Ongentys Drug Description: Detailed Datasheet
- Generic Name: opicapone capsules
- Brand Name: Ongentys
side effects drug center ongentys (opicapone capsules) drug
Drug Description
What is Ongentys and how is it used?
Ongentys is a prescription medicine used with levodopa and carbidopa in people with Parkinson’s disease (PD) who are having “OFF” episodes.
It is not known if Ongentys is safe and effective in children.
What are the possible side effects of Ongentys?
Ongentys may cause serious side effects, including:
- Falling asleep during normal activities. You may suddenly fall asleep while doing normal activities such as driving a car, talking or eating while taking Ongentys or other medicines used to treat Parkinson’s disease, without being drowsy or without warning. This may result in having accidents. Your chances of falling asleep while doing normal activities while taking Ongentys are higher if you take other medicines that cause drowsiness.
- Low blood pressure or dizziness. Low blood pressure, dizziness, light headedness, or fainting can happen with Ongentys. Tell your healthcare provider if you become dizzy, lightheaded, or faint while taking Ongentys.
- Uncontrolled sudden movements (dyskinesia). Ongentys may cause uncontrolled sudden movements or make such movements you already have worse or happen more often. Tell your healthcare provider if this happens.
- Seeing, hearing, believing, or feeling things that are not real or not true. Taking Ongentys may cause seeing, hearing, or feeling things that are not real (hallucinations), believing things that are not real (delusions), or aggressive behavior. Tell your healthcare provider if you have any of these changes in your behavior.
- Unusual urges (impulse control and compulsive disorders). Some people taking Ongentys may get urges to behave in a way unusual for them. Examples of this are unusual urges to gamble, increased sexual urges, strong urges to spend money, binge eating, and the inability to control these urges. If you or your family notice that you are developing any unusual behaviors, talk to your healthcare provider.
Tell your healthcare provider if you experience any of these side effects.
The most common side effects of Ongentys include:
- uncontrolled sudden movements (dyskinesia)
- constipation
- increase in a certain enzyme called blood creatine kinase
- low blood pressure
- weight loss
These are not all of the possible side effects of Ongentys. Call your healthcare provider for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
DESCRIPTION
ONGENTYS contains opicapone, a peripheral, selective and reversible catechol-O-methyltransferase (COMT) inhibitor. The chemical name of opicapone is 2,5-dichloro-3-(5-(3,4-dihydroxy-5-nitrophenyl)-1,2,4-oxadiazol3-yl)-4,6-dimethylpyridine-1-oxide with the following structure:
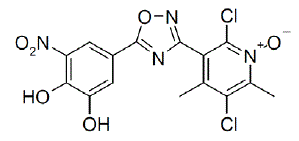 |
The opicapone molecular formula is C15H10Cl2N4O6; and its molecular weight is 413.17. Opicapone is a yellow powder/crystalline solid with limited aqueous solubility.
ONGENTYS capsules are intended for oral administration. Each capsule contains 25 mg or 50 mg of opicapone. ONGENTYS also contains the following inactive ingredients: lactose, magnesium stearate, pregelatinized starch, and sodium starch glycolate. The capsule shells contain: FD&C Blue#2, FD&C Red#3, gelatin, and titanium dioxide.
Indications & Dosage
INDICATIONS
ONGENTYS is indicated as adjunctive treatment to levodopa/carbidopa in patients with Parkinson’s disease (PD) experiencing “off” episodes.
DOSAGE AND ADMINISTRATION
Dosing And Administration Information
The recommended dosage of ONGENTYS is 50 mg administered orally once daily at bedtime. Patients should not eat food for 1 hour before and for at least 1 hour after intake of ONGENTYS [see CLINICAL PHARMACOLOGY].
Dosage Recommendations For Patients With Hepatic Impairment
In patients with moderate hepatic impairment (Child-Pugh B), the recommended dose of ONGENTYS is 25 mg orally once daily at bedtime [see Use In Specific Populations, CLINICAL PHARMACOLOGY].
Avoid use of ONGENTYS in patients with severe (Child-Pugh C) hepatic impairment [see Use In Specific Populations, CLINICAL PHARMACOLOGY].
Discontinuation And Missed Dose
When discontinuing ONGENTYS, monitor patients and consider adjustment of other dopaminergic therapies as needed. If a dose of ONGENTYS is missed, the next dose should be taken at the scheduled time the next day.
HOW SUPPLIED
Dosage Forms And Strengths
ONGENTYS capsules are available in the following strengths:
- 50 mg capsules with a dark blue opaque cap and dark pink opaque body; axially printed with “OPC” over “50” in white ink, on both the cap and body.
- 25 mg capsules with a light blue opaque cap and light pink opaque body; axially printed with “OPC” over “25” in blue ink, on both the cap and body.
