Breztri Aerosphere Drug Description: Detailed Datasheet
- Generic Name: budesonide, glycopyrrolate, and formoterol fumarate inhalation aerosol
- Brand Name: Breztri Aerosphere
side effects drug center breztri aerosphere (budesonide, glycopyrrolate, and formoterol fumarate inhalation aerosol) drug
Drug Description
What is Breztri Aerosphere and how is it used?
Breztri Aerosphere combines 3 medicines, an inhaled corticosteroid (ICS) medicine (budesonide), an anticholinergic medicine (glycopyrrolate), and a long-acting beta2-adrenergic agonist (LABA) medicine (formoterol fumarate) in 1 inhaler, delivered as a propelled spray.
- ICS medicines such as budesonide help to decrease inflammation in the lungs. Inflammation in the lungs can lead to breathing problems.
- Anticholinergic medicines, such as glycopyrrolate and LABA medicines, such as formoterol fumarate help the muscles around the airways in your lungs stay relaxed to prevent symptoms, such as wheezing, cough, chest tightness, and shortness of breath. These symptoms can happen when the muscles around the airways tighten. This makes it hard to breathe.
- Breztri Aerosphere is a prescription medicine used long term to treat people with chronic obstructive pulmonary disease (COPD). COPD is a chronic lung disease that includes chronic bronchitis, emphysema, or both.
- Breztri Aerosphere is used as 2 inhalations, 2 times each day (2 puffs in the morning and 2 puffs in the evening) to improve symptoms of COPD for better breathing and to reduce the number of flare-ups (the worsening of your COPD symptoms for several days).
- Breztri Aerosphere is not for the treatment of asthma. It is not known if Breztri Aerosphere is safe and effective in people with asthma. Breztri Aerosphere contains formoterol fumarate. LABA medicines such as formoterol fumarate when used alone increase the risk of hospitalizations and death from asthma problems. Breztri Aerosphere contains an ICS, an anticholinergic, and a LABA. When an ICS and LABA are used together, there is not a significant risk in hospitalizations and deaths from asthma problems.
- Breztri Aerosphere is not to be used to relieve sudden breathing problems and will not replace a rescue inhaler. Always have a rescue inhaler (an inhaled, short-acting bronchodilator) with you to treat sudden breathing problems. If you do not have a rescue inhaler, contact your healthcare provider to have one prescribed for you.
- Breztri Aerosphere should not be used in children. It is not known if Breztri Aerosphere is safe and effective in children.
- Do not use Breztri Aerosphere if you are allergic to budesonide, glycopyrrolate, formoterol, or any of the ingredients in BREZTRI AEROSPHERE. See “What are the ingredients in Breztri Aerosphere?” at the end of this Patient Information leaflet below for a complete list of ingredients in Breztri Aerosphere.
DESCRIPTION
Breztri Aerosphere (budesonide, glycopyrrolate and formoterol fumarate) Inhalation Aerosol is a pressurized metered-dose inhaler that delivers a combination of micronized budesonide [an inhaled corticosteroid (ICS)], micronized glycopyrrolate (an anticholinergic), and micronized formoterol fumarate [an inhaled long-acting beta2-adrenergic agonist (a LABA)] for oral inhalation.
Budesonide is a corticosteroid with the following chemical name: (RS)-11β, 16α, 17,21-Tetrahydroxypregna-1,4-diene-3,20-dione cyclic 16,17-acetal with butyraldehyde. Budesonide is a white to off-white, powder which is practically insoluble in water. The molecular formula is C25H34O6 and the molecular weight is 430.54. The structural formula is as follows:
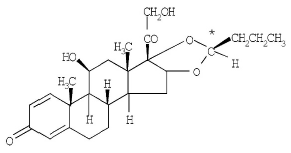 |
Budesonide contains nine chiral centers and is a mixture of the two epimers (22R and 22S). Glycopyrrolate is a quaternary ammonium salt with the following chemical name: (RS)-[3-(SR)- Hydroxy-1,1-dimethylpyrrolidinium bromide] α-cyclopentylmandelate. Glycopyrrolate is a powder that is freely soluble in water. The molecular formula is C19H28BrNO3, and the molecular weight is 398.33 g/mol. The structural formula is as follows:
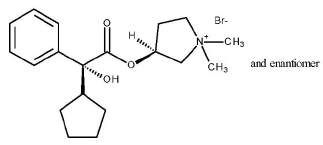 |
Glycopyrrolate contains two chiral centers and is a racemate of a 1:1 mixture of the R,S and S,R diastereomers. The active moiety, glycopyrronium, is the positively charged ion of glycopyrrolate.
Formoterol fumarate has the chemical name N-[2-Hydroxy-5-[(1RS)-1-hydroxy-2-[[(1RS)-2-(4- methoxyphenyl)-1-methylethyl]-amino] ethyl]phenyl] formamide, (E)-2-butenedioate dihydrate. Formoterol fumarate is a powder that is slightly soluble in water. The molecular formula is (C19H24N2O4)2·C4H4O4·2H2O and the molecular weight is 840.91 g/mol. The structural formula is as follows:
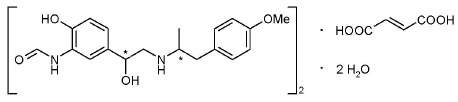 |
Formoterol fumarate contains two chiral centers and consists of a single enantiomeric pair (a racemate of R,R and S,S).
Breztri Aerosphere is formulated as a hydrofluoroalkane (HFA 134a) propelled pressurized metered dose inhaler containing 28 or 120 inhalations. The canister has an attached dose indicator and is supplied with a white plastic actuator body and mouthpiece with a light grey dust cap.
After priming, each actuation of the inhaler meters 182 mcg of budesonide, 10.4 mcg of glycopyrrolate (equivalent to 8.2 mcg of glycopyrronium), and 5.5 mcg of formoterol fumarate (equivalent to 4.7 mcg of formoterol) from the valve which delivers 160 mcg of budesonide, 9.0 mcg of glycopyrrolate (equivalent to 7.2 mcg of glycopyrronium), and 4.8 mcg of formoterol fumarate (equivalent to 4.1 mcg of formoterol) from the actuator. The actual amount of drug delivered to the lung may depend on patient factors, such as the coordination between actuation of the device and inspiration through the delivery system. Breztri Aerosphere also contains porous particles that form a co-suspension with the drug crystals. The porous particles are comprised of the phospholipid, 1,2-distearoyl-sn-glycero-3-phosphocholine (DSPC), and calcium chloride. Porous particles and HFA 134a are excipients in the formulation.
Indications & Dosage
INDICATIONS
BREZTRI AEROSPHERE is indicated for the maintenance treatment of patients with chronic obstructive pulmonary disease (COPD).
Limitations Of Use
BREZTRI AEROSPHERE is not indicated for the relief of acute bronchospasm or for the treatment of asthma [see WARNINGS AND PRECAUTIONS].
DOSAGE AND ADMINISTRATION
Recommended Dosage And Administration
The recommended dosage of BREZTRI AEROSPHERE is budesonide 320 mcg, glycopyrrolate 18 mcg and formoterol fumarate 9.6 mcg (administered as 2 inhalations of BREZTRI AEROSPHERE [budesonide/glycopyrrolate/formoterol fumarate 160 mcg/9 mcg/4.8 mcg]) twice daily in the morning and in the evening by oral inhalation. Do not take more than two inhalations twice daily.
After inhalation, rinse mouth with water without swallowing.
Preparation
Prime BREZTRI AEROSPHERE before using for the first time. Priming BREZTRI AEROSPHERE is essential to ensure appropriate drug content in each actuation. Prime BREZTRI AEROSPHERE by releasing 4 sprays into the air away from the face, shaking well before each spray.
If the inhaler has not been used for more than 7 days, is dropped, or after weekly cleaning, prime the inhaler again by releasing 2 sprays into the air away from the face, shaking well before each spray.
Dose Counter
BREZTRI AEROSPHERE canister has an attached dose indicator, which indicates how many inhalations remain. The dose indicator display will move after every tenth actuation. When nearing the end of the usable inhalations, the color behind the number in the dose indicator display window changes to red.
BREZTRI AEROSPHERE should be discarded when the dose indicator display window shows zero.
HOW SUPPLIED
Dosage Forms And Strengths
Inhalation Aerosol: a pressurized metered dose inhaler that delivers a combination of 160 mcg budesonide, 9 mcg glycopyrrolate, and 4.8 mcg formoterol fumarate per inhalation.
Storage And Handling
BREZTRI AEROSPHERE Inhalation Aerosol:
- 160 mcg budesonide, 9.0 mcg glycopyrrolate, and 4.8 mcg formoterol fumarate per inhalation
- is supplied as a pressurized aluminum canister with an attached dose indicator, a white plastic actuator and mouthpiece, and a light grey dust cap.
- contains 28 or 120 inhalations per canister.
- each 120-inhalation canister has a net fill weight of 10.7 grams (NDC 0310-4616-12).
- each 28-inhalation canister (institutional pack) has a net fill weight of 5.9 grams (NDC 0310- 4616-39).
- each canister of BREZTRI AEROSPHERE is packaged in a foil pouch with desiccant sachet and is placed into a carton.
- each carton contains one canister and Patient Information.
The BREZTRI AEROSPHERE canister should only be used with the BREZTRI AEROSPHERE actuator, and the BREZTRI AEROSPHERE actuator should not be used with any other inhalation drug product.
The correct amount of medication in each inhalation cannot be assured after the label number of inhalations from the canister have been used, when the dose indicator display window shows zero, even though the canister may not feel completely empty. BREZTRI AEROSPHERE should be discarded when the dose indicator display window shows zero or 3 months (for the 120-inhalation canister) or 3 weeks (for the 28-inhalation canister) after removal from the foil pouch, whichever comes first. Never immerse the canister into water to determine the amount remaining in the canister (“float test”).
Store at controlled room temperature 20°C to 25°C (68°F to 77°F); excursions permitted to 15°C to 30°C (59°F to 86°F) [see USP]. Keep in a dry place away from heat and sunlight.
For best results, the canister should be at room temperature before use. Shake well before using. Keep out of reach of children.
CONTENTS UNDER PRESSURE
Do not puncture. Do not use or store near heat or open flames. Exposure to temperatures above 120°F (49°C) may cause bursting. Never throw canister into fire or incinerator. Avoid spraying in eyes.
Manufactured for: AstraZeneca Pharmaceuticals LP, Wilmington, DE 19850. Manufactured by: AstraZeneca Dunkerque Production (AZDP), Dunkerque, France. Revised: Jul 2020
Side Effects
The following adverse reactions are discussed in greater detail in other sections of the labeling.
- Serious asthma-related events - hospitalizations, intubations, death [see WARNINGS AND PRECAUTIONS]
- Candida albicans infection [see WARNINGS AND PRECAUTIONS]
- Increased risk of pneumonia in COPD [see WARNINGS AND PRECAUTIONS]
- Immunosuppression and risk of infections [see WARNINGS AND PRECAUTIONS]
- Hypercorticism and adrenal suppression [see WARNINGS AND PRECAUTIONS]
- Paradoxical bronchospasm [see WARNINGS AND PRECAUTIONS]
- Hypersensitivity reactions including anaphylaxis [see CONTRAINDICATIONS and WARNINGS AND PRECAUTIONS]
- Cardiovascular effects [see WARNINGS AND PRECAUTIONS]
- Reduction in bone mineral density [see WARNINGS AND PRECAUTIONS]
- Worsening of narrow-angle glaucoma and cataracts [see WARNINGS AND PRECAUTIONS]
- Worsening of urinary retention [see WARNINGS AND PRECAUTIONS]
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared with rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of BREZTRI AEROSPHERE is based on the safety data from one 52-week exacerbation trial (Trial 1) and one 24-week lung function trial with a 28-week safety extension study, resulting in up to 52 weeks of treatment (Trial 2). In Trials 1 and 2, a total of 2783 subjects have received at least 1 dose of BREZTRI AEROSPHERE 320 mcg/18 mcg/9.6 mcg [see Clinical Studies].
In Trials 1 and 2, subjects received one of the following treatments: BREZTRI AEROSPHERE 320 mcg/18 mcg/9.6 mcg, glycopyrrolate and formoterol fumarate [GFF MDI 18 mcg/9.6 mcg], or budesonide and formoterol fumarate [BFF MDI 320 mcg/9.6 mcg]. Each treatment was administered twice daily.
In Trial 1, a 52-week, randomized, double-blind clinical trial, a total of 2144 subjects with COPD received at least 1 dose of BREZTRI AEROSPHERE 320 mcg/18 mcg/9.6 mcg (mean age: 64.7 years, 84.9% Caucasian, 59.7% male across all treatments) [see Clinical Studies].
In Trial 2, a 24-week, randomized, double-blind clinical trial, with a 28-week long-term safety extension resulting in up to 52 weeks of treatment, a total of 639 subjects received at least 1 dose of BREZTRI AEROSPHERE 320 mcg/18 mcg/9.6 mcg (mean age: 65.2 years, 50.1% Caucasian, 71.2% male across all treatments) [see Clinical Studies].
