton at room temperature and out of direct sunlight for 15 to 30 minutes to warm.
- DO NOT remove the syringes from the carton while allowing SKYRIZI to reach room temperature.
- DO NOT warm SKYRIZI in any other way (for example, DO NOT warm it in a microwave or in hot water).
Important Information:
- The liquid should look clear to slightly yellow and may contain tiny white or clear particles.
- DO NOT use SKYRIZI if the liquid is cloudy or contains flakes or large particles.
- DO NOT use SKYRIZI if the expiration date (EXP:) shown on the carton and prefilled syringe has passed.
- DO NOT use SKYRIZI if the syringe has been dropped or damaged.
- DO NOT use SKYRIZI if carton perforations are broken. Return product to the pharmacy.
Storage Information:
- Store SKYRIZI in your refrigerator between 36° F to 46°F (2° to 8°C).
- DO NOT shake SKYRIZI.
- Keep SKYRIZI in the original carton to protect from light until time to use.
- SKYRIZI is not made with natural rubber latex.
- DO NOT use if liquid has been frozen (even if thawed).
Keep SKYRIZI and all medicines out of the reach of children.
Call your healthcare provider or (866) SKYRIZI or (866) 759-7494 if you need help or do not know how to proceed
Gather the supplies for the injections and place the following on a clean, flat surface:
- 2 prefilled syringes and 2 alcohol swabs (included)
- 2 cotton balls or gauze pads (not included)
- FDA-cleared sharps disposal container (not included)
Wash and dry your hands.
Start with one prefilled syringe for first injection.
 |
 |
Pick from the 3 injectable areas:
- Front of left thigh or right thigh
- Your abdomen (belly) at least 2 inches from your navel (belly button)
 |
 |
Wipe the injection site in a circular motion with the alcohol swab (before both injections)
- DO NOT touch or blow on the injection site after it is cleaned. Allow the skin to dry before injecting.
- DO NOT inject through clothes.
- DO NOT inject into skin that is sore, bruised, red, hard, scarred, or has stretch marks, or into areas affected by psoriasis.
Hold the prefilled syringe with covered needle facing down, as shown.
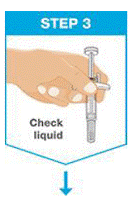
Check the liquid in the prefilled syringe.
- It is normal to see one or more bubbles in the window.
- The liquid should look clear to slightly yellow and may contain tiny white or clear particles.
- DO NOT use the prefilled syringe if liquid is cloudy or contains flakes or large particles.
 |
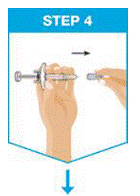
Remove the needle cover.
- Hold the syringe in one hand between the finger grip and needle cover.
- With the other hand, gently pull the needle cover straight off.
- DO NOT hold or pull plunger rod when removing the needle cover.
- You may see a drop of liquid at the end of the needle. This is normal.
- Throw away the needle cover.
- DO NOT touch the needle with your fingers or let the needle touch anything.
 |
Hold the body of the prefilled syringe in one hand between the thumb and index fingers.
Gently pinch the area of cleaned skin with your other hand and hold it firmly.
Insert the needle into the skin at about a 45-degree angle using a quick, short movement. Hold angle steady.
 |
Slowly push the plunger rod all the way in until all of the liquid is injected, and the syringe is empty.
Pull the needle out of the skin while keeping the syringe at the same angle.
Release the plunger rod and allow the prefilled syringe to move up until the entire needle is covered by the needle guard.
The prefilled syringe needle guard will not activate unless all the liquid has been injected.
- Press a cotton ball or gauze pad over the injection site and hold for 10 seconds.
- DO NOT rub the injection site. You may have slight bleeding. This is normal.
 |
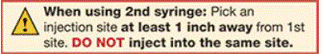
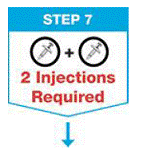
Repeat Steps 2 through 6 with the 2nd prefilled syringe for a full dose.
- Use the 2nd prefilled syringe right after using the 1st syringe.
 |
Put your used prefilled syringes in a FDA-cleared sharps disposal container right away after use.
- DO NOT throw away (dispose of) used prefilled syringes in the household trash.
For more information, see “Used SKYRIZI Prefilled Syringe Disposal” section.
 |
Questions About Using SKYRIZI
Q. What if I need help on how to inject SKYRIZI?
A. Call your healthcare provider or (866) SKYRIZI or (866) 759-7494 if you need help.
Q. What should I do with both used prefilled syringes after my injections?
A. Throw away (dispose of) both used prefilled syringes in a sharps disposal container and not your household trash. You can sign up to receive sharps containers for SKYRIZI syringe disposal at no additional cost by going to www.SKYRIZI.com or calling (866) SKYRIZI or (866) 759-7494.
Q. How do I know when the injection is complete?
A. The injection is complete when the prefilled syringe is empty, the plunger rod is pushed all the way in, and the syringe needle guard is activated.
Used SKYRIZI Prefilled Syringe Disposal
If you do not have a FDA-cleared sharps disposal container, you may use a household container that is:
- made of a heavy-duty plastic,
- can be closed with a tight-fitting, puncture-resistant lid, without sharps being able to come out,
- upright and stable during use,
- leak-resistant, and
- properly labeled to warn of hazardous waste inside the container.
When your sharps disposal container is almost full, you will need to follow your community guidelines for the right way to dispose of your sharps disposal container. There may be state or local laws about how you should throw away used needles and syringes.
For more information about safe sharps disposal, and for specific information about sharps disposal in the state that you live in, go to the FDA’s website at: www.fda.gov/safesharpsdisposal.
Do not recycle your used sharps disposal container.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