ONGENTYS (opicapone) capsules are available as:
- 50 mg hard gelatin capsules, Size 1; dark blue opaque cap and dark pink opaque body; axially printed with “OPC” over “50” in white ink, on both the cap and body
- Bottle of 30 with child-resistant closure: NDC 70370-3050-2
- 25 mg hard gelatin capsules, Size 1; light blue opaque cap and light pink opaque body; axially printed with “OPC” over “25” in blue ink, on both the cap and body
- Bottle of 30 with child-resistant closure: NDC 70370-3025-2
Storage And Handling
Store at a temperature below 30°C (86°F).
Distributed by: Neurocrine Biosciences, Inc. San Diego, CA 92130. Revised: Apr 2020
Side Effects
The following clinically significant adverse reactions are discussed in more detail in other sections of the labeling:
- Cardiovascular Effects with Concomitant Use of Drugs Metabolized by Catechol-O-Methyltransferase (COMT) [see WARNINGS AND PRECAUTIONS]
- Falling Asleep During Activities of Daily Living and Somnolence [see WARNINGS AND PRECAUTIONS]
- Hypotension/Syncope [see WARNINGS AND PRECAUTIONS]
- Dyskinesia [see WARNINGS AND PRECAUTIONS]
- Hallucinations and Psychosis [see WARNINGS AND PRECAUTIONS]
- Impulse Control/Compulsive Disorders [see WARNINGS AND PRECAUTIONS]
- Withdrawal-Emergent Hyperpyrexia and Confusion [see WARNINGS AND PRECAUTIONS]
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of ONGENTYS was evaluated in 265 patients with Parkinson’s disease (PD) in two 14-15 week placebo-and active-controlled (Study 1) or placebo-controlled (Study 2) studies [see Clinical Studies]. All patients were taking a stable dose of levodopa and a DOPA decarboxylase inhibitor, alone or in combination with other PD medications. In Study 1 and Study 2, the mean age of patients was 63.6 years, 59% of patients were male, and 89% of patients were Caucasian. At baseline, the mean duration of PD was 7.6 years.
Adverse Reactions Leading To Discontinuation Of Treatment
In Study 1 and Study 2, a total of 8% of ONGENTYS 50 mg-treated patients and 6% of patients who received placebo discontinued due to adverse events. The most common adverse reaction leading to discontinuation was dyskinesia, reported in 3% of ONGENTYS 50 mg-treated patients and 0.4% of patients who received placebo.
Common Adverse Reactions
Adverse reactions that occurred in the pooled studies at an incidence of at least 2% and greater than placebo are presented in Table 1. The most common adverse reactions (incidence at least 4% and greater than placebo) were dyskinesia, constipation, blood creatine kinase increased, hypotension/syncope, and weight decreased.
Table 1: Adverse Reactions with an Incidence of at Least 2% in Patients Treated with ONGENTYS and Greater than on Placebo, in Pooled Study 1 and Study 2
| Adverse Reactions | ONGENTYS 50 mg N=265 % | Placebo N=257 % |
| Nervous system disorders | ||
| Dyskinesia | 20 | 6 |
| Dizziness | 3 | 1 |
| Gastrointestinal disorders | ||
| Constipation | 6 | 2 |
| Dry mouth | 3 | 1 |
| Psychiatric disorders | ||
| Hallucination1 | 3 | 1 |
| Insomnia | 3 | 2 |
| Investigations | ||
| Blood creatine kinase increased | 5 | 2 |
| Weight decreased | 4 | 0 |
| Vascular disorders | ||
| Hypotension/syncope2 | 5 | 1 |
| Hypertension | 3 | 2 |
| 1 Includes hallucinations, hallucinations visual, hallucinations auditory, and hallucinations mixed 2 Includes hypotension, orthostatic hypotension, syncope, and presyncope | ||
Drug Interactions
Non-Selective Monoamine Oxidase (MAO) Inhibitors
Both ONGENTYS and non-selective MAO inhibitors (e.g., phenelzine, isocarboxazid, and tranylcypromine) inhibit catecholamine metabolism, leading to increased levels of catecholamines. Concomitant use may increase the risk of possible arrhythmias, increased heart rate, and excessive changes in blood pressure.
Concomitant use of ONGENTYS with non-selective MAO inhibitors is contraindicated [see CONTRAINDICATIONS]. Selective MAO-B inhibitors can be used concomitantly with ONGENTYS.
Effect Of ONGENTYS On Other Drugs
Drugs Metabolized By Catechol-O-Methyltransferase (COMT)
Concomitant use of ONGENTYS with drugs metabolized by COMT may affect the pharmacokinetics of those drugs, which may increase the risk of possible arrhythmias, increased heart rate, and excessive changes in blood pressure [see WARNINGS AND PRECAUTIONS]. Drugs known to be metabolized by COMT should be administered with caution. Monitor for changes in heart rate, rhythm, and blood pressure in patients concomitantly treated with ONGENTYS and drugs metabolized by COMT [see WARNINGS AND PRECAUTIONS].