The incidence of adverse reactions from the 52-week trial (Trial 1) is presented in Table 1 for subjects treated with BREZTRI AEROSPHERE 320 mcg/18 mcg/9.6 mcg, GFF MDI 18 mcg/9.6 mcg, or BFF MDI 320 mcg/9.6 mcg.
Table 1: Adverse reactions occurring at an incidence of ≥ 2% of subjects and more common in BREZTRI AEROSPHERE compared to GFF MDI and BFF MDI (Trial 1)
| Adverse Reaction | BREZTRI AEROSPHERE1 320 mcg/18 mcg/9.6 mcg N=2144 (%) | GFF MDI1 18 mcg/9.6 mcg N=2125 (%) | BFF MDI1 320 mcg/9.6 mcg N=2136 (%) |
| Upper Respiratory Tract Infection | 123 (5.7) | 102 (4.8) | 115 (5.4) |
| Pneumonia | 98 (4.6) | 61 (2.9) | 107 (5.0) |
| Back pain | 67 (3.1) | 55 (2.6) | 64 (3.0) |
| Oral candidiasis | 65 (3.0) | 24 (1.1) | 57 (2.7) |
| Influenza | 63 (2.9) | 42 (2.0) | 61 (2.9) |
| Muscle spasms | 60 (2.8) | 19 (0.9) | 53 (2.5) |
| Urinary tract infection | 58 (2.7) | 60 (2.8) | 41 (1.9) |
| Cough | 58 (2.7) | 50 (2.4) | 51 (2.4) |
| Sinusitis | 56 (2.6) | 47 (2.2) | 55 (2.6) |
| Diarrhea | 44 (2.1) | 37 (1.7) | 38 (1.8) |
| 1 BREZTRI AEROSPHERE = budesonide/glycopyrrolate/formoterol fumarate 320 mcg/18 mcg/9.6 mcg; GFF MDI = glycopyrrolate/formoterol fumarate 18 mcg/9.6 mcg; BFF MDI = budesonide/formoterol fumarate 320 mcg/9.6 mcg; all treatments were administered twice daily. | |||
In 24-week data from Trial 2, adverse reactions that occurred in subjects treated with BREZTRI AEROSPHERE 320 mcg/18 mcg/9.6 mcg (n=639) at an incidence of ≥ 2% included dysphonia (3.3%) and muscle spasms (3.3%).
Additional Adverse Reactions
Other adverse reactions that have been associated with one or more of the individual components of BREZTRI AEROSPHERE include: hyperglycemia, anxiety, insomnia, headache, palpitations, nausea, hypersensitivity, depression, agitation, restlessness, nervousness, tremor, dizziness, angina pectoris, tachycardia, cardiac arrhythmias (e.g., atrial fibrillation, supraventricular tachycardia, and extrasystoles), throat irritation, bronchospasm, dry mouth, bruising, urinary retention, chest pain, sign or symptoms of systemic glucocorticoid steroid effects (e.g., hypofunctional adrenal gland), and abnormal behavior.
Drug Interactions
No formal drug interaction studies have been performed with BREZTRI AEROSPHERE.
Inhibitors Of Cytochrome P450 3A4
The main route of metabolism of corticosteroids, including budesonide, a component of BREZTRI AEROSPHERE, is via cytochrome P450 isoenzyme 3A4 (CYP3A4). After oral administration of ketoconazole, a strong inhibitor of CYP3A4, the mean plasma concentration of orally administered budesonide increased. Concomitant administration of a CYP3A4 inhibitor may inhibit the metabolism of, and increase the systemic exposure to, budesonide. Caution should be exercised when considering the coadministration of BREZTRI AEROSPHERE with long-term ketoconazole and other known strong CYP3A4 inhibitors (e.g., ritonavir, atazanavir, clarithromycin, indinavir, itraconazole, nefazodone, nelfinavir, saquinavir, telithromycin) [see WARNINGS AND PRECAUTIONS].
Adrenergic Drugs
If additional adrenergic drugs are to be administered by any route, they should be used with caution because the sympathetic effects of formoterol, a component of BREZTRI AEROSPHERE, may be potentiated [see WARNINGS AND PRECAUTIONS].
Xanthine Derivatives, Steroids, Or Diuretics
Concomitant treatment with xanthine derivatives, steroids, or diuretics may potentiate the hypokalemic effect of beta2-adrenergic agonists such as formoterol, a component of BREZTRI AEROSPHERE.
Non-Potassium Sparing Diuretics
The hypokalemia and/or ECG changes that may result from the administration of non-potassium sparing diuretics (such as loop or thiazide diuretics) can be acutely worsened by beta2-agonists, especially when the recommended dose of the beta2-agonist is exceeded.
Monoamine Oxidase Inhibitors, Tricyclic Antidepressants, QTc Prolonging Drugs
BREZTRI AEROSPHERE, as with other beta2-agonists, should be administered with extreme caution to patients being treated with monoamine oxidase inhibitors or tricyclic antidepressants or other drugs known to prolong the QTc interval because the action of adrenergic agonists on the cardiovascular system may be potentiated by these agents. Drugs that are known to prolong the QTc interval may be associated with an increased risk of ventricular arrhythmias.
Beta-adrenergic Receptor Blocking Agents
Beta-adrenergic receptor antagonists (beta-blockers) and BREZTRI AEROSPHERE may interfere with the effect of each other when administered concurrently. Beta-blockers not only block the therapeutic effects of beta2-agonists, but may produce severe bronchospasm in COPD patients. Therefore, patients with COPD should not normally be treated with beta-blockers. However, under certain circumstances, e.g., as prophylaxis after myocardial infarction, there may be no acceptable alternatives to the use of betablockers in patients with COPD. In this setting, cardioselective beta-blockers could be considered, although they should be administered with caution.
Anticholinergics
There is a potential for an additive interaction with concomitantly used anticholinergic medications. Therefore, avoid coadministration of BREZTRI AEROSPHERE with other anticholinergic-containing drugs as this may lead to an increase in anticholinergic adverse effects [see WARNINGS AND PRECAUTIONS and ADVERSE REACTIONS].
Warnings & Precautions
WARNINGS
Included as part of the PRECAUTIONS section.
PRECAUTIONS
Serious Asthma-Related Events – Hospitalizations, Intubations, Death
The safety and efficacy of BREZTRI AEROSPHERE in patients with asthma have not been established. BREZTRI AEROSPHERE is not indicated for the treatment of asthma.
Use of long-acting beta2-adrenergic agonists (LABA) as monotherapy [without inhaled corticosteroid (ICS)] for asthma is associated with an increased risk of asthma-related death. Available data from controlled clinical trials also suggest that use of LABA as monotherapy increases the risk of asthmarelated hospitalization in pediatric and adolescent patients. These findings are considered a class effect of LABA monotherapy. When a LABA is used in fixed-dose combination with ICS, data from large clinical trials do not show a significant increase in the risk of serious asthma-related events (hospitalizations, intubations, death) compared with ICS alone.
Available data do not suggest an increased risk of death with use of LABA in patients with COPD.
Deterioration Of Disease and Acute Episodes
BREZTRI AEROSPHERE should not be initiated in patients with acutely deteriorating COPD, which may be a life-threatening condition. BREZTRI AEROSPHERE has not been studied in patients with acutely deteriorating COPD. The use of BREZTRI AEROSPHERE in this setting is not appropriate.
BREZTRI AEROSPHERE should not be used for the relief of acute symptoms, i.e., as rescue therapy for the treatment of acute episodes of bronchospasm. BREZTRI AEROSPHERE has not been studied in the relief of acute symptoms and extra doses should not be used for that purpose. Acute symptoms should be treated with an inhaled short-acting beta2-agonist.
When beginning treatment with BREZTRI AEROSPHERE, patients who have been taking inhaled, shortacting beta2-agonists on a regular basis (e.g., four times a day) should be instructed to discontinue the regular use of these drugs and use them only for symptomatic relief of acute respiratory symptoms. When prescribing BREZTRI AEROSPHERE, the healthcare provider should also prescribe an inhaled, short acting beta2-agonist and instruct the patient on how it should be used. Increasing inhaled beta2-agonist use is a signal of deteriorating disease for which prompt medical attention is indicated.
COPD may deteriorate acutely over a period of hours or chronically over several days or longer. If BREZTRI AEROSPHERE no longer controls symptoms, or the patient’s inhaled, short-acting beta2- agonist becomes less effective or the patient needs more inhalations of short-acting beta2-agonist than usual, these may be markers of deterioration of disease. In this setting, re-evaluate the patient and the COPD treatment regimen at once. The daily dosage of BREZTRI AEROSPHERE should not be increased beyond the recommended dose.
Avoid Excessive Use Of BREZTRI AEROSPHERE And Avoid Use With Other Long-Acting Beta2-Agonists
As with other inhaled drugs containing beta2-adrenergic agents, BREZTRI AEROSPHERE should not be used more often than recommended, at higher doses than recommended, or in conjunction with other medications containing LABA, as an overdose may result. Clinically significant cardiovascular effects and fatalities have been reported in association with excessive use of inhaled sympathomimetic drugs. Patients using BREZTRI AEROSPHERE should not use another medicine containing a LABA (e.g., salmeterol, formoterol fumarate, arformoterol tartrate, indacaterol) for any reason [see DRUG INTERACTIONS].
Oropharyngeal Candidiasis
BREZTRI AEROSPHERE contains budesonide, an ICS. Localized infections of the mouth and pharynx with Candida albicans have occurred in subjects treated with orally inhaled drug products containing budesonide. When such an infection develops, it should be treated with appropriate local or systemic (i.e., oral) antifungal therapy while treatment with BREZTRI AEROSPHERE continues. In some cases, therapy with BREZTRI AEROSPHERE may need to be interrupted. Advise the patient to rinse his/her mouth with water without swallowing following administration of BREZTRI AEROSPHERE to help reduce the risk of oropharyngeal candidiasis.
Pneumonia
Lower respiratory tract infections, including pneumonia, have been reported following the inhaled administration of corticosteroids. Physicians should remain vigilant for the possible development of pneumonia in patients with COPD as the clinical features of pneumonia and exacerbations frequently overlap.
In a 52-week trial of subjects with COPD (n = 8,529), the incidence of confirmed pneumonia was 4.2% for BREZTRI AEROSPHERE 320 mcg/18 mcg/9.6 mcg (n = 2144), 3.5% for budesonide, glycopyrrolate and formoterol fumarate [BGF MDI 160 mcg/18 mcg/9.6 mcg] (n = 2124), 2.3% for GFF MDI 18 mcg/9.6 mcg (n = 2125) and 4.5% for BFF MDI 320 mcg/9.6 mcg (n = 2136).
Fatal cases of pneumonia occurred in 2 subjects receiving BGF MDI 160 mcg/18 mcg/9.6 mcg, 3 subjects receiving GFF MDI 18 mcg/9.6 mcg, and no subjects receiving BREZTRI AEROSPHERE 320 mcg/18 mcg/9.6 mcg.
In a 24-week trial of subjects with COPD (n = 1,896), the incidence of confirmed pneumonia was 1.9% for BREZTRI AEROSPHERE 320 mcg/18 mcg/9.6 mcg (n = 639), 1.6% for glycopyrrolate and formoterol fumarate [GFF MDI 18 mcg/9.6 mcg] (n = 625) and 1.9% for budesonide and formoterol fumarate [BFF MDI 320 mcg/9.6 mcg] (n = 320). There were no fatal cases of pneumonia in the study.
Immunosuppression And Risk Of Infections
Patients who are using drugs that suppress the immune system are more susceptible to infection than healthy individuals. Chicken pox and measles, for example, can have a more serious or even fatal course in susceptible children or adults using corticosteroids. In such children or adults who have not had these diseases or been properly immunized, particular care should be taken to avoid exposure. How the dose, route, and duration of corticosteroid administration affects the risk of developing a disseminated infection is not known. The contribution of the underlying disease and/or prior corticosteroid treatment to the risk is also not known. If a patient is exposed to chickenpox, prophylaxis with varicella zoster immune globulin (VZIG) may be indicated. If exposed to measles, prophylaxis with pooled intramuscular immunoglobulin (IG) may be indicated (see the respective package inserts for complete VZIG and IG prescribing information). If chicken pox develops, treatment with antiviral agents may be considered.
ICS should be used with caution, if at all, in patients with active or quiescent tuberculosis infections of the respiratory tract; untreated systemic fungal, bacterial, viral, or parasitic infections; or ocular herpes simplex.
Transferring Patients From Systemic Corticosteroid Therapy
HPA Suppression/Adrenal Insufficiency
Particular care is needed for patients who have been transferred from systemically active corticosteroids to ICS because deaths due to adrenal insufficiency have occurred in patients during and after transfer from systemic corticosteroids to less systemically available ICS. After withdrawal from systemic corticosteroids, a number of months are required for recovery of hypothalamic-pituitary-adrenal (HPA) function.