Warnings & Precautions
WARNINGS
Included as part of the "PRECAUTIONS" Section
PRECAUTIONS
Cardiovascular Effects With Concomitant Use Of Drugs Metabolized By Catechol-O-Methyltransferase (COMT)
Possible arrhythmias, increased heart rate, and excessive changes in blood pressure may occur with concomitant use of ONGENTYS and drugs metabolized by COMT (e.g., isoproterenol, epinephrine, norepinephrine, dopamine, and dobutamine), regardless of the route of administration (including inhalation). Monitor patients treated concomitantly with ONGENTYS and drugs metabolized by COMT [see CONTRAINDICATIONS, DRUG INTERACTIONS].
Falling Asleep During Activities Of Daily Living And Somnolence
Patients treated with dopaminergic medications and medications that increase levodopa exposure, including ONGENTYS, have reported falling asleep while engaged in activities of daily living, including the operation of motor vehicles, which sometimes has resulted in accidents. Patients may not perceive warning signs, such as excessive drowsiness, or they may report feeling alert immediately prior to the event.
Before initiating treatment with ONGENTYS, advise patients of the potential to develop drowsiness and specifically ask about factors that may increase the risk for somnolence with dopaminergic therapy, such as concomitant sedating medications or the presence of a sleep disorder. If a patient develops daytime sleepiness or episodes of falling asleep during activities that require full attention (e.g., driving a motor vehicle, conversations, eating), consider discontinuing ONGENTYS or adjusting other dopaminergic or sedating medications. If a decision is made to continue ONGENTYS, patients should be advised not to drive and to avoid other potentially dangerous activities.
Hypotension/Syncope
In Study 1 and Study 2 [see Clinical Studies], hypotension (orthostatic and non-orthostatic), syncope, and presyncope occurred in 5% of patients treated with ONGENTYS 50 mg compared to 1% of patients who received placebo. Monitor patients for hypotension (orthostatic and non-orthostatic) and advise patients about the risk for syncope and presyncope. If these adverse reactions occur, consider discontinuing ONGENTYS or adjusting the dosage of other medications that can lower blood pressure.
Dyskinesia
ONGENTYS potentiates the effects of levodopa [see CLINICAL PHARMACOLOGY] and may cause dyskinesia or exacerbate pre-existing dyskinesia.
In controlled clinical trials (Study 1 and Study 2) [see Clinical Studies], dyskinesia occurred in 20% of patients treated with ONGENTYS 50 mg compared to 6% of patients who received placebo. Dyskinesia was also the most common adverse reaction leading to discontinuation of ONGENTYS [see ADVERSE REACTIONS].
Reducing the patient’s daily levodopa dosage or the dosage of another dopaminergic drug may mitigate dyskinesia that occurs during treatment with ONGENTYS.
Hallucinations And Psychosis
In Study 1 and Study 2, hallucinations (hallucinations, auditory hallucinations, visual hallucinations, mixed hallucinations) occurred in 3% of patients treated with ONGENTYS 50 mg compared to 1% of patients who received placebo. Delusions, agitation, or aggressive behavior occurred in 1% of patients treated with ONGENTYS 50 mg, and in no patient who received placebo. Consider stopping ONGENTYS if hallucinations or psychotic-like behaviors occur.
Patients with a major psychotic disorder should ordinarily not be treated with ONGENTYS because of the risk of exacerbating the psychosis with an increase in central dopaminergic tone. In addition, treatments for psychosis that antagonize the effects of dopaminergic medications may exacerbate the symptoms of PD.
Impulse Control/Compulsive Disorders
Patients treated with ONGENTYS can experience intense urges to gamble, increased sexual urges, intense urges to spend money, binge eating, and/or other intense urges, and the inability to control these urges while taking one or more dopaminergic therapies that increase central dopaminergic tone. In some cases, these urges were reported to have stopped when the dose was reduced, or the medication was discontinued. Because patients may not recognize these behaviors as abnormal, it is important for prescribers to specifically ask patients or their caregivers about the development of new or increased gambling urges, sexual urges, uncontrolled spending, or other urges while being treated with ONGENTYS.
In Study 1 and Study 2, impulse control disorders occurred in 1% of patients treated with ONGENTYS 50 mg, and in no patient who received placebo. Re-evaluate the patient’s current therapy(ies) for Parkinson’s disease and consider stopping ONGENTYS if a patient develops such urges while taking ONGENTYS.
Use with caution in Parkinson’s patients with suspected or diagnosed dopamine dysregulation syndrome.
Withdrawal-Emergent Hyperpyrexia And Confusion
A symptom complex resembling neuroleptic malignant syndrome (characterized by elevated temperature, muscular rigidity, altered consciousness, and autonomic instability), with no other obvious etiology, has been reported in association with rapid dose reduction, withdrawal of, or changes in drugs that increase central dopaminergic tone. In the controlled clinical studies of ONGENTYS, patients discontinued ONGENTYS treatment without dose tapering or gradual withdrawal. There were no reports of neuroleptic malignant syndrome in ONGENTYS controlled clinical studies. When discontinuing ONGENTYS, monitor patients and consider adjustment of other dopaminergic therapies as needed [see DOSAGE AND ADMINISTRATION].
Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (PATIENT INFORMATION).
Administration
Instruct patients and/or caregivers that ONGENTYS capsules should be taken at bedtime. Inform patients to not eat food for 1 hour before and for at least 1 hour after intake of ONGENTYS with food [see DOSAGE AND ADMINISTRATION].
Concomitant Medications
Certain medications can cause an interaction with ONGENTYS. Advise patients and/or caregivers to inform their healthcare provider of all the medicines the patient is taking, including over-the-counter medicines, dietary supplements, and herbal products [see WARNINGS AND PRECAUTIONS and DRUG INTERACTIONS].
Falling Asleep During Activities Of Daily Living
Advise patients and/or caregivers that somnolence has been reported with ONGENTYS. Patients treated with dopaminergic medications have reported falling asleep while engaged in activities of daily living. These adverse reactions may affect some patients’ ability to drive and operate machinery safely [see WARNINGS AND PRECAUTIONS].
Hypotension/Syncope
Advise patients that ONGENTYS may cause hypotension or syncope [see WARNINGS AND PRECAUTIONS].
Dyskinesia
Advise patients that ONGENTYS may cause dyskinesia or exacerbate pre-existing dyskinesia [see WARNINGS AND PRECAUTIONS].
Hallucinations And Psychosis
Advise patients that ONGENTYS may cause hallucinations, delusions, or aggressive behavior and they should report any of these adverse reactions to their healthcare provider [see WARNINGS AND PRECAUTIONS].
Impulse Control/Compulsive Disorders
Inform patients of the potential for experiencing intense urges to gamble, increased sexual urges, intense urges to spend money, binge eating, and other intense urges and the inability to control these urges while taking ONGENTYS and one or more medications that increase central dopaminergic tone that are generally used for the treatment of PD. Advise patients that they should report any of these adverse reactions to their healthcare provider [see WARNINGS AND PRECAUTIONS].
Withdrawal-Emergent Hyperpyrexia And Confusion
Advise patients to contact their healthcare provider before stopping ONGENTYS. Tell patients to inform their healthcare provider if they develop symptoms such as fever, confusion, or severe muscle stiffness after stopping ONGENTYS [see WARNINGS AND PRECAUTIONS].
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment Of Fertility
Carcinogenesis
No increase in tumors was observed when opicapone was administered orally to mice (0, 100, 375, or 750 mg/kg/day) for up to 2 years (84-93 weeks at the high dose). The highest dose tested is approximately 70 times the recommended dose (RHD) in humans (50 mg/day) on a body surface area (mg/m2) basis.
No increase in tumors was observed when opicapone was administered orally to rats (0, 100, 500, or 1000 mg/kg/day) for 2 years. Plasma exposure (AUC) at the highest dose tested is approximately 24 times that in humans at the RHD (50 mg/day).
Mutagenesis
Opicapone was negative in in vitro (bacterial reverse mutation test (Ames), chromosomal aberrations in human peripheral blood lymphocytes) and in in vivo (rat bone marrow micronucleus) assays.
Impairment Of Fertility
In male and female rats, oral administration of opicapone (0, 100, 500, or 1000 mg/kg/day) prior to and during mating and continuing in females to gestation day 6, resulted in no adverse effects on fertility or general reproductive performance. Plasma exposure (AUC) at the highest dose tested is approximately 40 times that in humans at the RHD.
Use In Specific Populations
Pregnancy
Risk Summary
There are no adequate data on the developmental risk associated with use of ONGENTYS in pregnant women. In animal studies, oral administration of opicapone during pregnancy resulted in adverse effects on embryofetal development (increased incidence of fetal abnormalities) at clinically relevant plasma exposures in one of two species tested. In addition, opicapone is always given concomitantly with levodopa/carbidopa, which is known to cause developmental toxicity in rabbits (see Data).
The background risk of major birth defects and miscarriage in the U.S. general population is 2-4% and 15-20% of clinically recognized pregnancies, respectively. The background risk for major birth defects and miscarriage in patients with Parkinson’s disease is unknown.
Data
Animal Data
Oral administration of opicapone (0, 150, 375, or 1000 mg/kg/day) to pregnant rats throughout gestation resulted in no adverse effects on embryofetal development. Plasma exposure (AUC) at the highest dose tested (1000 mg/kg/day) was approximately 40 times that in humans at the recommended human dose (50 mg/day).
In pregnant rabbits, oral administration of opicapone (0, 100, 175, or 225 mg/kg/day) during the period of organogenesis resulted in increased incidence of structural abnormalities at all doses tested; maternal toxicity was observed at all but the lowest dose tested. A no-effect dose for adverse effects on embryofetal development was not identified. Plasma exposure (AUC) at the low-effect dose (100 mg/kg/day) was less than that in humans at the RHD.