Patients who have been previously maintained on 20 mg or more per day of prednisone (or its equivalent) may be most susceptible, particularly when their systemic corticosteroids have been almost completely withdrawn. During this period of HPA suppression, patients may exhibit signs and symptoms of adrenal insufficiency when exposed to trauma, surgery, or infection (particularly gastroenteritis) or other conditions associated with severe electrolyte loss. Although BREZTRI AEROSPHERE may provide control of COPD symptoms during these episodes, in recommended doses it supplies less than normal physiological amounts of glucocorticoid systemically and does not provide the mineralocorticoid activity that is necessary for coping with these emergencies.
During periods of stress, or a severe COPD exacerbation, patients who have been withdrawn from systemic corticosteroids should be instructed to resume oral corticosteroids (in large doses) immediately and to contact their healthcare practitioner for further instruction. These patients should also be instructed to carry a warning card indicating that they may need supplementary systemic corticosteroids during periods of stress, or a severe COPD exacerbation.
Patients requiring oral corticosteroids should be weaned slowly from systemic corticosteroid use after transferring to BREZTRI AEROSPHERE. Prednisone reduction can be accomplished by reducing the daily prednisone dose by 2.5 mg on a weekly basis during therapy with BREZTRI AEROSPHERE. Lung function (forced expiratory volume in 1 second [FEV1] or morning peak expiratory flow [PEF]), betaagonist use, and COPD symptoms should be carefully monitored during withdrawal of oral corticosteroids. In addition, patients should be observed for signs and symptoms of adrenal insufficiency, such as fatigue, lassitude, weakness, nausea and vomiting, and hypotension.
Unmasking Of Allergic Conditions Previously Suppressed By Systemic Corticosteroids
Transfer of patients from systemic corticosteroid therapy to BREZTRI AEROSPHERE may unmask allergic conditions previously suppressed by the systemic corticosteroid therapy (e.g., rhinitis, conjunctivitis, eczema, arthritis, eosinophilic conditions).
Corticosteroid Withdrawal Symptoms
During withdrawal from oral corticosteroids, some patients may experience symptoms of systemically active corticosteroid withdrawal (e.g., joint and/or muscular pain, lassitude, depression) despite maintenance or even improvement of respiratory function.
Hypercorticism And Adrenal Suppression
Inhaled budesonide is absorbed into the circulation and can be systemically active. Effects of budesonide on the HPA axis are not observed with the therapeutic doses of budesonide in BREZTRI AEROSPHERE. However, exceeding the recommended dosage or coadministration with a strong cytochrome P450 3A4 (CYP3A4) inhibitor may result in HPA dysfunction [see WARNINGS AND PRECAUTIONS and DRUG INTERACTIONS].
Because of the possibility of significant systemic absorption of ICS, patients treated with BREZTRI AEROSPHERE should be observed carefully for any evidence of systemic corticosteroid effects. Particular care should be taken in observing patients postoperatively or during periods of stress for evidence of inadequate adrenal response.
It is possible that systemic corticosteroid effects, such as hypercorticism and adrenal suppression (including adrenal crisis) may appear in a small number of patients who are sensitive to these effects. If such effects occur, appropriate therapy should be initiated as needed.
Drug Interactions With Strong Cytochrome P450 3A4 Inhibitors
Caution should be exercised when considering the coadministration of BREZTRI AEROSPHERE with long-term ketoconazole, and other known strong CYP3A4 inhibitors (e.g., ritonavir, atazanavir, clarithromycin, indinavir, itraconazole, nefazodone, nelfinavir, saquinavir, telithromycin) because adverse effects related to increased systemic exposure to budesonide may occur [see DRUG INTERACTIONS and CLINICAL PHARMACOLOGY].
Paradoxical Bronchospasm
As with other inhaled therapies, BREZTRI AEROSPHERE can produce paradoxical bronchospasm, which may be life-threatening. If paradoxical bronchospasm occurs following dosing with BREZTRI AEROSPHERE, it should be treated immediately with an inhaled, short-acting bronchodilator; BREZTRI AEROSPHERE should be discontinued immediately and alternative therapy should be instituted.
Hypersensitivity Reactions Including Anaphylaxis
Immediate hypersensitivity reactions have been reported after administration of budesonide, glycopyrrolate or formoterol fumarate, the components of BREZTRI AEROSPHERE. If signs suggesting allergic reactions occur, in particular, angioedema (including difficulties in breathing or swallowing, swelling of tongue, lips, and face), urticaria, or skin rash, BREZTRI AEROSPHERE should be stopped at once and alternative treatment should be considered [see CONTRAINDICATIONS].
Cardiovascular Effects
Formoterol fumarate, like other beta2-agonists, can produce a clinically significant cardiovascular effect in some patients as measured by increases in pulse rate, systolic or diastolic blood pressure, and also cardiac arrhythmias, such as supraventricular tachycardia and extrasystoles [see CLINICAL PHARMACOLOGY].
If such effects occur, BREZTRI AEROSPHERE may need to be discontinued. In addition, beta-agonists have been reported to produce electrocardiographic changes, such as flattening of the T wave, prolongation of the QTc interval, and ST segment depression, although the clinical significance of these findings is unknown. Therefore, BREZTRI AEROSPHERE should be used with caution in patients with cardiovascular disorders, especially coronary insufficiency, cardiac arrhythmias, and hypertension.
Reduction In Bone Mineral Density
Decreases in bone mineral density (BMD) have been observed with long-term administration of products containing ICS. The clinical significance of small changes in BMD with regard to long-term consequences such as fracture is unknown. Patients with major risk factors for decreased bone mineral content, such as prolonged immobilization, family history of osteoporosis, postmenopausal status, tobacco use, advanced age, poor nutrition, or chronic use of drugs that can reduce bone mass (e.g., anticonvulsants, oral corticosteroids) should be monitored and treated with established standards of care. Since patients with COPD often have multiple risk factors for reduced BMD, assessment of BMD is recommended prior to initiating BREZTRI AEROSPHERE and periodically thereafter. If significant reductions in BMD are seen and BREZTRI AEROSPHERE is still considered medically important for that patient's COPD therapy, use of therapy to treat or prevent osteoporosis should be strongly considered.
In a subset of COPD patients in a 24-week trial with a 28-week safety extension that evaluated BREZTRI AEROSPHERE 320/18/9.6 mcg and GFF MDI 18/9.6 mcg, the effects on BMD endpoints were evaluated. BMD evaluations were performed at baseline and 52-weeks using dual energy x-ray absorptiometry (DEXA) scans. Mean percent changes in BMD from baseline was -0.1% for BREZTRI AEROSPHERE 320/18/9.6 mcg and 0.4% for GFF MDI 18/9.6 mcg [see Clinical Studies].
Glaucoma And Cataracts, Worsening Of Narrow-Angle Glaucoma
Glaucoma, increased intraocular pressure, and cataracts have been reported in patients with COPD following the long-term administration of ICS or with use of inhaled anticholinergics. BREZTRI AEROSPHERE should be used with caution in patients with narrow-angle glaucoma. Prescribers and patients should be alert for signs and symptoms of acute narrow-angle glaucoma (e.g., eye pain or discomfort, blurred vision, visual halos or colored images in association with red eyes from conjunctival congestion and corneal edema). Instruct patients to consult a physician immediately should any of these signs or symptoms develop. Consider referral to an ophthalmologist in patients who develop ocular symptoms or use BREZTRI AEROSPHERE long term.
In a 52-week trial that evaluated BREZTRI AEROSPHERE 320/18/9.6 mcg, GFF MDI 18/9.6 mcg, and BFF MDI 320/9.6 mcg in subjects with COPD, the incidence of cataracts ranged from 0.7% to 1.0% across groups.
Worsening Of Urinary Retention
BREZTRI AEROSPHERE, like all therapies containing an anticholinergic, should be used with caution in patients with urinary retention. Prescribers and patients should be alert for signs and symptoms of prostatic hyperplasia or bladder-neck obstruction (e.g., difficulty passing urine, painful urination), especially in patients with prostatic hyperplasia or bladder neck obstruction. Instruct patients to consult a physician immediately should any of these signs or symptoms develop.
Coexisting Conditions
BREZTRI AEROSPHERE, like all therapies containing sympathomimetic amines, should be used with caution in patients with convulsive disorders or thyrotoxicosis and in those who are unusually responsive to sympathomimetic amines. Doses of the related beta2-adrenoceptor agonist albuterol, when administered intravenously, have been reported to aggravate preexisting diabetes mellitus and ketoacidosis.
Hypokalemia And Hyperglycemia
Beta-adrenergic agonists may produce significant hypokalemia in some patients, possibly through intracellular shunting, which has the potential to produce adverse cardiovascular effects. The decrease in serum potassium is usually transient, not requiring supplementation. Beta2-agonist therapies may produce transient hyperglycemia in some patients.
Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (PATIENT INFORMATION and Instructions for Use).
Not For Treatment Of Acute Symptoms
Inform patients that BREZTRI AEROSPHERE is not meant to relieve acute symptoms of COPD and extra doses should not be used for that purpose. Advise patients to treat acute symptoms with an inhaled, short-acting beta2-agonist such as albuterol. Provide patients with such medication and instruct them in how it should be used.
Instruct patients to seek medical attention immediately if they experience any of the following:
- Decreasing effectiveness of inhaled, short-acting beta2-agonists
- Need for more inhalations than usual of inhaled, short-acting beta2-agonists
- Significant decrease in lung function as outlined by the health care practitioner
Tell patients they should not stop therapy with BREZTRI AEROSPHERE without physician guidance since symptoms may recur after discontinuation [see WARNINGS AND PRECAUTIONS].
Do Not Use Additional Long-acting Beta2-agonists Or Anticholinergics
Instruct patients not to use other LABA or anticholinergic medicines [see WARNINGS AND PRECAUTIONS].
Oropharyngeal Candidiasis
Inform patients that localized infections with Candida albicans occurred in the mouth and pharynx in some patients. If oropharyngeal candidiasis develops, it should be treated with appropriate local or systemic (i.e., oral) antifungal therapy while still continuing therapy with BREZTRI AEROSPHERE, but at times therapy with BREZTRI AEROSPHERE may need to be temporarily interrupted under close medical supervision. Advise patients to rinse the mouth with water without swallowing after inhalation to help reduce the risk of thrush [see WARNINGS AND PRECAUTIONS].
Pneumonia
Patients with COPD have a higher risk of pneumonia; instruct them to contact their healthcare providers if they develop symptoms of pneumonia [see WARNINGS AND PRECAUTIONS].
Immunosuppression And Risk Of Infections
Warn patients who are on immunosuppressant doses of corticosteroids to avoid exposure to chickenpox or measles and, if exposed, to consult their physicians without delay. Inform patients of potential worsening of existing tuberculosis, fungal, bacterial, viral, or parasitic infections, or ocular herpes simplex [see WARNINGS AND PRECAUTIONS].
Hypercorticism And Adrenal Suppression
Advise patients that BREZTRI AEROSPHERE may cause systemic corticosteroid effects of hypercorticism and adrenal suppression. Additionally, inform patients that deaths due to adrenal insufficiency have occurred during and after transfer from systemic corticosteroids. Patients should taper slowly from systemic corticosteroids if transferring to BREZTRI AEROSPHERE [see WARNINGS AND PRECAUTIONS].
Paradoxical Bronchospasm
As with other inhaled medicines, BREZTRI AEROSPHERE can cause paradoxical bronchospasm. If paradoxical bronchospasm occurs, instruct patients to discontinue BREZTRI AEROSPHERE and contact their healthcare provider right away [see WARNINGS AND PRECAUTIONS].
Hypersensitivity Reactions, Including Anaphylaxis
Advise patients that hypersensitivity reactions (e.g., anaphylaxis, angioedema, rash, urticaria) may occur after administration of BREZTRI AEROSPHERE. Instruct patients to discontinue BREZTRI AEROSPHERE if such reactions occur [see WARNINGS AND PRECAUTIONS].
Reduction In Bone Mineral Density
Advise patients who are at an increased risk for decreased BMD that the use of corticosteroids may pose an additional risk [see WARNINGS AND PRECAUTIONS].
Ocular Effects Such As Cataracts Or Glaucoma
Inform patients that long-term use of ICS may increase the risk of some eye problems (cataracts or glaucoma); consider regular eye examinations.
Instruct patients to be alert for signs and symptoms of acute narrow-angle glaucoma (e.g., eye pain or discomfort, blurred vision, visual halos or colored images in association with red eyes from conjunctival congestion, and corneal edema). Instruct patients to consult a physician immediately if any of these signs or symptoms develops [see WARNINGS AND PRECAUTIONS].
Worsening Of Urinary Retention
Instruct patients to be alert for signs and symptoms of urinary retention (e.g., difficulty passing urine, painful urination). Instruct patients to consult a physician immediately if any of these signs or symptoms develop [see WARNINGS AND PRECAUTIONS].