Oral administration of opicapone (0, 150, 375, or 1000 mg/kg/day) throughout gestation and lactation resulted in no adverse effects on pre-and postnatal development; however, effects on neurobehavioral development in the offspring were not rigorously assessed. Plasma exposure (AUC) at the highest dose tested (1000 mg/kg/day) was approximately 40 times that in humans at the RHD.
Opicapone is always given concomitantly with levodopa/carbidopa, which is known to cause visceral and skeletal malformations in rabbits. The developmental toxicity of opicapone in combination with levodopa/carbidopa was not assessed in animals.
Lactation
Risk Summary
There are no data on the presence of opicapone in human milk, the effects on the breastfed infant, or the effects on milk production. In lactating rats, oral administration of opicapone resulted in levels of opicapone or metabolites in milk similar to those in maternal plasma. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for ONGENTYS and any potential adverse effects on the breastfed infant from ONGENTYS or from the underlying maternal condition.
Pediatric Use
Safety and effectiveness in pediatric patients have not been established.
Geriatric Use
No dose adjustment is required for elderly patients. Of the total number of patients who received ONGENTYS 50 mg in Study 1 and Study 2, 52% of patients were 65 years and older. No overall differences in safety and effectiveness were observed between these patients and younger patients, but greater sensitivity to adverse reactions of some older individuals cannot be ruled out.
Renal Impairment
The renal route of elimination plays a minor role in the clearance of opicapone [see CLINICAL PHARMACOLOGY]. Avoid use of ONGENTYS in patients with end-stage renal disease (ESRD) (CLcr <15 mL/min). No dosage adjustment is required for patients with mild, moderate, or severe renal impairment. However, because of a potential for increased exposure, monitor patients with severe renal impairment for adverse reactions and discontinue ONGENTYS if tolerability issues arise.
Hepatic Impairment
Opicapone exposure is increased in patients with hepatic impairment [see CLINICAL PHARMACOLOGY]. Avoid use of ONGENTYS in patients with severe (Child-Pugh C) hepatic impairment. Dosage adjustment is recommended for patients with moderate (Child-Pugh B) hepatic impairment [see DOSAGE AND ADMINISTRATION]. No dosage adjustment is required in patients with mild (Child-Pugh A) hepatic impairment.
Overdosage & Contraindications
OVERDOSE
No specific antidotes for ONGENTYS are known. As a general measure, removal of ONGENTYS by gastric lavage and/or inactivation by administering activated charcoal should be considered. In managing overdose, provide supportive care, including close medical supervision and monitoring, and consider the possibility of multiple drug involvement. If an over-exposure occurs, call your poison control center at 1-800-222-1222 or www.poison.org.
CONTRAINDICATIONS
ONGENTYS is contraindicated in patients with:
- Concomitant use of non-selective monoamine oxidase (MAO) inhibitors [see DRUG INTERACTIONS].
- Pheochromocytoma, paraganglioma, or other catecholamine secreting neoplasms.
Clinical Pharmacology
Mechanism Of Action
Opicapone is a selective and reversible inhibitor of catechol-O-methyltransferase (COMT).
COMT catalyzes the transfer of the methyl group of S-adenosyl-L-methionine to the phenolic group of substrates that contain a catechol structure. Physiological substrates of COMT include DOPA, catecholamines (dopamine, norepinephrine, and epinephrine), and their hydroxylated metabolites. When decarboxylation of levodopa is prevented by carbidopa, COMT becomes the major metabolizing enzyme for levodopa, catalyzing its metabolism to 3-methoxy-4-hydroxy-L-phenylalanine (3-OMD).
Pharmacodynamics
COMT Activity
Once-daily administration of ONGENTYS 50 mg caused inhibition of COMT activity in erythrocytes; the maximal inhibition seen was 84% and was maintained >65% over a 24-hour dosing interval in patients with Parkinson’s disease. Following termination of treatment, COMT inhibition slowly returns to baseline levels, with >35% inhibition still observed 5 days after the last dose.
Effects On Levodopa
Peak (Cmax) and overall levodopa exposure (AUC) increased by 43-44% and 62-94%, respectively, in PD patients following once-daily administration of ONGENTYS at bedtime with levodopa/carbidopa administered every three or every four hours, as compared to after administration of levodopa/carbidopa alone.
Cardiac Electrophysiology
At a dose 16 times the recommended dosage, ONGENTYS does not prolong the QT interval to any clinically relevant extent.
Pharmacokinetics
Opicapone demonstrates dose-proportional pharmacokinetics over a 25 mg (0.5 times the recommended dosage) to 50 mg dose range. The pharmacokinetics of opicapone are similar in both PD patients and healthy subjects.
Absorption
After single-dose administration of ONGENTYS 50 mg, the median (range) plasma Tmax value was 2.0 (1.04.0) hours.