Risks Associated With Beta-agonist Therapy
Inform patients of adverse effects associated with beta2-agonists, such as palpitations, chest pain, rapid heart rate, tremor, or nervousness. Instruct patients to consult a healthcare practitioner immediately should any of these signs or symptoms develop [see WARNINGS AND PRECAUTIONS].
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment Of Fertility
No studies of carcinogenicity, mutagenicity, or impairment of fertility were conducted with BREZTRI AEROSPHERE; however, separate studies of budesonide, glycopyrrolate, and formoterol fumarate are described below.
Budesonide
Long-term studies were conducted in rats and mice using oral administration to evaluate the carcinogenic potential of budesonide.
In a 2-year study in Sprague-Dawley rats, budesonide caused a statistically significant increase in the incidence of gliomas in male rats at an oral dose of 50 mcg/kg (approximately equivalent to the MRHDID on a mcg/m² basis). No tumorigenicity was seen in male and female rats at respective oral doses up to 25 and 50 mcg/kg (approximately equivalent to the MRHDID on a mcg/m² basis). In two additional 2-year studies in male Fischer and Sprague-Dawley rats, budesonide caused no gliomas at an oral dose of 50 mcg/kg (approximately equivalent to the MRHDID on a mcg/m² basis). However, in the male Sprague- Dawley rats, budesonide caused a statistically significant increase in the incidence of hepatocellular tumors at an oral dose of 50 mcg/kg (approximately equivalent to the MRHDID on a mcg/m² basis). The concurrent reference corticosteroids (prednisolone and triamcinolone acetonide) in these two studies showed similar findings.
In a 91-week carcinogenicity study in mice, budesonide produced no treatment-related increases in the incidence of tumors at oral doses up to 200 mcg/kg (approximately 2 times the MRHDID on a mcg/m² basis).
Budesonide was not mutagenic or clastogenic in the Ames Salmonella/microsome plate test, mouse micronucleus test, mouse lymphoma test, chromosome aberration test in human lymphocytes, sex-linked recessive lethal test in Drosophila melanogaster, and DNA repair analysis in rat hepatocyte culture.
Fertility and reproductive performance were unaffected in rats at subcutaneous doses up to 80 mcg/kg (approximately equal to the MRHDID on a mcg/m² basis). However, it caused a decrease in prenatal viability and viability in the pups at birth and during lactation, along with a decrease in maternal body24 weight gain, at subcutaneous doses of 20 mcg/kg and above (0.3 times the MRHDID on a mcg/m² basis). No such effects were noted at 5 mcg/kg (0.08 times the MRHDID on a mcg/m² basis).
Glycopyrrolate
Long-term studies were conducted in mice using inhalation administration and rats using oral administration to evaluate the carcinogenic potential of glycopyrrolate.
In a 24-month inhalation carcinogenicity study in B6C3F1 mice, glycopyrrolate produced no evidence of tumorigenicity when administered to males or females at doses up to 705 and 335 mcg/kg/day, respectively (approximately 95 and 45 times the MRHDID of glycopyrrolate on a mcg/m² basis, respectively).
In a 24-month carcinogenicity study in rats, glycopyrrolate produced no evidence of tumorigenicity when administered to males or females by oral gavage at dosages up to 40,000 mcg/kg/day (approximately 11,000 times the MRHDID of glycopyrrolate on a mcg/m² basis).
Glycopyrrolate was not mutagenic or clastogenic in the Ames Salmonella/microsome plate test, in vitro mammalian cell micronucleus assay in TK6 cells, or in vivo micronucleus assay in rats.
Fertility and reproductive performance indices were unaffected in male and female rats that received glycopyrrolate by the subcutaneous route at doses up to 10,000 μg/kg/day (approximately 2700 times the MRHDID on a mcg/m² basis).
Formoterol Fumarate
Long-term studies were conducted in mice using oral administration and rats using inhalation administration to evaluate the carcinogenic potential of formoterol fumarate.
In a 24-month carcinogenicity study in CD-1 mice, formoterol fumarate at oral doses of 100 mcg/kg and above (approximately 25 times MRHDID on a mcg/m² basis) caused a dose-related increase in the incidence of uterine leiomyomas.
In a 24-month carcinogenicity study in Sprague-Dawley rats, an increased incidence of mesovarian leiomyoma and uterine leiomyosarcoma were observed at the inhaled dose of 130 mcg/kg (approximately 65 times the MRHDID on a mcg/m² basis). No tumors were seen at 22 mcg/kg (approximately 10 times the MRHDID on a mcg/m² basis).
Other beta-agonist drugs have similarly demonstrated increases in leiomyomas of the genital tract in female rodents. The relevance of these findings to human use is unknown.
Formoterol fumarate was not mutagenic or clastogenic in Ames Salmonella/microsome plate test, mouse lymphoma test, chromosome aberration test in human lymphocytes, or rat micronucleus test.
A reduction in fertility and/or reproductive performance was identified in male rats treated with formoterol at an oral dose of 15,000 mcg/kg, (approximately 2600 times the MRHDID on an AUC basis). No such effect was seen at 3,000 mcg/kg (approximately 1500 times the MRHDID on a mcg/m² basis). In a separate study with male rats treated with an oral dose of 15,000 mcg/kg (approximately 8000 times the MRHDID on a mcg/m² basis), there were findings of testicular tubular atrophy and spermatic debris in the testes and oligospermia in the epididymides. No effect on fertility was detected in female rats at doses up to 15,000 mcg/kg (approximately 1400 times the MRHDID on an AUC basis).
Use In Specific Populations
Pregnancy
Risk Summary
There are no adequate and well-controlled studies with BREZTRI AEROSPHERE or with two of its individual components, glycopyrrolate or formoterol fumarate, in pregnant women to inform a drugassociated risk; however, studies are available for the other component, budesonide.
In animal reproduction studies, budesonide alone, administered by the subcutaneous route, caused structural abnormalities, was embryocidal, and reduced fetal weights in rats and rabbits at 0.3 and 0.75 times maximum recommended human daily inhaled dose (MRHDID), respectively, but these effects were not seen in rats that received inhaled doses up to 4 times the MRHDID. Studies of pregnant women who received inhaled budesonide alone during pregnancy have not shown increased risk of abnormalities. Experience with oral corticosteroids suggests that rodents are more prone to teratogenic effects from corticosteroid exposure than humans.
Formoterol fumarate alone, administered by the oral route in rats and rabbits, caused structural abnormalities at 1500 and 61,000 times the MRHDID, respectively. Formoterol fumarate was also embryocidal, increased pup loss at birth and during lactation, and decreased pup weight in rats at 110 times the MRHDID. These adverse effects generally occurred at large multiples of the MRHDID when formoterol fumarate was administered by the oral route to achieve high systemic exposures. No structural abnormalities, embryocidal, or developmental effects were seen in rats that received inhalation doses up to 350 times the MRHDID.
Glycopyrrolate alone, administered by the subcutaneous route in rats and rabbits, did not cause structural abnormalities or affect fetal survival at exposures approximately 2700 and 5400 times from MRHDID, respectively. Glycopyrrolate had no effects on the physical, functional, and behavioral development of rat pups with exposures up to 2700 times the MRHDID.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Clinical Considerations
Labor Or Delivery
There are no well-controlled human trials that have investigated the effects of BREZTRI AEROSPHERE on preterm labor or labor at term. Because of the potential for beta-agonist interference with uterine contractility, use of BREZTRI AEROSPHERE during labor should be restricted to those patients in whom the benefits clearly outweigh the risks.
Data
Human Data
Studies of pregnant women have not shown that inhaled budesonide increases the risk of abnormalities when administered during pregnancy. The results from a large population-based prospective cohort epidemiological study reviewing data from three Swedish registries covering approximately 99% of the pregnancies from 1995-1997 (i.e., Swedish Medical Birth Registry; Registry of Congenital Malformations; Child Cardiology Registry) indicate no increased risk for congenital malformations from the use of inhaled budesonide during early pregnancy. Congenital malformations were studied in 2014 infants born to mothers reporting the use of inhaled budesonide for asthma in early pregnancy (usually 10-12 weeks after the last menstrual period), the period when most major organ malformations occur. The rate of recorded congenital malformations was similar compared to the general population rate (3.8% vs. 3.5%, respectively). In addition, after exposure to inhaled budesonide, the number of infants born with orofacial clefts was similar to the expected number in the normal population (4 children vs. 3.3, respectively).
These same data were utilized in a second study bringing the total to 2,534 infants whose mothers were exposed to inhaled budesonide. In this study, the rate of congenital malformations among infants whose mothers were exposed to inhaled budesonide during early pregnancy was not different from the rate for all newborn babies during the same period (3.6%).
Animal Data
Budesonide
In a fertility and reproduction study male rats were subcutaneously dosed for 9 weeks and females for 2 weeks prior to pairing and throughout the mating period. Females were dosed up until weaning of their offspring. Budesonide caused a decrease in prenatal viability and viability of the offspring at birth and during lactation, along with a decrease in maternal body weight gain, at a dose 0.3 times the MRHDID (on a mcg/m² basis at maternal subcutaneous doses of 20 mcg/kg/day and above). No such effects were noted at a dose 0.08 times the MRHDID (on a mcg/m² basis at a maternal subcutaneous dose of 5 mcg/kg/day).
In an embryo-fetal development study in pregnant rabbits dosed during the period of organogenesis from gestation days 6 to 18, budesonide produced fetal loss, decreased fetal weight, and skeletal abnormalities at a dose 0.75 times the MRHDID (on a mcg/m² basis at a maternal subcutaneous dose of 25 mcg/kg/day). In an embryo-fetal development study in pregnant rats dosed during the period of organogenesis from gestation days 6-15, budesonide produced similar adverse fetal effects at doses approximately 8 times the MRHDID (on a mcg/m² basis at a maternal subcutaneous dose of 500 mcg/kg/day). In another embryo-fetal development study in pregnant rats, no structural abnormalities or embryocidal effects were seen at doses up to 4 times the MRHDID (on a mcg/m² basis at maternal inhalation doses up to 250 mcg/kg/day).
In a peri-and post-natal development study, rats dosed from gestation day 15 to postpartum day 21, budesonide had no effects on delivery, but did affect growth and development of offspring. Offspring survival was reduced, and surviving offspring had decreased mean body weights at birth and during lactation at doses 0.3 times the MRHDID and higher (on a mcg/m² basis at maternal subcutaneous doses of 20 mcg/kg/day and higher). These findings occurred in the presence of maternal toxicity.
Formoterol Fumarate
In a fertility and reproduction study, male rats were orally dosed for at least 9 weeks and females for 2 weeks prior to pairing and throughout the mating period. Females were either dosed up to gestation day 19 or up until weaning of their offspring. Males were dosed up to 25 weeks. Umbilical hernia was observed in rat fetuses at oral doses 1500 times the MRHDID (on a mcg/m² basis at maternal oral doses of 3000 mcg/kg/day and higher). Brachygnathia was observed in rat fetuses at a dose 8000 times the MRHDID (on a mcg/m² basis at a maternal oral dose of 15,000 mcg/kg/day). Pregnancy was prolonged at a dose 8000 times the MRHDID (on a mcg/m² basis at a maternal oral dose of 15,000 mcg/kg/day). Fetal and pup deaths occurred at doses approximately 1500 times the MRHDID and higher (on a mcg/m² basis at oral doses of 3000 mcg/kg/day and higher) during gestation.
In an embryo-fetal development study in pregnant rats dosed during the period of organogenesis from gestation days 6 to 15, no structural abnormalities, embryocidal effects, or developmental effects were seen at doses up to 350 times the MRHDID (on a mcg/m² basis with maternal inhalation doses up to 690 mcg/kg/day).
In an embryo-fetal development study in pregnant rabbits dosed during the period of organogenesis from gestation days 6 to 18, subcapsular cysts on the liver were observed in the fetuses at a dose 61,000 times the MRHDID (on a mcg/m² basis with a maternal oral dose of 60,000 mcg/kg/day). No teratogenic effects were observed at doses up to 3500 times the MRHDID (on a mcg/m² basis at maternal oral doses up to 3500 mcg/kg/day).
In a pre- and post-natal development study, pregnant female rats received formoterol at oral doses of 0, 210, 840, and 3400 mcg/kg/day from gestation day 6 (completion of implantation) through the lactation period. Pup survival was decreased from birth to postpartum day 26 at doses 110 times the MRHDID and higher (on a mcg/m² basis at maternal oral doses of 210 mcg/kg/day and higher), although there was no evidence of a dose-response relationship. There were no treatment-related effects on the physical, functional, and behavioral development of rat pups.
Glycopyrrolate
In an embryo-fetal development study in pregnant rats dosed during the period of organogenesis from gestation days 6 to 17, glycopyrrolate produced no structural abnormalities or effects on fetal survival; however, slight reductions of fetal body weight in the presence of maternal toxicity at the highest tested dose that was 2700 times the MRHDID (on a mcg/m² basis at a maternal subcutaneous dose of 10,000 mcg/kg/day). Fetal body weights were unaffected with doses up to 270 times the MRHDID (on a mcg/m² basis with maternal subcutaneous doses up to 1000 mcg/kg/day). Maternal toxicity was observed with doses 270 times the MRHDID and higher (on a mcg/m² basis with maternal subcutaneous doses of 1000 mcg/kg/day and higher).
In an embryo-fetal development study in pregnant rabbits dosed during the period of organogenesis from gestation days 6 to 18, glycopyrrolate produced no structural abnormalities or effects on fetal survival; however, slight reductions of fetal body weight in the presence of maternal toxicity at the highest tested dose that was 5400 times the MRHDID (on a mcg/m² basis at a maternal subcutaneous dose of 10,000 mcg/kg/day). Fetal body weights were unaffected with doses up to 540 times the MRHDID (on a mcg/m² basis with maternal subcutaneous doses up to 1000 mcg/kg/day). Maternal toxicity was observed with doses 540 times the MRHDID and higher (on a mcg/m² basis with maternal subcutaneous doses of 1000 mcg/kg/day and higher).
In a pre- and post-natal development study, pregnant female rats received glycopyrrolate at doses of 100, 1000, and 10,000 mcg/kg/day from gestation day 6 through the lactation period. Pup body weight gain was slightly reduced from birth through the lactation period at a dose 2700 times the MRHDID (on a mcg/m² basis with a maternal subcutaneous dose of 10,000 mcg/kg/day); however, pup body weight gain was unaffected after weaning. There were no treatment-related effects on the physical, functional, and behavioral development of pups with doses up to 2700 times the MRHDID (on a mcg/m² basis with maternal subcutaneous doses up to 10,000 mcg/kg/day). Maternal toxicity was observed from gestation days 6 to 18 with doses 270 times the MRHDID and higher (on a mcg/m² basis with maternal subcutaneous doses of 1000 mcg/kg/day and higher).
Lactation
Risk Summary
There are no available data on the effects of BREZTRI AEROSPHERE, budesonide, glycopyrrolate, or formoterol fumarate on the breastfed child or on milk production. Budesonide, like other ICS, is present in human milk [see Data]. There are no available data on the presence of glycopyrrolate or formoterol fumarate in human milk. Formoterol fumarate and glycopyrrolate have been detected in the plasma of undosed rat pups suckling from exposed dams [see Data]. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for BREZTRI AEROSPHERE and any potential adverse effects on the breast-fed child from BREZTRI AEROSPHERE or from the underlying maternal condition.
Data
Human Data
Human data with budesonide delivered via dry powder inhaler indicates that the total daily oral dose of budesonide available in breast milk to the infant is approximately 0.3% to 1% of the dose inhaled by the mother. For BREZTRI AEROSPHERE, the dose of budesonide available to the infant in breast milk, as a percentage of the maternal dose, would be expected to be similar.
There is no available human data for formoterol or glycopyrrolate.
Animal Data
In the fertility and reproduction study in rats, plasma levels of formoterol were measured in pups on postnatal day 15 [see Use In Specific Populations]. It was estimated that the maximum plasma concentration that the pups received from the maternal animal, at the highest dose of 15 mg/kg, after nursing was 4.4% (0.24 nmol/L for a litter vs. 5.5 nmol/L for the mother).
In the reproductive/developmental toxicity study in rats, plasma levels of glycopyrrolate were measured in pups on post-natal day 4. The maximum concentration in the pups was 6% of the maternal dose of 10 mg/kg/day (pup plasma concentration of 96 ng/mL at 1 hour after dosing corresponded with 1610 ng/Ml in the dam at 0.5 hours after dosing).
Pediatric Use
BREZTRI AEROSPHERE is not indicated for use in children. The safety and effectiveness of BREZTRI AEROSPHERE have not been established in pediatric patients.
Geriatric Use
Based on available data, no adjustment of the dosage of BREZTRI AEROSPHERE in geriatric patients is necessary, but greater sensitivity in some older individuals cannot be ruled out.
In Trials 1 and 2, 1100 subjects and 343 subjects, respectively, aged 65 years and older were administered BREZTRI AEROSPHERE 320 mcg/18 mcg/9.6 mcg twice daily. In both trials, no overall differences in safety or effectiveness were observed between these subjects and younger subjects.
Hepatic Impairment
Formal pharmacokinetic studies using BREZTRI AEROSPHERE have not been conducted in patients with hepatic impairment. However, since budesonide and formoterol fumarate are predominantly cleared by hepatic metabolism, impairment of liver function may lead to accumulation of budesonide and formoterol fumarate in plasma. Therefore, patients with severe hepatic disease should be closely monitored.
Renal Impairment
Formal pharmacokinetic studies using BREZTRI AEROSPHERE have not been conducted in patients with renal impairment. In patients with severe renal impairment (creatinine clearance of ≤30 mL/min/1.73 m²) or end-stage renal disease requiring dialysis, BREZTRI AEROSPHERE should only be used if the expected benefit outweighs the potential risk [see CLINICAL PHARMACOLOGY].
Overdosage & Contraindications
OVERDOSE
No cases of overdose have been reported with BREZTRI AEROSPHERE. BREZTRI AEROSPHERE contains budesonide, glycopyrrolate, and formoterol fumarate; therefore, the risks associated with overdosage for the individual components described below apply to BREZTRI AEROSPHERE. Treatment of overdosage consists of discontinuation of BREZTRI AEROSPHERE together with institution of appropriate symptomatic and/or supportive therapy. The judicious use of a cardioselective beta-receptor blocker may be considered, bearing in mind that such medication can produce bronchospasm. Cardiac monitoring is recommended in case of overdosage.
Budesonide
If used at excessive doses for prolonged periods, systemic corticosteroid effects, such as hypercorticism may occur [see WARNINGS AND PRECAUTIONS].
Glycopyrrolate
High doses of glycopyrrolate, a component of BREZTRI AEROSPHERE, may lead to anticholinergic signs and symptoms such as nausea, vomiting, dizziness, lightheadedness, blurred vision, increased intraocular pressure (causing pain, vision disturbances or reddening of the eye), obstipation, or difficulties in voiding.
Formoterol Fumarate
An overdose of formoterol fumarate would likely lead to an exaggeration of effects that are typical for beta2-agonists: seizures, angina, hypertension, hypotension, tachycardia, atrial and ventricular tachyarrhythmias, nervousness, headache, tremor, palpitations, muscle cramps, nausea, dizziness, sleep disturbances, metabolic acidosis, hyperglycemia, hypokalemia. As with all sympathomimetic medications, cardiac arrest, and even death may be associated with overdosage of formoterol fumarate.
CONTRAINDICATIONS
BREZTRI AEROSPHERE is contraindicated in patients who have demonstrated hypersensitivity to budesonide, glycopyrrolate, formoterol, or any of the excipients [see WARNINGS AND PRECAUTIONS and DESCRIPTION].
Clinical Pharmacology
Mechanism Of Action
BREZTRI AEROSPHERE
BREZTRI AEROSPHERE contains budesonide, glycopyrrolate, and formoterol fumarate. The mechanism of action described below for the individual components applies to BREZTRI AEROSPHERE. These drugs represent three different classes of medications (a synthetic corticosteroid, an anticholinergic, and a long-acting selective beta2-adrenoceptor agonist) that have different effects on clinical physiology and inflammatory indices of COPD.
Budesonide
Budesonide is an anti-inflammatory corticosteroid that exhibits potent glucocorticoid activity and weak mineralocorticoid activity. In standard in vitro and animal models, budesonide has approximately a 200- fold higher affinity for the glucocorticoid receptor and a 1000-fold higher topical anti-inflammatory potency than cortisol (rat croton oil ear edema assay). As a measure of systemic activity, budesonide is 40 times more potent than cortisol when administered subcutaneously and 25 times more potent when administered orally in the rat thymus involution assay.
In glucocorticoid receptor affinity studies, the 22R epimer of budesonide was two times as active as the 22S epimer. In vitro studies indicated that the two forms of budesonide do not interconvert.
Inflammation is an important component in the pathogenesis of COPD. Corticosteroids have a wide range of inhibitory activities against multiple cell types (e.g., mast cells, eosinophils, neutrophils, macrophages, and lymphocytes) and mediators (e.g., histamine, eicosanoids, leukotrienes, and cytokines) involved in allergic and non-allergic-mediated inflammation. These anti-inflammatory actions of corticosteroids may contribute to their efficacy.
Glycopyrrolate
Glycopyrrolate is a long-acting antimuscarinic agent which is often referred to as an anticholinergic. It has similar affinity to the subtypes of muscarinic receptors M1 to M5. In the airways, it exhibits pharmacological effects through inhibition of the M3 receptor at the smooth muscle leading to bronchodilation. The competitive and reversible nature of antagonism was shown with human and animal origin receptors and isolated organ preparations. In preclinical in vitro as well as in vivo studies, prevention of methylcholine and acetylcholine-induced bronchoconstrictive effects was dose-dependent and lasted more than 12 hours. The clinical relevance of these findings is unknown. The bronchodilation following inhalation of glycopyrrolate is predominantly a site-specific effect.
Formoterol Fumarate
Formoterol fumarate is a long-acting selective beta2-adrenergic agonist (beta2-agonist) with a rapid onset of action. Inhaled formoterol fumarate acts locally in the lung as a bronchodilator. In vitro studies have shown that formoterol has more than 200-fold greater agonist activity at beta2-receptors than at beta1- receptors. The in vitro binding selectivity to beta2- over beta1-adrenoceptors is higher for formoterol than for albuterol (5 times), whereas salmeterol has a higher (3 times) beta2-selectivity ratio than formoterol.
Although beta2-receptors are the predominant adrenergic receptors in bronchial smooth muscle and beta1- receptors are the predominant receptors in the heart, there are also beta2-receptors in the human heart comprising 10% to 50% of the total beta-adrenergic receptors. The precise function of these receptors has not been established, but they raise the possibility that even highly selective beta2-agonists may have cardiac effects.
The pharmacologic effects of beta2-adrenoceptor agonist drugs, including formoterol fumarate, are at least in part attributable to stimulation of intracellular adenyl cyclase, the enzyme that catalyzes the conversion of adenosine triphosphate (ATP) to cyclic-3',5'-adenosine monophosphate (cyclic AMP). Increased cyclic AMP levels cause relaxation of bronchial smooth muscle and inhibition of release of mediators of immediate hypersensitivity from cells, especially from mast cells.
Pharmacodynamics
Cardiac Electrophysiology
A TQT study was not performed with BREZTRI AEROSPHERE as budesonide is not known to affect the QT interval. However, the potential for QTc interval prolongation with glycopyrrolate/formoterol fumarate was assessed in a double-blind, single-dose, placebo- and positive-controlled crossover trial in 69 healthy subjects. The largest mean (90% upper confidence bound) differences from placebo in baseline-corrected QTcI for 2 inhalations of glycopyrrolate/formoterol fumarate 9/4.8 mcg and glycopyrrolate/formoterol fumarate 72/19.2 mcg, were 3.1 (4.7) ms and 7.6 (9.2) ms, respectively, and excluded the clinically relevant threshold of 10 ms. A dose-dependent increase in heart rate was also observed. The largest mean (90% upper confidence bound) differences from placebo in baseline-corrected heart rate were 3.3 (4.9) beats/min and 7.6 (9.5) beats/min seen within 10 minutes of dosing with 2 inhalations of glycopyrrolate/formoterol fumarate 9/4.8 mcg and glycopyrrolate/formoterol fumarate 72/19.2 mcg, respectively.
Chronic Obstructive Pulmonary Disease
The effects of BREZTRI AEROSPHERE on cardiac rhythm in subjects with COPD was assessed using 24-hour Holter monitoring at Week 16 in a 52-week trial (Trial 1).
The Holter monitoring population in Trial 1 included 180 subjects on BREZTRI AEROSPHERE 320 mcg/18 mcg/9.6 mcg, 160 subjects on glycopyrrolate and formoterol fumarate [GFF MDI 18 mcg/9.6 mcg], and 183 subjects on budesonide/formoterol fumarate [BFF MDI 320 mcg/9.6 mcg]. No clinically meaningful effects on cardiac rhythm were observed.
HPA Axis Effects
Effects of BREZTRI AEROSPHERE on the HPA axis were assessed by measurement of 24-hour serum cortisol at Baseline and Week 24 in subjects with COPD. The geometric mean ratio (Week 24/Baseline) was 0.86 (Co-efficient of variation (CV) =39%) and 0.94 (CV=36.6%) for BREZTRI AEROSPHERE 320 mcg/18mcg/9.6mcg and GFF MDI 18 mcg/9.6 mcg, respectively.