Effect of Food
Following a moderate fat/moderate calorie meal, the mean peak plasma concentration (Cmax) for opicapone decreased 62%, the mean overall plasma exposure (AUC) decreased 31%, and the Tmax was delayed by 4 hours. In Study 1, ONGENTYS was administered without regard to food. In Study 2, ONGENTYS administration and food consumption were separated by 1 hour [see DOSAGE AND ADMINISTRATION, Clinical Studies].
Distribution
Opicapone is highly bound to plasma proteins (>99%), which is independent of concentration.
Elimination
The mean elimination half-life of opicapone is 1 to 2 hours.
Metabolism
Sulphation is the primary metabolic pathway of opicapone, based on clinical studies and in vitro assessments. Other metabolic pathways include glucuronidation, methylation (by COMT), reduction, and glutathione conjugation.
Excretion
After administration of a single dose of radiolabeled opicapone 100 mg (2 times the recommended dosage) to healthy subjects, approximately 70% of the dose was recovered in feces (22% as unchanged), 20% in expired air, and 5% in urine (<1% as unchanged).
Specific Populations
No clinically significant differences in the pharmacokinetics of opicapone were observed based on age (i.e., 18 to 40 years of age and ≥ 65 years of age), sex, or race/ethnicity (i.e., Japanese, Caucasian, Asian, and Black).
Renal Impairment
Based on population pharmacokinetic analyses, no clinically significant differences in the pharmacokinetics of opicapone were observed in patients with mild or moderate renal impairment (CLcr 30-89 mL/min using the Cockcroft-Gault equation) relative to those with normal renal function (CLcr >90 mL/min). Patients with severe renal impairment or ESRD (CLcr <30 mL/min) have not been studied [see Use In Specific Populations].
Hepatic Impairment
The single-dose pharmacokinetics of opicapone was evaluated in subjects with mild (Child-Pugh: A) and moderate (Child-Pugh: B) hepatic impairment. In subjects with mild hepatic impairment, the mean overall opicapone plasma exposure (AUC) increased by 35%, which is not expected to be clinically significant. In subjects with moderate hepatic impairment, the mean overall opicapone plasma exposure (AUC) increased by 84%. Dosage adjustment for ONGENTYS is required in subjects with moderate hepatic impairment [see DOSAGE AND ADMINISTRATION]. ONGENTYS has not been studied in patients with severe hepatic impairment (Child-Pugh: C) [see Use In Specific Populations].
Drug Interaction Studies
Clinical Studies
No clinically significant differences in the pharmacokinetics of opicapone were observed when administered concomitantly with quinidine (index substrate of P-gp [MDR1]), acetaminophen, or rasagiline.
No clinically significant differences in the pharmacokinetics of the following drugs were observed when administered concomitantly with opicapone: S-warfarin (index substrate of CYP2C9), R-Warfarin (substrate of CYP1A2 and CYP3A4), or repaglinide (index substrate of CYP2C8 and OATP1B1).
No clinically significant differences in the pharmacokinetics of the following drugs for the treatment of Parkinson’s disease were observed when administered concomitantly with opicapone: rasagiline, selegiline, pramipexole, ropinirole, or amantadine.
In Vitro Studie
Opicapone does not affect protein binding of warfarin, diazepam, digoxin, or tolbutamide, in vitro.
CYP Enzymes
Opicapone is not an inhibitor or inducer of major CYPs.
Transporter Systems
Opicapone is a substrate of P-gp (MDR1) (see Clinical Studies), BCRP, MRP2, OATP1B3, and OATP2B1. No clinically significant transporter mediated interaction is expected for opicapone. Opicapone is not an inhibitor of P-gp (MDR1), BCRP, OAT1, OAT3, OCT1, OCT2, OATP1B3, BSEP, MATE1, or MATE2-K.
Clinical Studies
The efficacy of ONGENTYS for the adjunctive treatment to levodopa/carbidopa in patients with Parkinson’s disease (PD) experiencing “off” episodes was evaluated in two double-blind, randomized, parallel-group, placebo-and active-controlled (Study 1, NCT01568073), or placebo-controlled (Study 2, NCT01227655) studies of 14-15 week duration. All patients were treated with levodopa/ DOPA decarboxylase inhibitor (DDCI) (alone or in combination with other PD medications). The double-blind period for each study began with a period for levodopa/DDCI dose adjustment (up to 3 weeks), followed by a stable maintenance period of 12 weeks.
Study 1
In Study 1, patients (n=600) were randomized to treatment with one of 3 doses of ONGENTYS. The intention to treat (ITT) population included patients treated with ONGENTYS 50 mg once daily (n=115) or placebo (n=120). Baseline demographic characteristics were similar across all treatment groups: approximately 60% of patients were male, mean age was 64 years, and all patients were Caucasian. Baseline PD characteristics in the treatment groups were: mean duration of PD of 7 years for ONGENTYS 50 mg compared to 7.7 years for placebo, and mean onset of motor fluctuations of 2.2 years prior to study enrollment. Eighty-two percent of patients in both groups used concomitant PD medications in addition to levodopa; the most commonly used were dopamine agonists (68%), amantadine (23%), MAO-B inhibitors (20%), and anticholinergics (5%).
The primary efficacy endpoint was the change in mean absolute OFF-time based on 24-hour patient diaries completed 3 days prior to each of the scheduled visits. ONGENTYS 50 mg significantly reduced mean absolute OFF-time compared to placebo (Table 2).
Table 2: Study 1 -Absolute OFF-time (Hours) Change from Baseline to Endpoint
| N | Baseline Mean (SE) | LS Mean Change from Baseline (SE) | Placebo-subtracted Difference (95% CI) | Adjusted p-value a | |
| Placebo | 120 | 6.17 hours (0.162) | -0.93 (0.223) | - | - |
| ONGENTYS 50 mg | 115 | 6.20 hours (0.166) | -1.95 (0.233) | -1.01 (-1.620, -0.407) | p=0.002 |
| CI=confidence interval; LS =least squares; N=total number of patients; SE=standard error. a Adjusted p values were calculated using a gatekeeping procedure controlling for multiplicity. | |||||
ON-time without troublesome dyskinesia was a secondary efficacy endpoint in Study 1 (Table 3).
Table 3: Study 1 -Absolute ON-time Without Troublesome Dyskinesia (Hours) Change from Baseline to Endpoint
| N | Baseline Mean (SE) | LS Mean Change from Baseline (SE) | Placebo-subtracted Difference (95% CI) | Nominal p-value a | |
| Placebo | 120 | 9.61 (0.191) | 0.75 (0.237) | - | - |
| ONGENTYS 50 mg | 115 | 9.54 (0.183) | 1.84 (0.247) | 1.08 (0.440, 1.728) | p=0.001 |
| CI=confidence interval; LS =least squares; N=total number of patients; SE=standard error. a Unadjusted p-value. | |||||
Study 2
In Study 2, patients (n=427) were randomized to treatment with either one of two doses of ONGENTYS once daily (n=283) or placebo (n=144). The intention to treat (ITT) study population included patients treated with ONGENTYS 50 mg once daily (n=147) or placebo (n=135). Baseline demographic characteristics (ONGENTYS 50 mg vs. placebo) were: mean age (66 years vs. 62 years), male (61% vs. 53%), Caucasian (78% vs. 66%) and Asian (21% vs. 31%). Baseline PD characteristics were generally similar across treatment groups with a mean duration of PD of 8.2 years, and a mean onset of motor fluctuations of 3.2 years prior to study enrollment. Eighty-five percent of patients treated with ONGENTYS 50 mg compared to 81% of patients who received placebo used concomitant PD medications in addition to levodopa; the most commonly used were dopamine agonists (70%), amantadine (21%), MAO-B inhibitors (20%), and anticholinergics (12%).
The primary efficacy endpoint was the change in mean absolute OFF-time based on 24-hour patient diaries completed 3 days prior to each of the scheduled visits. ONGENTYS 50 mg significantly reduced mean absolute OFF-time compared to placebo (Table 4).
Table 4: Study 2 -Absolute OFF-time (Hours) Change from Baseline to Endpoint
| N | Baseline Mean (SE) | LS Mean Change from Baseline (SE) | Placebo-subtracted Difference (95% CI) | Adjusted p-value a | |
| Placebo | 135 | 6.12 (0.200) | -1.07 (0.239) | - | - |
| ONGENTYS 50 mg | 147 | 6.32 (0.183) | -1.98 (0.230) | -0.91 (-1.523, -0.287) | p=0.008 |
| CI=confidence interval; LS =least squares; N=total number of patients; SE=standard error. a Adjusted p values were calculated using Dunnett's alpha level adjustment to control for multiplicity. | |||||
ON-time without troublesome dyskinesia was a secondary efficacy endpoint in Study 2 (Table 5).
Table 5: Study 2 -Absolute ON-time without troublesome dyskinesia (Hours) Change from Baseline to Endpoint
| N | Baseline Mean (SE) | LS Mean Change from Baseline (SE) | Placebo-subtracted Difference (95% CI) | Nominal p-value | |
| Placebo | 135 | 9.61 (0.206) | 0.80 (0.256) | - | - |
| ONGENTYS 50 mg | 147 | 9.37 (0.183) | 1.43 (0.247) | 0.62 (-0.039, 1.287) | p=0.065 (NS*) |
| CI=confidence interval; LS =least squares; N=total number of patients; SE=standard error. *= not statistically significant. | |||||
Medication Guide
PATIENT INFORMATION
ONGENTYS®
(on-JEN-tis)
(opicapone) capsules
What is ONGENTYS?
ONGENTYS is a prescription medicine used with levodopa and carbidopa in people with Parkinson’s disease (PD) who are having “OFF” episodes.
It is not known if ONGENTYS is safe and effective in children.