Pharmacokinetics
Linear pharmacokinetics were demonstrated for budesonide (80 to 320 mcg), glycopyrrolate (18 to 144 mcg), and formoterol fumarate (2.4 to 38.4 mcg). Pharmacokinetic information for glycopyrrolate and formoterol fumarate is for the active moieties, glycopyrronium and formoterol, respectively. The pharmacokinetics of budesonide, glycopyrronium, and formoterol from BREZTRI AEROSPHERE are comparable to the pharmacokinetics of budesonide, glycopyrronium, and formoterol when administered as budesonide/formoterol or glycopyrrolate/formoterol in studies of healthy subjects (single dose) and subjects with COPD (repeated dose).
The pharmacokinetics of the individual components of BREZTRI AEROSPHERE are presented below.
Absorption
Budesonide
Following inhaled administration of BREZTRI AEROSPHERE in subjects with COPD, Cmax occurred within 20 to 40 minutes. Steady state is estimated to be achieved after approximately 1 day of repeated dosing of BREZTRI AEROSPHERE via population pharmacokinetic analysis and the AUC0-12 is approximately 1.3 times higher than after the first dose.
Glycopyrrolate
Following inhaled administration of BREZTRI AEROSPHERE in subjects with COPD, Cmax occurred within 2 to 6 minutes. Steady state is estimated to be achieved after approximately 3 days of repeated dosing of BREZTRI AEROSPHERE via population pharmacokinetic analysis and the AUC0-12 is approximately 1.8 times higher than after the first dose.
Formoterol Fumarate
Following inhaled administration of BREZTRI AEROSPHERE in subjects with COPD, Cmax occurred within 20 to 60 minutes. Steady state is estimated to be achieved after approximately 2 days of repeated dosing with BREZTRI AEROSPHERE via population pharmacokinetic analysis and the AUC0-12 is approximately 1.4 times higher than after the first dose.
Distribution
Budesonide
The estimated budesonide apparent volume of distribution at steady-state in subjects with COPD is approximately 1200 L, via population pharmacokinetic analysis. Over the concentration range of 1-100 nmol/L, mean plasma protein binding of budesonide ranged from 86% to 87%.
Glycopyrrolate
The estimated glycopyrronium apparent volume of distribution at steady-state in subjects with COPD is approximately 5500 L, via population pharmacokinetic analysis. Over the concentration range of 2-500 nmol/L, plasma protein binding of glycopyrronium ranged from 43% to 54%.
Formoterol Fumarate
The estimated formoterol apparent volume of distribution at steady-state in subjects with COPD is approximately 2400 L, via population pharmacokinetic analysis. Over the concentration range of 10-500 nmol/L, plasma protein binding of formoterol ranged from 46% to 58%.
Elimination
Budesonide
Budesonide was excreted in urine and feces in the form of metabolites. Only negligible amounts of unchanged budesonide have been detected in the urine. The effective half-life of budesonide in subjects with COPD derived via population pharmacokinetic analysis was approximately 5 hours.
Glycopyrrolate
After IV administration of a 0.2 mg radiolabeled glycopyrronium, 85% of dose recovered was recovered in urine 48 hours post-dose and some of radioactivity was also recovered in bile. The effective half-life of glycopyrronium in subjects with COPD derived via population pharmacokinetics analysis was approximately 15 hours.
Formoterol Fumarate
The excretion of formoterol was studied in six healthy subjects following simultaneous administration of radiolabeled formoterol via the oral and IV routes. In that study, 62% of the drug related radioactivity of formoterol was excreted in the urine while 24% was eliminated in the feces. The effective half-life of formoterol in subjects with COPD derived via population pharmacokinetics analysis was approximately 10 hours.
Metabolism
Budesonide
In vitro studies with human liver homogenates have shown that budesonide was rapidly and extensively metabolized. Two major metabolites formed via CYP3A4 catalyzed biotransformation have been isolated and identified as 16α-hydroxyprednisolone and 6ß-hydroxybudesonide. The corticosteroid activity of each of these two metabolites was less than 1% of that of the parent compound. No qualitative differences between the in vitro and in vivo metabolic patterns were detected. Negligible metabolic inactivation was observed in human lung and serum preparations.
Glycopyrrolate
Based on information from the published literature, and an in vitro human hepatocyte study, metabolism plays a minor role in the overall elimination of glycopyrronium. CYP2D6 was found to be the predominant enzyme involved in the metabolism of glycopyrronium.
Formoterol Fumarate
The primary metabolism of formoterol is by direct glucuronidation and by Odemethylation followed by conjugation to inactive metabolites. Secondary metabolic pathways include deformylation and sulfate conjugation. CYP2D6 and CYP2C have been identified as being primarily responsible for O-demethylation.
Specific Populations
Population pharmacokinetic analysis showed no evidence of a clinically significant effect of age, sex, race/ethnicity, or body weight on the pharmacokinetics of budesonide, glycopyrronium, or formoterol.
Patients With Hepatic Impairment
Dedicated studies of BREZTRI AEROSPHERE evaluating effect of hepatic impairment on the pharmacokinetics of budesonide, glycopyrronium, and formoterol were not conducted.
Reduced liver function may affect the elimination of corticosteroids. Budesonide pharmacokinetics was affected by compromised liver function as evidenced by a doubled systemic availability after oral ingestion. The intravenous budesonide pharmacokinetics were, however, similar in cirrhotic patients and in healthy subjects.
As budesonide and formoterol are primarily eliminated via hepatic metabolism, an increased exposure can be expected in patients with severe hepatic impairment.
Patients With Renal Impairment
Studies with BREZTRI AEROSPHERE evaluating the effect of renal impairment on the pharmacokinetics of budesonide, glycopyrronium, and formoterol were not conducted.
The effect of renal impairment on the exposure to budesonide, glycopyrronium, and formoterol for up to 24 weeks was evaluated in a population pharmacokinetic analysis. Estimated glomerular filtration rate (eGFR) varied from 31-192 mL/min representing a range of moderate to no renal impairment. Simulation of the systemic exposure (AUC0-12) in subjects with COPD with moderate renal impairment (eGFR of 45 mL/min) indicates an approximate 68% increase for glycopyrronium compared to subjects with COPD with normal renal function (eGFR of >90 mL/min). Renal function was found not to significantly affect exposure to budesonide or formoterol after drug clearance adjusted by age or body weight in a population pharmacokinetic analysis.
Drug Interactions
No pharmacokinetic interaction has been observed between budesonide, glycopyrrolate, and formoterol fumarate when administered in combination by the inhaled route. Specific drug interaction studies of BREZTRI AEROSPHERE with other co-administered drugs have not been performed.
Ketoconazole And Itraconazole
Ketoconazole and itraconazole, strong inhibitors of cytochrome P450 (CYP) isoenzyme 3A4 (CYP3A4), the main metabolic enzyme for corticosteroids, increased plasma levels of orally ingested budesonide and orally inhaled budesonide, respectively.
Cimetidine
At recommended doses, cimetidine, a non-specific inhibitor of CYP enzymes, had a slight but clinically insignificant effect on the pharmacokinetics of oral budesonide.
Clinical Studies
The clinical efficacy of BREZTRI AEROSPHERE has been evaluated in two (Trial 1 and 2) randomized, double-blind, multicenter, parallel-group trials in subjects with moderate to very severe COPD who remained symptomatic while receiving 2 or more inhaled maintenance treatments for COPD for at least 6 weeks prior to screening.
Trial 1 (NCT02465567) was conducted over 52 weeks in a total of 8,588 subjects randomized (1:1:1:1) to receive BREZTRI AEROSPHERE (budesonide/glycopyrrolate/formoterol fumarate 320 mcg/18 mcg/9.6 mcg), budesonide, glycopyrrolate and formoterol fumarate [BGF MDI 160 mcg/18 mcg/9.6 mcg] (the BGF MDI 160 mcg/18 mcg/9.6 mcg dosing regimen is not approved), glycopyrrolate and formoterol fumarate [GFF MDI 18 mcg/9.6 mcg], or budesonide and formoterol fumarate [BFF MDI 320 mcg/9.6 mcg], all administered twice daily. GFF MDI and BFF MDI used the same inhaler and excipients as BREZTRI AEROSPHERE.
Trial 1 was conducted in subjects with a history of 1 or more moderate or severe exacerbations in the year prior to screening, post-bronchodilator FEV1/FVC ratio less than 0.7 and the post-bronchodilator FEV1 less than 65% predicted normal value.
The population demographics across all treatments in Trial 1 were: mean age of 65 years, 60% male, 85% Caucasian, and an average smoking history of 48 pack-years, with 41% identified as current smokers. The mean post-bronchodilator percent predicted FEV1 was 43% (range 16% to 73%). At study entry, the most common COPD medications were ICS + long-acting muscarinic antagonist (LAMA) + LABA (39%), ICS + LABA (31%), and LAMA + LABA (14%).
In Trial 1, the primary endpoint was the rate of moderate or severe COPD exacerbations for BREZTRI AEROSPHERE compared with GFF MDI and BFF MDI.
Trial 2 (NCT02497001) was conducted over 24 weeks, in a total of 1,896 subjects randomized (2:2:1:1) to receive BREZTRI AEROSPHERE (budesonide/glycopyrrolate/formoterol fumarate 320 mcg/18 mcg/9.6 mcg), glycopyrrolate and formoterol fumarate [GFF MDI 18 mcg/9.6 mcg], budesonide and formoterol fumarate [BFF MDI 320 mcg/9.6 mcg], or open-label active comparator, all administered twice daily. GFF MDI and BFF MDI used the same inhaler and excipients as BREZTRI AEROSPHERE. Trial 2 was conducted in subjects with a screening post-bronchodilator FEV1/FVC ratio less than 0.7 and post-bronchodilator FEV1 less than 80% predicted normal value. Subjects in Trial 2 were not required to have a history of moderate or severe exacerbations in the year prior to screening.
The population demographics across all treatments in Trial 2 were: mean age of 65 years, 71% male, 50% Caucasian, 45% Asian, and an average smoking history of 52 pack-years, with 40% identified as current smokers. The mean post-bronchodilator percent predicted FEV1 was 50% (range 22% to 84%). At study entry, the most common COPD medications were ICS + LAMA + LABA (27%), ICS + LABA (38%), and LAMA + LABA (20%).
In Trial 2, the primary endpoints were FEV1 area under the curve from 0-4 hours (FEV1 AUC0-4) at Week 24 for BREZTRI AEROSPHERE compared to BFF MDI and change from baseline in morning pre-dose trough FEV1 at Week 24 for BREZTRI AEROSPHERE compared to GFF MDI.
Lung Function
In Trial 1, a subset of subjects were included in a spirometric sub-study with primary endpoints of FEV1 AUC0-4 at Week 24 (mL) and change from baseline in morning pre-dose trough FEV1 at Week 24 (mL). BREZTRI AEROSPHERE demonstrated an increase in on-treatment FEV1 AUC0-4 and trough FEV1 at Week 24 relative to BFF MDI and GFF MDI (Table 2). The effects on lung function (mean change from baseline in on-treatment morning pre-dose trough FEV1) of BREZTRI AEROSPHERE compared with GFF MDI and BFF MDI were observed at all timepoints over the course of the study (Figure 1).
In Trial 2, BREZTRI AEROSPHERE demonstrated an increase in on-treatment FEV1 AUC0-4 at Week 24 relative to BFF MDI and an increase in mean change from baseline in morning pre-dose trough FEV1 at Week 24 compared with GFF MDI (Table 2). The comparison of BREZTRI AEROSPHERE with GFF MDI in mean change from baseline in morning pre-dose trough FEV1 at Week 24 was not statistically significant.
In both trials, there were consistent improvements in lung function in patient subgroups classified by age, sex, degree of airflow limitation (moderate, severe, and very severe), and previous ICS use.
Table 2: Change in FEV1 AUC0-4 and Least Square (LS) Mean Change from Baseline in Morning Pre-dose Trough FEV1 (mL) at Week 24 in Trial 1 (Spirometric Sub-study) and Trial 21
| Treatment | N | FEV1 AUC0-4 at Week 24 | N | Change from baseline in morning pre-dose trough FEV1 at Week 24 | ||
| Difference from | Difference from | |||||
| GFF MDI | BFF MDI | GFF MDI | BFF MDI | |||
| Trial 1 (Sub-study) | ||||||
| BREZTRI AEROSPHERE | 633 | N=588 53 mL (29, 77) | N=605 119 mL (95, 143) | 634 | N=586 35 mL (12, 57) | N=608 76 mL (54, 99) |
| Trial 2 | ||||||
| BREZTRI AEROSPHERE | 436 | N=403 5 mL (-25, 34) | N=201 116 mL (80, 152) | 565 | N=522 13 mL (-9, 36) | N=266 74 mL (47, 102) |
| 1The analysis excluded spirometry data collected after discontinuation of study treatment. | ||||||
Figure 1: Adjusted Mean Change from Baseline in Trough FEV1 Over Time (Trial 1)1
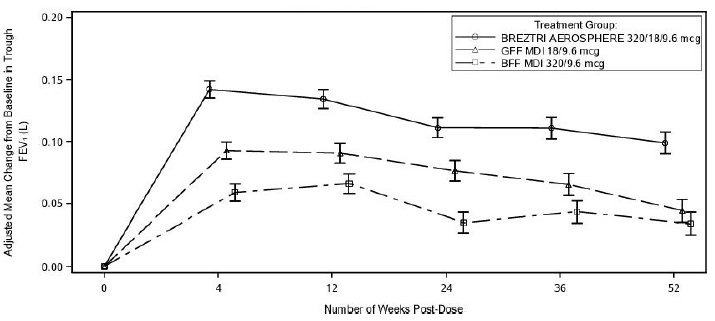 |
1The analysis excluded spirometry data collected after discontinuation of study treatment.
In Trial 2, the median time to onset on Day 1, defined as a 100 mL increase from baseline in FEV1, was within 5 minutes in subjects receiving BREZTRI AEROSPHERE 320 mcg/18 mcg/9.6 mcg. In Trial 1, subjects treated with BREZTRI AEROSPHERE 320 mcg/18 mcg/9.6 mcg on average used less daily rescue medication over 24 weeks compared to subjects treated with GFF MDI 18 mcg/9.6 mcg and BFF MDI 320 mcg/9.6 mcg.
Exacerbations
In Trial 1, the primary endpoint was the rate of on-treatment moderate or severe COPD exacerbations in subjects treated with BREZTRI AEROSPHERE 320 mcg/18 mcg/9.6 mcg compared with GFF MDI and BFF MDI.
Exacerbations were defined as worsening of 2 or more major symptoms (dyspnea, sputum volume, and sputum color) or worsening of any 1 major symptom together with any 1 of the following minor symptoms: cough, wheeze, sore throat, colds (nasal discharge and/or nasal congestion), and fever without other cause for at least 2 consecutive days. Exacerbations were considered to be moderate severity if treatment with systemic corticosteroids and/or antibiotics was required and were considered to be severe if they resulted in hospitalization or death.
In Trial 1, treatment with BREZTRI AEROSPHERE 320 mcg/18 mcg/9.6 mcg demonstrated a reduction in the rate of on-treatment moderate or severe COPD exacerbations over 52 weeks compared with GFF MDI and BFF MDI (see Table 3).
Table 3: Rates of moderate or severe exacerbations over 52 weeks in Trial 11
| Treatment2 (N) | Mean Annual Rate | Rate Ratio vs. Comparator (95% CI) | % Reduction in Exacerbation Rate (95% CI) | P-Value |
| BREZTRI AEROSPHERE (N=2137) | 1.08 | N/A | N/A | N/A |
| GFF MDI (N=2120) | 1.42 | 0.76 (0.69, 0.83) | 24 (17, 31) | p<0.0001 |
| BFF MDI (N=2131) | 1.24 | 0.87 (0.79, 0.95) | 13 (5, 21) | p=0.0027 |
| 1On-treatment analyses excluded exacerbation data collected after discontinuation of study treatment. 2 BREZTRI (BREZTRI AEROSPHERE) = budesonide/glycopyrrolate/formoterol fumarate 320 mcg/18 mcg/9.6 mcg; GFF MDI = glycopyrrolate/formoterol fumarate 18 mcg/9.6 mcg; BFF MDI = budesonide/formoterol fumarate 320 mcg/9.6 mcg | ||||
In Trial 2, treatment with BREZTRI AEROSPHERE 320 mcg/18 mcg/9.6 mcg reduced the annual rate of on-treatment moderate or severe COPD exacerbations compared with GFF MDI 18 mcg/9.6 mcg (rate ratio [95% CI]: 0.48 [0.37, 0.64]), and compared with BFF MDI 320 mcg/9.6 mcg (rate ratio [95% CI]: 0.82 [0.58, 1.17]). The comparison of BREZTRI AEROSPHERE with GFF MDI was not statistically significant due to failure higher in the analysis hierarchy.
Health-Related Quality Of Life
In both trials, health-related quality of life was assessed using the St. George’s Respiratory Questionnaire (SGRQ) responder analysis which was defined as an improvement in SGRQ score from baseline of 4 or more.
In Trial 1, the on-treatment percentage of SGRQ responders at Week 24 was greater for subjects treated with BREZTRI AEROSPHERE 320 mcg/18 mcg/9.6 mcg (50%) compared with both GFF MDI 18 mcg/9.6 mcg (43%; odds ratio 1.4; 95% CI: 1.2, 1.5) and BFF MDI 320 mcg/9.6 mcg (45%; odds ratio 1.2; 95% CI: 1.1, 1.4). Similar differences between treatments were observed at Week 52.
In Trial 2, the on-treatment percentage of SGRQ responders at Week 24 was greater for subjects treated with BREZTRI AEROSPHERE 320 mcg/18 mcg/9.6 mcg (50%) compared with both GFF MDI 18 mcg/9.6 mcg (44%; odds ratio 1.3; 95% CI: 1.0, 1.6) and BFF MDI 320 mcg/9.6 mcg (43%; odds ratio 1.3; 95% CI: 1.0, 1.7).
Medication Guide
PATIENT INFORMATION
BREZTRI AEROSPHERE™
(brez-TREE)
(budesonide, glycopyrrolate, and formoterol fumarate) inhalation aerosol, for oral inhalation use
What is BREZTRI AEROSPHERE?
BREZTRI AEROSPHERE combines 3 medicines, an inhaled corticosteroid (ICS) medicine (budesonide), an anticholinergic medicine (glycopyrrolate), and a long-acting beta2-adrenergic agonist (LABA) medicine (formoterol fumarate) in 1 inhaler, delivered as a propelled spray.
- ICS medicines such as budesonide help to decrease inflammation in the lungs. Inflammation in the lungs can lead to breathing problems.
- Anticholinergic medicines, such as glycopyrrolate and LABA medicines, such as formoterol fumarate help the muscles around the airways in your lungs stay relaxed to prevent symptoms, such as wheezing, cough, chest tightness, and shortness of breath. These symptoms can happen when the muscles around the airways tighten. This makes it hard to breathe.
- BREZTRI AEROSPHERE is a prescription medicine used long term to treat people with chronic obstructive pulmonary disease (COPD). COPD is a chronic lung disease that includes chronic bronchitis, emphysema, or both.
- BREZTRI AEROSPHERE is used as 2 inhalations, 2 times each day (2 puffs in the morning and 2 puffs in the evening) to improve symptoms of COPD for better breathing and to reduce the number of flare-ups (the worsening of your COPD symptoms for several days).
- BREZTRI AEROSPHERE is not for the treatment of asthma. It is not known if BREZTRI AEROSPHERE is safe and effective in people with asthma. BREZTRI AEROSPHERE contains formoterol fumarate. LABA medicines such as formoterol fumarate when used alone increase the risk of hospitalizations and death from asthma problems. BREZTRI AEROSPHERE contains an ICS, an anticholinergic, and a LABA. When an ICS and LABA are used together, there is not a significant risk in hospitalizations and deaths from asthma problems.
- BREZTRI AEROSPHERE is not to be used to relieve sudden breathing problems and will not replace a rescue inhaler. Always have a rescue inhaler (an inhaled, short-acting bronchodilator) with you to treat sudden breathing problems. If you do not have a rescue inhaler, contact your healthcare provider to have one prescribed for you.
- BREZTRI AEROSPHERE should not be used in children. It is not known if BREZTRI AEROSPHERE is safe and effective in children.
- Do not use BREZTRI AEROSPHERE if you are allergic to budesonide, glycopyrrolate, formoterol, or any of the ingredients in BREZTRI AEROSPHERE. See “What are the ingredients in BREZTRI AEROSPHERE?” at the end of this Patient Information leaflet below for a complete list of ingredients in BREZTRI AEROSPHERE.
Before using BREZTRI AEROSPHERE, tell your healthcare provider about all of your medical conditions, including if you:
- have heart problems.
- have high blood pressure.
- have seizures.
- have thyroid problems.
- have diabetes.
- have liver problems.
- have kidney problems.
- have weak bones (osteoporosis).
- have an immune system problem.
- have eye problems such as glaucoma or cataracts. BREZTRI AEROSPHERE may make your glaucoma worse.
- have prostate or bladder problems, or problems passing urine. BREZTRI AEROSPHERE may make these problems worse.
- have any type of viral, bacterial, parasitic, or fungal infection.
- are exposed to chickenpox or measles.
- are pregnant or plan to become pregnant. It is not known if BREZTRI AEROSPHERE may harm your unborn baby.
- are breastfeeding. It is not known if the medicines in BREZTRI AEROSPHERE pass into your breast milk and if they can harm your baby. You and your healthcare provider should decide if
- you will take BREZTRI AEROSPHERE while breastfeeding.
Tell your healthcare provider about all the medicines you take, including prescription and over-thecounter medicines, vitamins, and herbal supplements. BREZTRI AEROSPHERE and certain other medicines may interact with each other. This may cause serious side effects.
Especially tell your healthcare provider if you take:
- anticholinergics (including tiotropium, ipratropium, aclidinium, and umeclidinium)
- other LABAs (including salmeterol, formoterol fumarate, arformoterol tartrate, vilanterol, olodaterol, and indacaterol)
- atropine
- antifungal or anti-HIV medicines
Know the medicines you take. Keep a list of them to show your healthcare provider and pharmacist each time you get a new medicine.
How should I use BREZTRI AEROSPHERE?
Read the step-by-step instructions for using BREZTRI AEROSPHERE at the end of this Patient Information leaflet.
- Before using BREZTRI AEROSPHERE, make sure your healthcare provider has taught you how to use the inhaler and you understand how to use it correctly.
- Use BREZTRI AEROSPHERE exactly as your healthcare provider tells you to use it. Do not use BREZTRI AEROSPHERE more often than prescribed.
- Use 2 inhalations of BREZTRI AEROSPHERE, 2 times each day (2 puffs in the morning and 2 puffs in the evening).
- Do not take more than 2 inhalations of BREZTRI AEROSPHERE 2 times each day.
- If a dose (2 puffs) of BREZTRI AEROSPHERE is missed, it should be taken as soon as possible and the next dose should be taken at the usual time. Do not take more than one dose to make up for a forgotten dose.
- Rinse your mouth with water and spit the water out after each dose (2 puffs) of BREZTRI AEROSPHERE. Do not swallow the water. This will help to reduce the chance of getting a fungus infection (thrush) in the mouth and throat.
- If you take too much BREZTRI AEROSPHERE, call your healthcare provider or go to the nearest hospital emergency room right away if you have any unusual symptoms, such as worsening shortness of breath, chest pain, increased heart rate, or shakiness.
- Do not spray BREZTRI AEROSPHERE in your eyes. If BREZTRI AEROSPHERE gets in your eyes, rinse them well with water. If redness continues, call your healthcare provider.
- Do not use other medicines that contain a LABA or an anticholinergic for any reason. Ask your healthcare provider or pharmacist if any of your other medicines are LABA or anticholinergic containing medicines.
- Do not change or stop any medicines used to control or treat your breathing problems. Your healthcare provider will change your medicines as needed.
- BREZTRI AEROSPHERE does not relieve sudden breathing problems and you should not take extra doses of BREZTRI AEROSPHERE to relieve sudden symptoms. Always have a rescue inhaler with you to treat sudden symptoms. If you do not have a rescue inhaler, call your healthcare provider to have one prescribed for you.
- Call your healthcare provider or get emergency medical care right away if:
- your breathing problems get worse.
- you need to use your rescue inhaler more often than usual.
- your rescue inhaler does not work as well to relieve your symptoms.
What are the possible side effects of BREZTRI AEROSPHERE?
BREZTRI AEROSPHERE can cause serious side effects, including:
- fungal infection in your mouth or throat (thrush). Rinse your mouth with water without swallowing after using BREZTRI AEROSPHERE to help reduce your chance of getting thrush.
- pneumonia. People with COPD have a higher chance of getting pneumonia. BREZTRI AEROSPHERE may increase your chance of getting pneumonia. Call your healthcare provider if you notice any of the following symptoms:
- increase in mucus (sputum) production
- change in mucus color
- fever
- chills
- increased cough
- increased breathing problems
- weakened immune system and increased chance of getting infections (immunosuppression).
- reduced adrenal function (adrenal insufficiency). Adrenal insufficiency is a condition where the adrenal glands do not make enough steroid hormones. This can happen when you stop taking oral corticosteroid medicines (such as prednisone) and start taking a medicine containing an ICS (such as BREZTRI AEROSPHERE). During this transition period, when your body is under stress from fever, trauma (such as a car accident), infection, surgery, or worse COPD symptoms, adrenal insufficiency can get worse and may cause death.
Symptoms of adrenal insufficiency include:
- feeling tired
- lack of energy
- weakness
- nausea and vomiting
- low blood pressure (hypotension)
- sudden breathing problems immediately after inhaling your medicine. If you have sudden breathing problems immediately after inhaling your medicine, stop taking BREZTRI AEROSPHERE and call your healthcare provider right away.