Do not take ONGENTYS if you:
- take a type of medicine called a non-selective monoamine-oxidase (MAO) inhibitor, such as phenelzine, isocarboxazid, or tranylcypromine. Ask your healthcare provider or pharmacist if you are taking a non-selective MAO inhibitor.
- have a tumor that secretes hormones known as catecholamines. These tumors include pheochromocytoma (a type of adrenal gland tumor) and paraganglioma.
Before taking ONGENTYS, tell your healthcare provider about all of your medical conditions, including if you:
- have daytime sleepiness from a sleep disorder, have unexpected or unpredictable periods of sleep or sleepiness, take a medicine to help you sleep, or take any medicine that makes you feel sleepy.
- have or have had intense urges or unusual behaviors, including gambling, increased sex drive, binge eating, or compulsive shopping.
- have a history of uncontrolled sudden movements (dyskinesia).
- have or have had hallucinations or psychosis.
- have liver problems.
- have kidney problems.
- are pregnant or plan to become pregnant. It is not known if ONGENTYS will harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if ONGENTYS passes into breast milk. You and your healthcare provider should decide the best way to feed your baby if you take ONGENTYS.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Especially tell your healthcare provider if you take nonselective MAO inhibitors (such as phenelzine, tranylcypromine, and isocarboxazid) or catecholamine medicines (such as isoproterenol, epinephrine, norepinephrine, dopamine, and dobutamine), regardless of how you take the medicine (by mouth, inhaled, or by injection).
ONGENTYS and other medicines may affect each other causing side effects. ONGENTYS may affect the way other medicines work, and other medicines may affect how ONGENTYS works.
Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist when you get a new medicine.
How should I take ONGENTYS?
- Take ONGENTYS exactly as your healthcare provider tells you to.
- ONGENTYS should be taken 1 time each day at bedtime.
- Do not eat 1 hour before taking ONGENTYS and do not eat for at least 1 hour after taking ONGENTYS.
- If you miss a dose, take your usual dose of ONGENTYS on the next day at bedtime.
- Do not stop taking ONGENTYS or change your dose before talking to your healthcare provider.
- Your dose of other Parkinson’s disease medicines may change when stopping ONGENTYS. Tell your healthcare provider if you have symptoms of withdrawal such as fever, confusion, or severe muscle stiffness.
- If you take too much ONGENTYS, call your healthcare provider or Poison Control Center at 1-800-222-1222, or go to the nearest hospital emergency room right away.
What should I avoid while taking ONGENTYS?
- Do not drive, operate machinery, or do other dangerous activities until you know how ONGENTYS affects you.
What are the possible side effects of ONGENTYS?
ONGENTYS may cause serious side effects, including:
- Falling asleep during normal activities. You may suddenly fall asleep while doing normal activities such as driving a car, talking or eating while taking ONGENTYS or other medicines used to treat Parkinson’s disease, without being drowsy or without warning. This may result in having accidents. Your chances of falling asleep while doing normal activities while taking ONGENTYS are higher if you take other medicines that cause drowsiness.
- Low blood pressure or dizziness. Low blood pressure, dizziness, light headedness, or fainting can happen with ONGENTYS. Tell your healthcare provider if you become dizzy, lightheaded, or faint while taking ONGENTYS.
- Uncontrolled sudden movements (dyskinesia). ONGENTYS may cause uncontrolled sudden movements or make such movements you already have worse or happen more often. Tell your healthcare provider if this happens.
- Seeing, hearing, believing, or feeling things that are not real or not true. Taking ONGENTYS may cause seeing, hearing, or feeling things that are not real (hallucinations), believing things that are not real (delusions), or aggressive behavior. Tell your healthcare provider if you have any of these changes in your behavior.
- Unusual urges (impulse control and compulsive disorders). Some people taking ONGENTYS may get urges to behave in a way unusual for them. Examples of this are unusual urges to gamble, increased sexual urges, strong urges to spend money, binge eating, and the inability to control these urges. If you or your family notice that you are developing any unusual behaviors, talk to your healthcare provider.
Tell your healthcare provider if you experience any of these side effects.
The most common side effects of ONGENTYS include:
- uncontrolled sudden movements (dyskinesia)
- constipation
- increase in a certain enzyme called blood creatine kinase
- low blood pressure
- weight loss
These are not all of the possible side effects of ONGENTYS. Call your healthcare provider for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store ONGENTYS?
- Store ONGENTYS at a temperature below 86°F (30°C).
- Keep ONGENTYS and all medicines out of the reach of children.
General information about the safe and effective use of ONGENTYS.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use ONGENTYS for a condition for which it was not prescribed. Do not give ONGENTYS to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about ONGENTYS that is written for health professionals.
What are the ingredients in ONGENTYS?
Active ingredient: opicapone
Inactive ingredients: lactose, magnesium stearate, pregelatinized starch, and sodium starch glycolate. The capsule shells contain: FD&C Blue#2, FD&C Red#3, gelatin, and titanium dioxide.
This Patient Information has been approved by the U.S. Food and Drug Administration