- serious allergic reactions. Call your healthcare provider or get emergency medical care if you get any of the following symptoms of a serious allergic reaction:
- rash
- hives
- swelling of your face, mouth, and tongue
- breathing problems
- effects on your heart.
- increased blood pressure
- a fast or irregular heartbeat
- chest pain
- effects on your nervous system.
- tremor
- nervousness
- bone thinning or weakness (osteoporosis).
- new or worsened eye problems including acute narrow-angle glaucoma and cataracts.
Acute narrow-angle glaucoma can cause permanent loss of vision if not treated. Symptoms of acute narrow-angle glaucoma may include:- eye pain or discomfort
- nausea or vomiting
- blurred vision
- seeing halos or bright colors around lights
- red eyes
If you have these symptoms, call your healthcare provider right away before taking another dose.
- urinary retention. People who take BREZTRI AEROSPHERE may develop new or worsening urinary retention. Symptoms of urinary retention may include:
- difficulty urinating
- urinating frequently
- painful urination
- urination in a weak stream or drips
If you have these symptoms of urinary retention, stop taking BREZTRI AEROSPHERE and call your healthcare provider right away before taking another dose.
- changes in laboratory blood values, including high levels of blood sugar (hyperglycemia) and low levels of potassium (hypokalemia). Low levels of potassium may cause symptoms of muscle spasm, muscle weakness, or abnormal heart rhythm.
Common side effects of BREZTRI AEROSPHERE include
- upper respiratory tract infection
- pneumonia
- back pain
- thrush in your mouth and throat. Rinse your mouth with water without swallowing after use to help prevent this.
- joint pain
- flu
- headache
- high blood sugar levels
- muscle spasms
- cough
- inflammation of the sinuses
- diarrhea
- hoarseness
- painful and frequent urination (signs of a urinary tract infection)
- nausea
- difficulty sleeping
- feeling anxious
- awareness of your heart beating (palpitations)
Tell your healthcare provider about any side effect that bothers you or that does not go away.
These are not all the possible side effects of BREZTRI AEROSPHERE. Ask your healthcare provider or pharmacist for more information.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800- FDA-1088. You may also report side effects to AstraZeneca at 1-800-236-9933.
How should I store BREZTRI AEROSPHERE?
- Store BREZTRI AEROSPHERE at room temperature between 68°F to 77°F (20°C to 25°C). Keep in a dry place away from heat and sunlight.
- Store BREZTRI AEROSPHERE in the unopened foil pouch and only open when ready for use.
- Do not put a hole in the BREZTRI AEROSPHERE canister.
- Do not use or store BREZTRI AEROSPHERE near heat or a flame. Temperatures above 120°F (49°C) may cause the canister to burst.
- Do not throw the BREZTRI AEROSPHERE canister into a fire or an incinerator.
- Throw away BREZTRI AEROSPHERE 3 months after you open the foil pouch (for the 120- inhalation canister), or 3 weeks after you open the foil pouch (for the 28-inhalation canister), or when the dose indicator reaches zero “0”, whichever comes first.
- Keep BREZTRI AEROSPHERE and all medicines out of the reach of children.
General information about the safe and effective use of BREZTRI AEROSPHERE.
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use BREZTRI AEROSPHERE for a condition for which it was not prescribed. Do not give your BREZTRI AEROSPHERE to other people, even if they have the same condition that you have. It may harm them.
You can ask your healthcare provider or pharmacist for information about BREZTRI AEROSPHERE that is written for healthcare professionals.
What are the ingredients in BREZTRI AEROSPHERE?
Active ingredients: micronized budesonide, micronized glycopyrrolate, and micronized formoterol fumarate
Inactive ingredients: hydrofluoroalkane (HFA 134a) and porous particles (comprised of DSPC [1,2- Distearoyl-sn-glycero-3-phosphocholine] and calcium chloride)
Instructions for Use
BREZTRI AEROSPHERE™
(brez-TREE) (budesonide, glycopyrrolate, and formoterol fumarate) inhalation aerosol, for oral inhalation use
Read this Instructions for Use before you start using BREZTRI AEROSPHERE and each time you get a refill. There may be new information. This information does not take the place of talking to your doctor about your medical condition or treatment.
Important Information:
For oral inhalation use only.
Use BREZTRI AEROSPHERE exactly as your healthcare provider tells you to.
If you have any questions about the use of your inhaler, ask your healthcare provider or pharmacist.
Clean your inhaler 1 time each week. See Steps 1 to 8, “How to clean your BREZTRI AEROSPHERE inhaler”.
Parts of your BREZTRI AEROSPHERE inhaler (See Figure 1):
BREZTRI AEROSPHERE comes as a canister that fits into an actuator with a dose indicator.
Do not use the BREZTRI AEROSPHERE actuator with a canister of medicine from any other inhaler.
Do not use the BREZTRI AEROSPHERE canister with an actuator from any other inhaler.
Figure 1
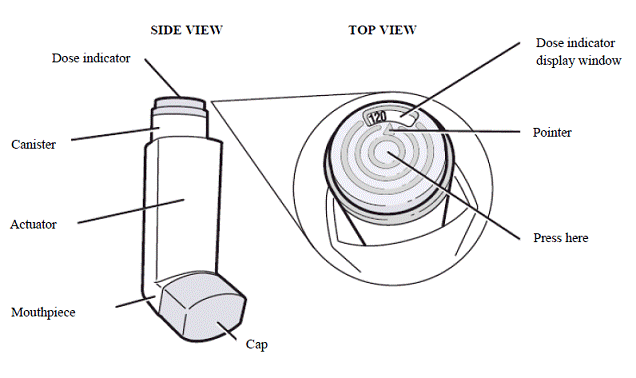 |
- BREZTRI AEROSPHERE comes with a dose indicator located on the top of the canister (See Figure 1). The dose indicator display window will show you how many puffs of medicine you have left. A puff of medicine is released each time you press the center of the dose indicator.
Before you use BREZTRI AEROSPHERE for the first time make sure that the pointer on the dose indicator should point to the right of the “120” inhalation mark in the dose indicator display window (See Figure 1). If you have a 7-day inhaler (28 inhalation canister), the pointer should point to the right of the “30” inhalation mark.
- The pointer will be pointing to 120 after 10 puffs are delivered from BREZTRI AEROSPHERE. This means that there are 120 puffs of medicine left in the canister (See Figure 2a).
- The pointer will be pointing between 100 and 120 after you take 10 more puffs. This means that there are 110 puffs of medicine left in the canister (See Figure 2b).
- The pointer will be pointing to 100 after you take 10 more puffs. This means that there are 100 puffs of medicine left in the canister (See Figure 2c).
Figure 2a, 2b and 2c
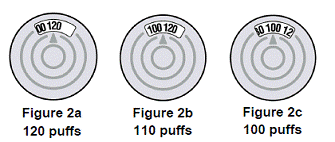 |
- The dose indicator display window will continue to move after every 10 puffs. The number in the dose indicator display window will continue to change after every 20 puffs.
Figure 2d
 |
- The color in the dose indicator display window will change to red, as shown in the shaded area, when there are only 20 puffs of medicine left in your inhaler (See Figure 2d).
- The dose indicator for the 7-day inhaler, 28 inhalation canister, moves after every 10 puffs; with markings for 30, 15 and 0 puffs. The color in the 7-day inhaler, 28 inhalation canister dose indicator display window will change to red when there are only 10 puffs of medicine left in your inhaler.
Preparing your BREZTRI AEROSPHERE inhaler for use:
- Your BREZTRI AEROSPHERE inhaler comes in a foil pouch that contains a drying packet (desiccant).
- Take the BREZTRI AEROSPHERE inhaler out of the foil pouch.
- Throw away the pouch and the drying packet. Do not eat or breathe in the contents of the drying packet.
- BREZTRI AEROSPHERE should be at room temperature before you use it.
Figure 3
 |
Priming your BREZTRI AEROSPHERE inhaler:
Before you use BREZTRI AEROSPHERE for the first time, you must prime the inhaler.
- Remove the cap from the mouthpiece (See Figure 3). Check inside the mouthpiece for objects before use.
- Hold the inhaler in the upright position away from your face and shake the inhaler well (See Figure 4).
Figure 4
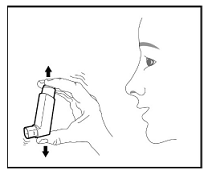 |
- Press down firmly on the center of the dose indicator until the canister stops moving in the actuator, to release a puff of medicine from the mouthpiece (See Figure 5). You may hear a soft click from the dose indicator as it counts down during use.
Figure 5
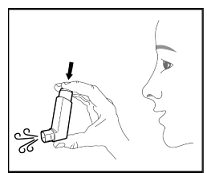 |
- Repeat the priming steps 3 more times (See Figure 4 and Figure 5). Shake the inhaler well before each priming puff.
- After priming 4 times, the dose indicator should be pointing to the right of “120” (See Figure 1) and your inhaler is now ready to use. If you have a 7-day inhaler (28 inhalation canister), the pointer should point to the right of the “30” inhalation mark.
- Replace the cap until you are ready to use your BREZTRI AEROSPHERE inhaler.
Using your BREZTRI AEROSPHERE inhaler:
Step 1: Remove the cap from the mouthpiece (See Figure 6).
Figure 6
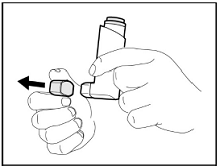 |
Step 2: Shake the inhaler well before each use (See Figure 7).
Figure 7
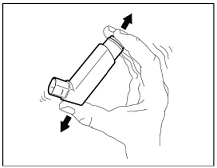 |
Step 3: Hold the inhaler with the mouthpiece pointing towards you and breathe out as fully as you can through your mouth (See Figure 8).
Figure 8
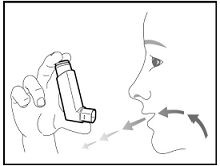 |
Step 4: Close your lips around the mouthpiece and tilt your head back, keeping your tongue below the mouthpiece (See Figure 9).
Figure 9
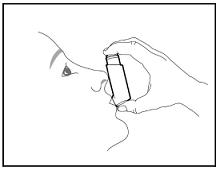 |
Step 5: While breathing in deeply and slowly, press down on the center of the dose indicator until the canister stops moving in the actuator and a puff of medicine has been released (See Figure 10). Then stop pressing the dose indicator.
Figure 10
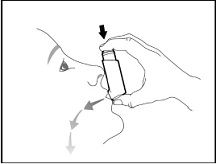 |
Step 6: When you have finished breathing in, remove the mouthpiece from your mouth. Hold your breath as long as you comfortably can, up to 10 seconds (See Figure 11).
Figure 11
 |
Step 7: Breathe out gently (See Figure 12). Repeat Steps 2 through 7 to take your second puff of BREZTRI AEROSPHERE.
Figure 12
 |
Step 8: Replace the cap over the mouthpiece right away after use (See Figure 13).
Figure 13
 |
Step 9: Rinse your mouth with water to remove any excess medicine. Do not swallow.
It is important to store BREZTRI AEROSPHERE in a dry place.
How to clean your BREZTRI AEROSPHERE inhaler:
Clean the inhaler once each week. It is very important to keep your inhaler clean so that medicine will not build-up and block the spray through the mouthpiece (See Figure 14).
Figure 14
 |
Step 1: Take the canister out of the actuator (See Figure 15). Do not clean the canister or let it get wet.
Figure 15
 |
Step 2: Take the cap off the mouthpiece.
Step 3: Hold the actuator under the tap and run warm water through it for about 30 seconds. Turn the actuator upside down and run warm water through it again for about 30 seconds (See Figure 16).
Figure 16
 |
Step 4: Shake off as much water from the actuator as you can.
Step 5: Look into the actuator and the mouthpiece to make sure any medicine build-up has been completely washed away. If there is any build-up, repeat Steps 3 through 5 in the section “How to clean your BREZTRI AEROSPHERE inhaler”.
Step 6: Let the actuator air-dry completely, such as overnight (See Figure 17). Do not put the canister back into the actuator if it is still wet.
Figure 17
 |
Step 7: When the actuator is dry, gently press the canister down in the actuator (See Figure 18). Do not press down too hard on the canister. This could cause a puff of medicine to be released.
Figure 18
 |
Step 8: Re-prime your BREZTRI AEROSPHERE inhaler after each cleaning. To re-prime the inhaler, shake the inhaler well and press down on the center of the dose indicator 2 times to release a total of 2 puffs into the air away from your face. Your inhaler is now ready to use.
If BREZTRI AEROSPHERE is not used for more than 7 days, or is dropped, you will need to re-prime it before use.
To re-prime the inhaler, shake the inhaler well and press down on the center of the dose indicator 2 times to release a total of 2 puffs into the air away from your face. Your inhaler is now ready to use again.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
